Abstract
Global climate change is threatening the persistence of coral reefs as associated summer heatwaves trigger the loss of microalgal endosymbionts (Symbiodiniaceae) from the coral tissues, or coral bleaching. We infected aposymbiotic juveniles of the coral Acropora tenuis with either wildtype (WT10) or heat-evolved (SS1 or SS8) Symbiodiniaceae strains Cladocopium proliferum (formerly referred to as Cladocopium goreaui and Cladocopium C1acro). After 10 months at 27 °C, SS8-juveniles were 2 × larger than SS1- or WT10-juveniles. In response to a simulated heatwave (31 °C for 41 days), the WT10-juveniles bleached and showed a decline in respiration while cell densities and respiration in both SS-juvenile groups remained unchanged compared to the controls. These results reveal that some heat-evolved strains can increase the bleaching tolerance of juvenile corals without a trade-off against growth. This response is opposite to the lower nutrient provisioning often reported for naturally thermotolerant Symbiodiniaceae (e.g. genus Durusdinium), thereby offering enhanced fitness to the host without the ecological consequences of diminished growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reef-building corals are suffering unprecedented mortality caused by the increase in the frequency and intensity of marine heatwaves (Heron et al. 2017). These mass mortality events are caused by coral bleaching, the loss of Symbiodiniaceae from the coral tissue (Weis 2008). Under ambient conditions, Symbiodiniaceae share photosynthates with their coral hosts, fulfilling most of their nutritional requirements (Yellowlees et al. 2008). However, when corals are heat-stressed, damage to the Symbiodiniaceae photosystems (Weis 2008) results in the excess production of reactive oxygen species which is often followed by the loss of Symbiodiniaceae, coral starvation and ultimately mortality if temperatures remain elevated for too long (Wiedenmann et al. 2013; Rädecker et al. 2021). Future projections suggest that even under a 1.5 °C warming scenario, coral reefs will decline by 70–90% (IPCC 2018). This mounting threat has precipitated the need to identify and establish novel approaches to mitigate current and future reef degradation. These approaches include the enhancement of coral thermal tolerance via bioengineering methods (i.e. assisted evolution (van Oppen et al. 2015)).
Experimental evolution of Symbiodiniaceae across replicate cultures and strains can increase in vitro thermal tolerance (Chakravarti and van Oppen 2018; Buerger et al. 2020). Following introduction of heat-evolved coral symbionts of the common and widely distributed species Cladocopium proliferum (Butler et al. 2023) into Acropora tenuis larvae, about a third of the strains increased the thermal bleaching tolerance of larvae (Buerger et al. 2020). Survival was also enhanced at the juvenile phase of A. tenuis (Quigley and van Oppen 2022). Here we show enhanced thermal tolerance in A. tenuis juveniles infected with heat-evolved C. proliferum without a reduction in coral growth at ambient temperature.
Methods
Methods for coral spawning, symbiont inoculation into coral juveniles, heat stress experiment, juvenile size, Symbiodiniaceae cell density, stable isotope incubations, and photosynthetic performance, and respiration are detailed in the Supplementary Information (SI). Heat-evolved (SS1 and SS8) and wildtype (WT10) C. proliferum were generated as previously described (Chakravarti and van Oppen 2018; Buerger et al. 2020; SI).
