Abstract
The present study aims at assessing the thermal tolerance of the black coral Antipathella wollastoni (Gray, 1857), which forms extensive forests in multiple Macaronesian islands. Fragments of A. wollastoni were exposed for 15 d to temperature conditions ranging from 19 to 26.5 °C, and multiple endpoints were investigated. No mortality was observed, and total antioxidant capacity remained unaffected by temperature increase. Respiration rates described a Gaussian relationship and tissue necrosis a linear increase with temperature. Increased temperature triggered the formation of bailout propagules, a process that may be used by the species as an escape strategy under unfavourable environmental conditions. Propagules of A. wollastoni were described for the first time. Altogether, A. wollastoni was suggested to have low vulnerability to increased temperatures, owing to its wide thermal window of performance (9.7 °C) and thermal safety margin (1.2 °C), similar to its congeneric Mediterranean Antipathella subpinnata.
Resumen
El presente estudio pretende evaluar la tolerancia térmica del coral negro Antipathella wollastoni (Gray, 1857), que forma extensos bosques en múltiples islas de la Macaronesia. Se expusieron fragmentos de A. wollastoni durante 15 días a condiciones de temperatura que oscilaban entre 19 y 26,5 °C, y se investigaron múltiples puntos finales. No se observó mortalidad, y la capacidad antioxidante total no se vio afectada por el aumento de temperatura. Las tasas de respiración describieron una relación gaussiana y la necrosis tisular un aumento lineal con la temperatura. El aumento de la temperatura desencadenó la formación de propágulos de resistencia, un proceso que podría ser utilizado por la especie como estrategia de escape en condiciones ambientales desfavorables. Los propágulos de A. wollastoni se han descrito además por primera vez. Globalmente, se sugirió que A. wollastoni tiene una baja vulnerabilidad al aumento de las temperaturas, debido a su amplia ventana térmica de rendimiento (9,7 °C) y a su margen térmico de seguridad (1,2 °C), similares a los de su congénere mediterránea Antipathella subpinnata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antipatharians, also known as black corals, are sessile colonial hexacorals. They produce a black spiny skeleton made of chitin and scleroproteins and are commonly considered as azooxanthellate (Wagner et al. 2012). They can form dense aggregations of one or more species, sometimes called “marine animal forests” (MAFs; sensu Rossi et al. 2017). These can modify environmental conditions locally and act as biodiversity hotspot in all oceans (Bo et al. 2012, 2014a, b, 2015; Oakley 1988; Tazioli et al. 2007; Warner 2005), generally from shallow depths to the deep sea (Bo et al. 2018; Cairns 2007; Tazioli et al. 2007; Wagner et al. 2012). From a conservation perspective, antipatharians are included in the CITES convention, due to their exploitation (Wagner et al. 2012). At the same time, their slow growth and long life led to their inclusion as vulnerable marine ecosystems (VMEs) by FAO and OSPAR.
Due to their erect position and branching morphology, these underwater forests are particularly vulnerable to fishing activities such as bottom trawling and trammel nets (Bo, et al. 2014a, b; Gori et al. 2019; Moccia et al. 2022). In addition, antipatharians possess a range of characteristics that make them extremely sensitive to changes: slow growth, delayed first reproduction, low survivorship and recruitment of larvae and extreme longevity (Parker 1997; Wagner et al. 2012). For these reasons, antipatharians are listed in multiple international agreements for marine ecosystem conservation (e.g. CITES Appendix II; Rossi et al. 2017), but this does not protect them from ongoing global changes, such as ocean warming (OW).
The few studies on the impacts of increased temperatures on antipatharians are recent and have revealed mixed effects (Godefroid et al. 2023). Among studying other variables, these studies have also produced thermal performance curves (TPCs) that allow describing the metabolic performance of an organism across temperatures. TPCs are characterized by a thermal optimum (Topt) that represents the temperature at which performance is maximum and by a thermal breadth (Tbr) that describes the width of the thermal window of performance. In a first study, a mesophotic (80 m) species of the genus Stichopathes in the tropics (Mo’orea, French Polynesia, Pacific Ocean) was exposed to a range of temperature conditions for 16 days (Godefroid et al. 2022a). Results showed that this species lived close to its Topt (28.8 °C), with many effects observed beyond this limit, including reduced metabolism, impaired healing capacity, increased tissue necrosis, mucus and antioxidant production. In addition, the Tbr of this tropical species was narrow (4.4 °C), which in general characterises species that are well adapted to their local thermal conditions. A similar experiment (15 days heat stress) was performed on the mesophotic (70 m) branched Antipathella subpinnata (Ellis and Solander, 1786) from the Western Mediterranean (Bordighera, Italy), reaching different conclusions. A. subpinnata showed a wide Tbr (6.1 °C) with no observed effects, nor signs of stress, at the experimental temperatures, even at 3 °C above the annual maximum temperature (Godefroid et al. 2022b). A wide Tbr is likely more favourable under OW as it implies that the species is able to tolerate a wider range of temperatures and that temperature increase will have little impact on its performance, compared to a species with a narrow Tbr.
Antipatharia have a very wide bathymetric and geographic distribution, and thus such differences in physiological tolerance are expected among species. However, due to the paucity of studies available, it is difficult to infer if these different responses are due to phylogenetic or environmental factors. Therefore, the present study aims at assessing the thermal tolerance of the congeneric Antipathella wollastoni (Gray, 1857), which forms extensive forests in subtropical waters of the Canarian Archipelago (Bianchi et al. 2000; Czechowska et al. 2020), as well as in other islands of the Macaronesian region (e.g. Azores; de Matos et al. 2014). This is also particularly timely as the warming rate in the Canary Islands upwelling system is the highest of all four Eastern Boundary Upwelling systems (Arístegui et al. 2009). More specifically, the present study aims to reproduce an experimental design similar to that used in previous studies (15 days exposure to a range of temperature conditions), to ensure comparability across experiments.
Material and methods
Sampling site and antipatharian collection
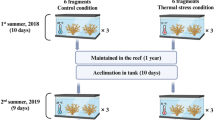
Fragments of Antipathella wollastoni were collected at 25 m depth on 23rd January 2022, in Baja de Gando (27°55' 56.1" N, 15°21' 11.0" W), located on the East coast of Gran Canaria Island (Canary Islands, Spain). The annual range of temperature in the study site is 18.4–24.7°C (see Fig. S1), and at the time of collection, the seawater temperature was 19 °C. In total, seven fragments (ca. 100 mm height) were collected from eight different colonies (n = 56). Fragments were collected in plastic bags filled with seawater from the site of collection and then transported in a refrigerated box to the experimental facility of the University Institute of Sustainable Aquaculture and Marine Ecosystems (IU-ECOAQUA), within 1 h. Fragments were individually tagged, attached to a support using holdfast EPOXY resin (Holdfast, Aquarium Systems, France), and placed in an open-circuit system composed of seven fibreglass aquaria (35L, salinity 36.8), under identical temperature relative to the site of collection (19 °C). One fragment of each colony was placed per aquarium, so each aquarium contained eight fragments from different colonies. Seawater in the system was filtered using a biological filter (Biological Filter, Aqua Medic, Bissendorf, Germany) and a skimmer (AQUA OCEAN PRO-SKP900, Spain). Seawater temperature was controlled using a chiller (Johnson Controls A350, ± 0.5 °C, America), and aeration was provided in each aquarium. All aquariums were illuminated for 10 h each day by blue-light fluorescent tubes (T8 10.000 K, Power Luw Pro, Spain), based on the local photoperiod. Fragments were fed two times a day (in the morning and at dawn) with a mix composed of live rotifers, freshly hatched Artemia, copepods (Ocean Prime, Copepods 500–700 μm, Groningen, The Netherlands) and phytoplankton (Tropic Marin, Phyton 60 G, Germany). Seawater parameters were checked daily using a handheld meter (WTW Multi 350i for temperature, pH and conductivity) and colorimetric tests (for nitrates and nitrites), to ensure good seawater quality.