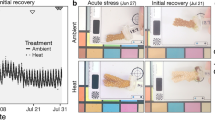
After ~ 10 months of growth, the juveniles were subjected to a heat stress experiment, which started on 10 October 2019 (D0) and lasted for 41 days (4 days of ramping plus 37 days at elevated temperature). Juveniles that were settled onto polystyrene black sheets were transferred to individual, closed plastic containers placed within individual 45 L experimental tanks. These tanks were set at constant flow-through to help maintain the temperature within each container. Three replicate tanks were used for each temperature treatment (27 °C or 31 °C), with n = 1–2 replicate containers per strain, with multiple juveniles in each. The position of the containers within each tank was randomised and individual air lines were supplied. HOBO pendant temperature loggers were added to each tank and Osram brand T5 lights (HO 24 W/865) set at 60 PAR (µmol m−2 s−1) were positioned above each. Light intensity was confirmed with a ULM-500 light meter with a US-SQS/L sensor (Walz). Temperature in the heat treatment was ramped from 27 to 31 °C at a rate of 1 °C per day, and measurements (survival, growth, symbiont cell densities, photophysiology, respiration, nutrient transfer) started once the heat stress treatment reached 31 °C (15 October 2019: D5). Measurements were then taken at the following timepoints: day 5 (15 October), and days 38, 39, 40, 41 (between 17 and 20 of November 2019). Measurements were staggered over four days given the amount of time each required. Specifically, photographs and PSII maximum quantum yields (Fv/Fm) were collected throughout the heat stress period, stable isotope incubation and cell counts were carried out on days 5 and 39, and respirometry on days 40–41. Physiological and metabolic measurements were performed at similar times of the day to avoid biases linked to circadian rhythms. Symbiont cell densities in the juvenile corals and symbiont photophysiology were used as proxies for bleaching. Other traits, like chlorophyll a, were not measured given the young age of the juveniles and therefore, low biomass available.
All statistical analyses were run in R version v.3.2.3 (R Core Team 2013). Values were calculated as percentage change in the trait between the first (D5) and the final (D41) day of heating. Differences between treatments were assessed using linear models using the nlme package (Pinheiro et al. 2014) with interactive fixed effects of culture (WT10, SS1, SS8) × temperature (27 °C or 31 °C).
Results and discussion
SS-juveniles grow as fast or faster than WT-juveniles at ambient temperature
Symbiodiniaceae exhibit a high level of physiological diversity, with naturally thermotolerant strains typically translocating less carbon to their host at ambient temperatures compared with thermosensitive symbionts, translating to slower host growth (Cantin et al. 2009). To explore whether this trait trade-off has evolved during the experimental heat selection of the Symbiodiniaceae strains, coral juveniles infected with either SS1, SS8 or WT10 derived from the same clonal culture of Cladocopium proliferum were maintained at ambient conditions for 10 months at which time their size was measured. Contrary to our expectations, the SS8-juveniles were twice as large (~ 3.6 mm2) as juveniles infected with SS1 or WT10 (both ~ 1.7 mm2, lm, p < 1e-07; Fig. 1).
SS-juveniles are more bleaching tolerant than WT-juveniles
We have previously shown that SS1 and SS8 enhance thermal bleaching tolerance (Buerger et al. 2020) and survival (Quigley and van Oppen 2022) of A. tenuis larvae or juveniles but the trade-off with growth was unassessed. We therefore subjected the three host-symbiont pairs to a simulated heatwave at a maximum temperature of 31 °C that lasted for 40 days, with a subset of the juveniles kept at 27 °C as controls. To measure the extent of bleaching, the in hospite symbiont cell densities in the three host-symbiont pairs exposed to elevated or ambient temperatures were assessed at day 5 and 39 (D5 and D39). When the maximum temperature was reached on D5, the average symbiont cell density in A. tenuis juveniles did not differ between temperature treatments for any of the host-symbiont pairs nor among host-symbiont pairs within a temperature treatment (Fig. 2, average across the three ~ 50,000 ± 10,000 cells/mm2 at 27 °C and ~ 36,000 ± 6,000 at 31 °C). By D39, cell densities at 27 °C had increased slightly for WT10-juveniles and decreased slightly for the SS-juveniles, however, these changes were not statistically significant (p = 0.19–1.0 for all pairwise comparisons for differences within timepoint).
At D39, however, symbiont cell densities in WT10-juveniles were 2.6-times lower at 31 °C compared to 27 °C (linear model (lm), p = 0.0438), implying that WT10-juveniles lost cells in this timepoint in response to the elevated temperature treatment. In contrast, SS-juveniles (SS1- and SS8-juveniles) showed no difference in Symbiodiniaceae densities at 31 °C relative to 27 °C nor between temperature treatments on D39 (p = 0.49–1.0 for all pairwise comparisons). This confirms previous independent results that these heat-evolved symbionts confer increased tolerance to both the larval (Buerger et al. 2020) and juvenile (Quigley and van Oppen 2022) life stages of this coral species.