Experimental design
Fragments were maintained for one month at collection site temperature (19 °C). Then, temperature was gradually increased in the aquariums by 0.5 °C per day, until reaching their respective target temperature, at which they were maintained for 15 days. Six temperature treatments were established: two aquariums were maintained at control temperature (19 °C) and five aquariums with increased temperatures (20.5, 22, 23.5, 25 and 26.5 °C). The maximum temperature used during the experiments did not exceed 26.5° because for other congeneric species, higher temperatures lead to the death of the colonies (Godefroid et al. 2022b). Each aquarium contained seven fragments from different colonies. Temperature treatments were selected based on the maximum seasonal temperature experienced by the coral in the environment, + 3 °C. Seawater delivered to all aquariums was cooled at 19 °C using a chiller (Johnson control A350; ± 0.5°C) and was heated in individual aquariums using one or two 100 W heating resistances connected to a temperature controller (Inkbird ITC-308; ± 0.3 °C).
Response variables
Standard metabolic rate (SMR) was estimated from rates of oxygen uptake using closed respirometry, at day 15. An 80-L tank equipped with a circulation pump and a bubbling system to ensure complete temperature homogenisation was used as a temperature-controlled water bath. Seawater temperature in the tank was controlled using two 100 W heating resistances connected to a temperature controller (Inkbird ITC-308; ± 0.3°C). The respirometry trials generally started around 8 AM, allowing a minimum of 12 h of digestion after the last feeding. In total, nine cylindrical respirometry chambers (400 mL) were used during each trial and positioned inside the tank. Seven were used to measure the respiration rate of the fragments (one per chamber), and two were filled with seawater only (controls). Each respirometry chamber contained a magnetic stir bar which maintained constant homogenization of the dissolved gas, a magnetic stir plate placed below the tank, and an oxygen sensor spot (PreSens, SP-PSt3-NAU-D5-YOP-SA, Germany) that allowed the connection with a mono-canal oxygen meter (Stand-alone Fibre Optic Oxygen Meter, Fibox 4, PreSens Precision Sensing GmbH, Regensburg, Germany), via an optic fibre. Oxygen saturation was measured every 10 minutes in each chamber, for a total duration of 2 h. At the end of measurements, the total chambers (fragment + chamber + seawater) and the fragments were weighed (wet weight). A photograph of all cut branches laid in two dimensions (with a scale; resolution 1 mm) was taken and then analysed to measure the total length of all ramifications, using the software ImageJ (Schneider et al., 2012). A difference was made between live and dead (only skeleton) portions of the ramifications, and only the total length of live ramifications (the portion of breathing tissue) was used for normalizing respiration rates. These normalization methods allowed comparison with previous results (Godefroid et al. 2022a, b).
Oxygen saturation measurements were converted to oxygen concentration (in µmol L-1) based on the volume of seawater in the experimental chambers, calculated from the density of seawater and the weight of seawater (Millero and Huang 2009; Millero and Poisson 1981). The rate of oxygen consumption was calculated from the slope of the linear regression of oxygen concentration with time. Oxygen consumption rate of the fragments was corrected using the average consumption rate of the two seawater-only chambers and normalized by the wet weight and total length of the live ramifications of the fragment. Oxygen consumption rates were expressed in µmol O2 hr-1 g-1 and in µmol O2 h-1 cm-1.
At the end of the respirometry assay, a 2–3-cm piece was cut from each coral fragment and placed in a tube with phosphate buffer (50 mM), for biomarker analysis. Tissues were separated from the skeleton and homogenized using a micro pestle. Homogenates were centrifuged for 10 min at 4 °C (10000 g, Eppendorf Centrifuge 5430R), and the supernatant was transferred into a new tube and stored at − 80 c°C until analysis. Total protein content and antioxidant capacity in the supernatant were measured as reported in Godefroid et al. (2022a, b). Briefly, total protein content was determined using a commercial reagent kit based on the Bradford assay (Pierce BCA Protein Assay Kit, ThermoFisher Scientific Inc., USA) with bovine serum albumin (BSA) as standard (2 mg/mL). Protein contents were used for biomarker normalization. Measurement of total antioxidant capacity (TAC) was carried out using OxiSelect Total Antioxidant Capacity Assay Kit (Cell Biolabs Inc., USA), an electron-transfer-based assay (Huang et al. 2005) that measures the capacity of an antioxidant solution to reduce an oxidant (copper (II) reduced into copper (I)). The chromatic change is proportional with the sample antioxidant concentration. Absorbance was measured at 490 nm in a microplate reader (Beckman Coulter paradigm), and values were compared to uric acid standard curves. Results were normalized to the protein content and expressed as “mM Copper Reducing Equivalents per g of protein”.
Tissue necrosis (i.e. the partial loss of live tissues around the skeleton) was quantified at the end of the experiment (day 15), by using the same photographs taken at the end of the respirometry test, and reported as a proportion: tissue necrosis (%) = (length of necrosed ramifications/total length of all ramifications)*100.
Mortality (i.e. 100% tissue loss) was also checked daily for all fragments and reported on a binary scale (dead/alive).
Fragments produced bailout propagules that either sank at the bottom of the aquaria or floated and were caught in a square-shaped trap connected to the aquarium water outflow. The trap was covered with a microplankton net (0.2 µm) in order to retain the produced propagules within the trap (Coppari et al. 2020). Retrieving of the propagules at the bottom of the aquaria could not be done in a quantitative way, preventing a quantitative analysis of the phenomenon. Propagules were observed with a magnifying lens to study their morphology (Leica EZ4 W Stereo Microscope w/Integrated WiFi Camera, Microscope Central, Feasterville, Pennsylvania). The shape of the propagules was categorized in four main classes, based on Coppari et al. (2020): circular shape (C), elongated shape (E), polypoid shape (P) and “other” (O). Propagule size was measured from pictures using the Software ImageJ (Schneider et al., 2012).
Data analysis
The relationship between respiration rate and temperature was analysed using a symmetrical Gaussian function (Rodolfo-Metalpa et al. 2014), fitted by nonlinear regression with the R package nlstools (Baty et al. 2015):
where P is the temperature dependent physiological response, Topt is the optimal temperature at which this response is maximal (Pmax) and c indicates the standard deviation of the curve. Tbr was estimated from c, as the temperature range in which the organism’ performance is ≥75% of Pmax. The standard errors of the parameters estimated were also obtained with the model.
Results for the effects of temperature on biochemical markers and tissue necrosis were analysed using linear least square regression model (y = ax + b), using the package lsmeans (Russel 2016), with temperature as the independent variable (n = 6) and the biomarker as the (y) dependent variable. Symmetrical Gaussian (nonlinear regression) model was also tested on biochemical markers results but the model did not converge.
Analysis of residuals was carried out for all regression analyses, and no trends were evidenced. All analyses and figures were performed using the software R (R Development Core Team 2017).