The photochemical efficiency of the Symbiodiniaceae photosystem II (maximum quantum yield, Fv/Fm) tends to decline when corals experience heat stress (Ferrier-Pagès et al. 2010) and this measure is therefore commonly used to assess thermal stress in corals (Cantin et al. 2009; Wiedenmann et al. 2013; Buerger et al. 2020). Fv/Fm values were unusually low over the course of the experiment across all juvenile treatments and at both temperatures (0.1–0.3, Fig. S1, while values for healthy corals are typically larger than 0.5 (Suggett et al. 2015)) and this trait was therefore not analysed further. Lower and variable PAM values relative to adults have previously been observed in young juvenile corals inoculated with cultured symbionts and may be indicative of early onset of symbiosis of individual or specific strains (Quigley et al. 2017; Brunner et al. 2022) (Figure S1).
As an additional measure of stress, we assessed dark respiration on D5 and at the end of the experiment (D40–41) (Fig. S2). Overall, respiration was low. On D5, there was no significant difference in O2 consumption between the 27 °C and 31 °C treatment for any of the three juvenile groups, nor between any pair of juvenile groups at each temperature (Fig. S2A), which may have been due to the small sample size and high level of variation observed. Despite the low experimental replication, WT10-juveniles exhibited higher respiration compared to SS8-juveniles at the ambient temperature on D40–41 (p = 0.03), while there was no significant difference in respiration between SS1- and SS8- juveniles (p = 0.48). In addition, although respiration at 31 °C compared to 27 °C was slightly lower for WT10-juveniles at the final timepoint, this difference was not statistically significant (p = 0.12). There was also no significant difference detected in the SS-juveniles (p = 0.86 and 0.99 for SS1- and SS8-juveniles, respectively). We hypothesise that the reduced respiration at elevated temperature can be ascribed to their loss of symbionts. The large changes in respiration for WT10-juveniles occurred at the same time as the largest decrease in symbiont cell densities (2.6-times decrease in density) in the juveniles at D39.
Enhanced tolerance to elevated temperature of SS-juveniles does not come at a cost of reduced growth rate at ambient temperature
Growth, as measured in percentage change in surface area, was not only measured prior to, but also during the 41-day long heatwave experiment. The growth of the coral juveniles did not differ statistically between ambient and elevated temperatures (Fig. 3A), or among juveniles harbouring SS1, SS8 or WT10 at either 27 °C or 31 °C (lm, p = 0.162–1). Our 41 day-long experiment may have been too short to reveal differences in growth between small coral juveniles. Survival was also measured over the course of the heatwave experiment, but there were no statistical differences among the three groups at ambient or elevated temperature, nor between ambient and elevated temperatures for each of the three groups (Fig. 3B; lm, p = 0.5–0.7).
Fitness traits of A. tenuis juveniles infected with Cladocopium proliferum strains WT10, SS1 or SS8 over the course of the heat stress experiment at ambient and elevated temperature treatments. A Percentage change in surface area (± SE, n = 18- 46 juveniles per treatment combination) between the start of the heat stress experiment and D38 and B percentage of survival (± SE, n = 4 juveniles per treatment combination)
To quantify the nutrient fluxes between Symbiodiniaceae and their hosts, coral juveniles were co-incubated with stable isotope tracers. Levels of 13C enrichment were very low and could not be interpreted confidently (Supplementary Results, Fig. S3), but 15N enrichment (via assimilation of ammonium) was high and heterogeneously distributed between host cells and algal symbionts (Fig. 4). No clear trend emerged from the 15N enrichment in the Symbiodiniaceae cells. Indeed, on D5 at ambient temperature, WT10 Symbiodiniaceae were significantly more enriched in 15N than SS08 (Fig. 4A; lm, p < 0.05), but this trend was reversed at 31° C, when SS08 symbionts were 38.6% more enriched than WT10 (Fig. 4A; lm, p < 0.05). Further, at the end of the experiment (D39), the host tissue adjacent to the algal symbionts was 36.3 to 39.1% more enriched in SS01 and SS08 compared to WT10, at both ambient and elevated temperatures (Fig. 4B, lm, p < 0.05). As the Symbiodiniaceae themselves assimilated approximately three-times less nitrogen than these gastrodermal hotspots, it is unlikely the heat-evolved strains translocated more nitrogen to the host tissues and other physiological or microbial processes may be responsible for these differences.