Results and discussion
The present study assessed the thermotolerance of a major engineer species at mesophotic depths in the Canary Islands archipelago, the branched antipatharian Antipathella wollastoni. The relationship between oxygen consumption rate and temperature was described by a symmetrical Gaussian curve (Fig. 1a), with its maximum (Pmax ; 0.02 ± 0.001 µmol O2 h-1 cm-1; mean ± se; p < 0.001) at 25.9 ± 2.7 °C (Topt; p < 0.001). The standard deviation of the curve (c) was estimated at 6.4 ± 2.7°C (p < 0.05; Table S2). So, Topt for respiration (25.9 °C) is 1.2 °C above maximum environmental temperature (24.7 °C), suggesting low susceptibility to increasing temperatures. In a similar experiment on the congeneric Antipathella subpinnata from the Mediterranean Sea, Topt (16.4 °C) was 0.4 °C above maximum environmental temperature (16 °C; Soto-Navarro et al. 2020). So, A. wollastoni has larger thermal safety margin (1.2 °C; defined here as the difference between the maximum temperature in the environment and Topt) and Tbr (9.7 °C) than A. subpinnata (0.4 and 6.1 °C, respectively). This thermal strategy (large thermal safety margin, wide thermal performance window) is often viewed as being favourable under ocean warming, suggesting that A. wollastoni is likely to be more heat tolerant than A. subpinnata in future ocean conditions. However, in the latter species, when accounting for the other endpoints, no other signs of stress, such as bailout, necrosis, mortality and effects on antioxidant responses, were observed during the heat stress experiment, even 4.5 °C above the mean annual temperature (Godefroid et al. 2022b), while effects were observed in the present experiment.
Effects of temperature on Antipathella wollastoni fragments (total of seven fragments from different colonies on each aquarium) a Oxygen consumption rate (µmol O2 h-1 cm-1). b Total antioxidant capacity (mmol copper reducing equivalents g-1 protein). c Proportion of tissue necrosis (%). d The four categories of bailout propagules are elongated (E), circular (C), polypoid (P) and other (O), based on Coppari et al. (2020) (Scale: 5mm).
Because A. wollastoni can be found in the shallow subtidal from some Atlantic tropical islands, such as São Tomé and Principe and Cape Verde (Wirtz 2018; Brito and Ocaña, 2004), where they experience temperatures > 22 ºC all year round, the distribution range of this coral corroborates the large thermal tolerance we have here demonstrated.
Temperature had no effects on the total antioxidant capacity of the fragments (linear regression, p = 0.17; Fig. 1b; Table S3), and mortality (in the sense of 100% tissue loss on fragments) was not observed in any treatment over the course of the experiment. However, temperature significantly increased the proportion of tissue necrosis (linear regression, p < 0.001; Fig. 1c; Table S4) and bailout propagules were observed in all treatments over the course of the experiment, except in the controls. In total, 148 brown-orange propagules were collected. All were actively moving and rotated on different axes. Four main morphologies were distinguished: circular (5%; n = 8); elongated (23%; n = 34); polypoid (28%; n = 41); and other (44%; n = 65) (Fig. 1d). All propagules were measured, according to their morphology, and their average size ranged between 0.4 and 1.09 mm (Table 1).
Polyp bailout, the active detachment from the mother colony (Sammarco 1982), is an extreme response used as a survival and escape strategy under stressful conditions (Fordyce et al. 2017; Perez et al. 2014). It is a well-known process in tropical and temperate scleractinian corals, as well as in octocorals (Capel et al. 2014; Fordyce et al. 2017; Kruzic 2007; Rakka et al. 2019; Sammarco 1982; Serrano et al. 2018; Shapiro et al. 2016; Wells and Tonra 2020). It was previously observed in antipatharians under experimental conditions, either by mechanical induction in Antipathes sp. (Bo 2008), Antipathes caribbeana and Plumapathes pennacea (Gonçalves 2016), or under stressful conditions in the laboratory in Antipathella fiordensis from New-Zealand (Miller and Grange 1997; Parker et al. 1997) and Antipathella subpinnata from the Mediterranean Sea (Coppari et al. 2020). The formation of propagules (that either sank or floated) by the fragments of A. wollastoni was observed in all temperature treatments except the controls, so even under temperatures covered by the annual temperature range in the environment (18.4–24.7 °C). This suggests that temperature could trigger the formation of propagules but suggests that other factors linked to the experiment also contributed, explaining the formation of propagules at temperatures within the annual temperature range. One factor may be the rate of temperature increase (+ 0.5 °C d−1) that may have been too fast for this species, suggesting low thermal acclimation capacity. Another factor may be a delayed effect of sampling and transport or rearing conditions per se, as observed in other species (Coppari et al. 2020; Serrano et al. 2018). The difficulty in maintaining these species in aquaria may also explain tissue necrosis, although in small proportion, observed at day 15 in the controls (19 °C; 8.1 ± 4.8%). It is worth noting that our experimental design was limited by the use of only one aquarium (with 8 replicated coral fragments) per thermal treatment. Future studies should circumvent this limitation, whether logistically feasible, by having more than one tank/aquarium per thermal treatment. Despite these limitations, the results obtained in this study are important and a good basis for improving research on the effects of temperature on antipatharians.
References
Arístegui J, Barton ED, Álvarez-Salgado XA, Santos AMP, Figueiras FG, Kifani S, Hernández-León S, Mason E, Machú E, Demarcq H (2009) Sub-regional ecosystem variability in the canary current upwelling. Prog Oceanogr 83(1–4):33–48. https://doi.org/10.1016/j.pocean.2009.07.031
Baty F, Ritz C, Charles S, Brutsche M, Flandrois JP, Delignette-Muller ML (2015) A toolbox for nonlinear regression in R: the package nlstools. J Stat Softw 66:1–21
Bianchi CN, Morri RHC, Wirtz P (2000) The subtidal epibenthic communities off Puerto Del Carmen (Lanzarote, Canary Islands). Arquipelago Life Mar Sci 2:145–155
Bo M, Canese S, Spaggiari C, Pusceddu A, Bertolino M, Angiolillo M, Giusti M, Loreto MF, Salvati E, Greco S, Bavestrello G (2012) Deep coral oases in the south tyrrhenian sea. PLoS ONE 7(11):e49870. https://doi.org/10.1371/journal.pone.0049870
Bo M, Bava S, Canese S, Angiolillo M, Cattaneo-Vietti R, Bavestrello G (2014) Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol Conserv 171:167–176. https://doi.org/10.1016/j.biocon.2014.01.011
Bo M, Canese S, Bavestrello G (2014) Discovering Mediterranean black coral forests: Parantipathes larix (Anthozoa: Hexacorallia) in the Tuscan Archipelago Italy. Italian J Zool 81(1):112–125. https://doi.org/10.1080/11250003.2013.859750
Bo M, Bavestrello G, Angiolillo M, Calcagnile L, Canese S, Cannas R, Cau A, D’Elia M, D’Oriano F, Follesa MC, Quarta G, Cau A (2015) Persistence of pristine deep-sea coral gardens in the mediterranean sea (SW Sardinia). PLOS ONE 10(3):e0119393. https://doi.org/10.1371/journal.pone.0119393
Bo M, Bavestrello G, Angiolillo M, Calcagnile L, Canese S, Cannas R, Cau A, D’Elia M, D’Oriano F, Follesa MC, Quarta G, Cau A (2018) Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS One 10:e0119393
Bo M (2008) Taxonomy and ecology of antipatharians. Ph.D. Dissertation in Marine Biology and Ecology, Universita` Politecnica delle Marche, Ancona, Italy, p 212.