Nitrogen assimilation (atom % ± SE, n = 25 for Symbiodiniaceae, n = 47 for host hotspots) by the juveniles and their algal symbionts. Nitrogen assimilation in (a) in hospite Cladocopium proliferumo and b hotspots in the gastroderm, within 10 µm from the algal symbionts, during the heat stress experiment. Asterisks (*) denote significant differences (lm, p < 0.05). Note: the scale of the y-axes differs between panels (a) and (b). Representative nanoscale secondary ion mass spectrometry (NanoSIMS) images showing (c–e) 15N/14N ratio in the algal symbionts (circled in white), and hotspots (white arrows). The 15N/14N images are displayed as Hue Saturation Intensity (HSI) where the colour scale of the ratio, with natural abundance in blue, changing to pink with increasing 15N levels. Scale bar = 10 µm
The heat-evolved Cladocopium strains provided measurable benefits to the coral holobiont under ambient and elevated temperatures, including faster growth under long-term ambient conditions (SS8-juveniles), the maintenance of symbiont cell densities in juveniles under heat stress and equivalent respiration under ambient and elevated temperatures (SS1- and SS8-juveniles). These patterns confirm previous results with heat-evolved strains (Buerger et al 2022; Quigley and van Oppen 2022). Our results suggest that heat-evolved Symbiodiniaceae can enhance the tolerance of Acropora coral juveniles without a trade-off in growth. Importantly, this contrasts with the traits previously described for naturally thermotolerant Symbiodiniaceae in the genus Durusdinium, with important implications for ecosystem health. Symbioses of Acropora corals with Durusdinium are characterised by comparatively high thermal tolerance but low symbiont-to-host carbon translocation and growth at ambient temperature compared with heat-sensitive Cladocopium (Cantin et al. 2009). In contrast, Pocillopora corals in the far eastern Pacific harbouring Durusdinium glynnii have higher thermal tolerance compared with conspecifics that house Cladocopium latusorum, but show no metabolic trade-off (Turnham et al. 2023). Durusdinium has recently been introduced into the Caribbean (Thornhill et al. 2014), and become more prevalent and abundant over time in some regions possibly due to increasing anthropogenic stressors (Pettay et al. 2015). An increase in Durusdinium abundance in Caribbean corals is concerning, specifically due to the early evidence of the trade-off between thermal tolerance and growth in this genus (but see Turnham et al. 2023), which, when modelled, negatively impact resilience and long-term recovery from decreased growth (Ortiz et al. 2013). These results suggest that the experimental evolution of Symbiodiniaceae followed by their reintroduction into coral hosts may provide a valuable tool for reef restoration initiatives.
Data availability
All code and data will be available via Github upon publication.