Brito A, Ocaña O (2004) Corales de las Islas Canarias: antozoos con esqueleto de los fondos litorales y profundos. Francisco Lemus, Editor
Cairns SD (2007) Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull Mar Sci 81(3):12
Capel KCC, Migotto AE, Zilberberg C, Kitahara MV (2014) Another tool towards invasion? Polyp “bail-out” in Tubastraea coccinea. Coral Reefs 33(4):1165–1165. https://doi.org/10.1007/s00338-014-1200-z
Coppari M, Fumarola L, Bramanti L, Romans P, Pillot R, Bavestrello G, Bo M (2020) Unveiling asexual reproductive traits in black corals: Polyp bail-out in Antipathella subpinnata. Coral Reefs. https://doi.org/10.1007/s00338-020-02018-1
Czechowska K, Feldens P, Tuya F, Cosme de Esteban M, Espino F, Haroun R, Schönke M, Otero-Ferrer F (2020) Testing side-scan sonar and multibeam echosounder to study black coral gardens: a case study from macaronesia. Remote Sens 12(19):3244. https://doi.org/10.3390/rs12193244
de Matos V, Gomes-Pereira JN, Tempera F, Ribeiro PA, Braga-Henriques A, Porteiro F (2014) First record of Antipathella subpinnata (Anthozoa, Antipatharia) in the Azores (NE Atlantic), with description of the first monotypic garden for this species. Deep Sea Res Part II: Topic Stud Oceanogr 99:113–121. https://doi.org/10.1016/j.dsr2.2013.07.003
Expósito FJ, González A, Pérez JC, Díaz JP, Taima D (2015) High-resolution future projections of temperature and precipitation in the canary islands. J Clim 28(19):7846–7856. https://doi.org/10.1175/JCLI-D-15-0030.1
Fordyce AJ, Camp EF, Ainsworth TD (2017) Polyp bailout in Pocillopora damicornis following thermal stress. F1000Research. https://doi.org/10.5256/F1000RESEARCH.11522.D161213
Godefroid M, Hédouin L, Mercière A, Dubois P (2022) Thermal stress responses of the antipatharian Stichopathes sp. from the mesophotic reef of Mo’orea, French Polynesia. Sci Total Environ 820:153094. https://doi.org/10.1016/j.scitotenv.2022.153094
Godefroid M, Zeimes T, Bramanti L, Romans P, Bo M, Toma M, Guillaumot C (2022) Low vulnerability of the Mediterranean antipatharian Antipathella subpinnata (Ellis & Solander, 1786) to ocean warming. Ecol Model 475:110209
Godefroid M, Gouveia A, Otero-Ferrer F, Espino F, Tuya F, Dubois P (2023) Higher daily temperature range at depth is linked with higher thermotolerance in antipatharians from the canary islands. J Therm Biol 115:103593. https://doi.org/10.1016/j.jtherbio.2023.103593
Gonçalves JF (2016) On the origin of bilaterality: insights from the study of black corals (Cnidaria: Antipatharia). Populations and Evolution [q-bio.PE]. Université Pierre et Marie Curie––Paris VI, English.
Gori A, Grinyó J, Dominguez-Carrió C, Ambroso S, López-González PJ, Gili JM, Bo M (2019) 20 Gorgonian and Black Coral Assemblages in Deep Coastal Bottoms and Continental Shelves of the Mediterranean Sea. Mediterranean Cold-Water Corals: Past,Present and Future. Springer, Cham, pp 245–248
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53(6):1841–1856
Kruzic P (2007) Polyp expulsion of the coral Cladocora caespitose (Anthozoa, Scleractinia) in extreme sea temperature conditions. Natura Croatica 16:211
Miller K, Grange KR (1997) Population genetic studies of antipatharian black corals from Doubtful and Nancy Sounds, Fiordland, New Zealand. In: Proceedings of the 6th international conference on coelenterate biology, pp 353–363.
Millero FJ, Huang F (2009) The density of seawater as a function of salinity (5 to 70 g kg−1) and temperature (273.15 to 363.15 K). Ocean Science 5(2):91–100
Millero FJ, Poisson A (1981) International one-atmosphere equation of state of seawater. Deep Sea Res Part A Oceanogr Res P 28(6):625–629
Moccia D, Carugati L, Follesa MC, Cannas R, Carbonara P, Pusceddu A, Cau A (2022) Environmental status and geomorphological characterisation of seven black coral forests on the sardinian continental shelf (NW Mediterranean Sea). Biology 11(5):732. https://doi.org/10.3390/biology11050732
Oakley SG (1988) Settlement and growth of Antipathes pennacea on a shipwreck. Coral Reefs 7(2):77–79. https://doi.org/10.1007/BF00301644
Parker NR, Mladenov PV, Grange KR (1997) Reproductive biology of the antipatharian black coral Antipathes fiordensis in Doubtful Sound, Fiordland New Zealand. Mar Biol 130(1):11–22
Perez K III, Rodgers KS, Jokiel PL, Lager CV, Lager DJ (2014) Effects of terrigenous sediment on settlement and survival of the reef coral Pocillopora damicornis. PeerJ 2:e387
Rakka M, Bilan M, Godinho A, Movilla J, Orejas C, Carreiro-Silva M (2019) First description of polyp bailout in cold-water octocorals under aquaria maintenance. Coral Reefs 38(1):15–20. https://doi.org/10.1007/s00338-018-01760-x
Rodolfo-Metalpa R, Hoogenboom MO, Rottier C, Ramos-Esplá A, Baker AC, Fine M, Ferrier-Pagès C (2014) Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob Change Biol 20(10):3036–3049. https://doi.org/10.1111/gcb.12571
Rossi S, Bramanti L, Gori A, Orejas C (eds) (2017) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots. Springer, Cham. https://doi.org/10.1007/978-6733-319-17001-5
Russel LV (2016) Least-squares means: the R package lsmeans. J Stat softw 69:1–33
Sammarco P (1982) Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar Ecol Prog Ser 10:57–65. https://doi.org/10.3354/meps010057
Serrano E, Coma R, Inostroza K, Serrano O (2018) Polyp bail-out by the coral Astroides calycularis (Scleractinia, Dendrophylliidae). Mar Biodivers 48(3):1661–1665. https://doi.org/10.1007/s12526-017-0647-x
Shapiro OH, Kramarsky-Winter E, Gavish AR, Stocker R, Vardi A (2016) A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat Commun. https://doi.org/10.1038/ncomms10860
Soto-Navarro J, Jordá G, Amores A, Cabos W, Somot S, Sevault F, Macías D, Djurdjevic V, Sannino G, Li L, Sein D (2020) Evolution of mediterranean sea water properties under climate change scenarios in the Med-CORDEX ensemble. Clim Dyn 54(3–4):2135–2165. https://doi.org/10.1007/s00382-019-05105-4
Tazioli S, Bo M, Boyer M, Rotinsulu H, Bavestrello G (2007) Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zool Stud 46(2):227–241
Wagner D, Luck DG, Toonen RJ (2012) The Biology and Ecology of Black Corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Advances in Marine Biology. Elsevier, London, pp 67–132
Warner GF (2005) The occurrence of black corals in Jamaican reef environments, with special reference to Stichopathes lutkeni (Antipatharia: Antipathidae). Revista Biol Trop 53:6
Wells CD, Tonra KJ (2020) Polyp bailout and reattachment of the abundant Caribbean octocoral Eunicea flexuosa. BioRxiv. https://doi.org/10.1101/2020.07.04.187930
Wirtz P (2018) New records of marine invertebrates from São Tomé and Príncipe (Eastern tropical Atlantic). Arquipel Life Mar Sci 35:41–46
Acknowledgments
M. Godefroid is holder of a Belgian FRIA grant (number 1.E.066.19F). Ph. Dubois is a Research Director of the National Fund for Scientific Research (FRS-FNRS; Belgium). This work was supported by the Royal Belgian Zoological Society and the FNRS project COBICO [grant number T0084.18]. Financial support was partially provided by the EU (BEST initiatives) B-Charmed project and through the 2015-2016 BiodivERsA COFUND call for research proposals, with the national funders Agencia Española de Investigación PCI2022-133015 (RestoreSeas Peoject). We also thank Dr. Rafael Gines for the disposal of IU-ECOAQUA biosecurity facilities to run the experiments and Marcial Cosme de Esteban for his assistance during respirometry measurements.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gouveia, A., Godefroid, M., Dubois, P. et al. Thermal stress response of Antipathella wollastoni (Gray, 1857) from the Canary Islands archipelago. Coral Reefs 42, 1263–1269 (2023). https://doi.org/10.1007/s00338-023-02415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02415-2