References
Buerger P, Alvarez-Roa C, Coppin CW, Pearce SL, Chakravarti LJ, Oakeshott JG, Edwards OR, van Oppen MJH (2020) Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci Adv 6:eaba2498
Buerger P, Vanstone R, Maire J, van Oppen MJH (2022) Long-term heat selection of the coral endosymbiont Cladocopium C1acro (Symbiodiniaceae) stabilizes associated bacterial communities. Int J Mol Sci 23(9):4913
Butler CC, Turnham KE, Lewis AM, Nitschke MR, Warner ME, Kemp DW, Hoegh-Guldberg O, Fitt WK, van Oppen MJH, LaJeunesse TC (2023) Formal recognition of host-generalist species of dinoflagellate (Cladocopium Symbiodiniaceae) mutualistic with Indo-Pacific reef corals. J Phycol. https://doi.org/10.1111/jpy.13340
Brunner CA, Ricardo GF, Uthicke S, Negri AP, Hoogenboom MO (2022) Effects of climate change and light limitation on coral recruits. Mar Ecol Prog Ser 690:65–82
Cantin N, van Oppen M, Willis B, Mieog J, Negri A (2009) Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28:405–414
Chakravarti LJ, van Oppen MJH (2018) Experimental evolution in coral photosymbionts as a tool to increase thermal tolerance. Front Mar Sci 5:227
Ferrier-Pagès C, Rottier C, Beraud E, Levy O (2010) Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: effect on the rates of photosynthesis. J Exp Mar Biol Ecol 390:118–124
Heron SF, Eakin CM, Douvere F, Anderson KD, Day J, Geiger E, Hoegh-Guldberg O, van Hooidonk R, Hughes TP, Marshall P, Obura DO (2017) Impacts of climate change on world heritage coral reefs : a first global scientific assessment. UNESCO World Heritage Centre, Paris
IPCC (2018) Summary for Policymakers. Global warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change. World Meteorological Organization Technical Document, p 32
Ortiz JC, González-Rivero M, Mumby PJ (2013) Can a thermally tolerant symbiont improve the future of C aribbean coral reefs? Glob Chang Biol 19:273–281
Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC (2015) Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc Natl Acad Sci 112:7513–7518
Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) Nlme: linear and nonlinear mixed effects models. R package version 3.1–118
Quigley KM, van Oppen MJH (2022) Predictive models for the selection of thermally tolerant corals based on offspring survival. Nat Commun 13:1–13
Quigley KM, Bay LK, Willis BL (2017) Temperature and water quality-related patterns in sediment-associated symbiodinium communities impact symbiont uptake and fitness of juveniles in the genus Acropora. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00401
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina J-B (2021) Heat stress destabilizes symbiotic nutrient cycling in corals. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2022653118
Suggett DJ, Goyen S, Evenhuis C, Szabó M, Pettay DT, Warner ME, Ralph PJ (2015) Functional diversity of photobiological traits within the genus Symbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytol 208:370–381
Thornhill DJ, Lewis AM, Wham DC, LaJeunesse TC (2014) Host-specialist lineages dominate the adaptive radiation of reef coral endosymbionts. Evolution 68:352–367
Turnham KE, Aschaffenburg MD, Pettay DT, Paz-García DA, Reyes-Bonilla H, Pinzón J, Timmins E, Smith RT, McGinley MP, Warner ME (2003) LaJeunesse TC (2023) High physiological function for corals with thermally tolerant, host-adapted symbionts. Proc R Soc B 290:20231021
van Oppen OJ, Putnam H, Gates R (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci 112:2307–2313
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211:3059–3066
Wiedenmann J, D’Angelo C, Smith EG, Hunt AN, Legiret F-E, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:160–164
Yellowlees D, Rees TAV, Leggat W (2008) Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31:679–694
Acknowledgements
The authors would like to thank Sven Uthicke for the use of the respirometry chambers, Sam Noonan for his advice on respirometry, and Rachael Collins for her help in performing the ImageJ measurements of the coral juveniles. We would like to thank Paul Guagliardo and Jeremy Bougoure for acquiring the NanoSIMS data. We would also like to thank Nick Carey for his help with the respiration data and for developing the respR package, and the AIMS workshop for bespoke machining of respirometry vial adaptors and holders.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by the Paul G. Allen Family Foundation and the Australian Institute of Marine Science. MJHvO acknowledges Australian Research Council Laureate Fellowship FL180100036. KMQ acknowledges Australian Research Council DECRA Fellowship DE230100284.
Author information
Authors and Affiliations
Contributions
KQ, CAR, MP, and MvO conceived and designed the experiment; CAR and KQ conducted the experiment, KQ and CAR carried out the data analysis; MP and JBR carried out the sample preparation and NanoSIMS data analysis. KQ, JBR and MvO wrote the manuscript. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Corals were collected under the following Great Barrier Reef Marine Park permit to AIMS G12/ 35236.1
Consent for publication
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quigley, K.M., Alvarez-Roa, C., Raina, JB. et al. Heat-evolved microalgal symbionts increase thermal bleaching tolerance of coral juveniles without a trade-off against growth. Coral Reefs 42, 1227–1232 (2023). https://doi.org/10.1007/s00338-023-02426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02426-z