Abstract
Population irruptions of crown-of-thorns seastar (CoTS, Acanthaster spp.) represent a perennial threat to Indo-Pacific coral reefs. Age determination of CoTS is challenging, thereby hindering understanding and management of this nuisance species. Telomeres, which are protective DNA structure found at the ends of eukaryotic chromosomes that shorten at each cell division, have been used to estimate age in wild animals. To investigate the use of telomeres in CoTS, we optimized a quantitative PCR protocol to measure relative telomere length (rTL) in CoTS for the first time. Comparing rTL among four age groups (4, 7, 16, > 24 months post-settlement), we found that adult CoTS generally exhibit shorter rTL than juveniles, which is the first evidence of age-related telomere attrition in CoTS. However, there was large within-age class variation, and no significant relationships were found between adult CoTS rTL and potential age-indicating external features. Furthermore, we found accelerated telomere attrition under sub-optimal diet, where individuals that were fed crustose coralline algae for 16 months exhibited shorter rTL than their counterparts fed on coral. A positive correlation was found between rTL of tube feet and pyloric caeca, suggesting synchronization of telomere dynamics across somatic tissues in CoTS. Overall, our results suggest that rTL could be used to classify CoTS into broad age groups, though individual variation constrains the ability to resolve specific cohorts. The present study contributes to the understanding of telomere dynamics in marine invertebrates, while laying the groundwork for future research into rTL as biomarker for age and potentially stress for CoTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crown-of-thorns seastars (CoTS; Acanthaster spp.) are highly efficient predators of reef-building scleractinian corals (Pratchett et al. 2014). During periodic population irruptions, local densities of CoTS can exceed 1000 individuals per hectare (Chesher 1969). These population irruptions have caused extensive decline in coral cover, contributing to reef degradation throughout the Indo-Pacific region (De’ath et al. 2012; Nakamura et al. 2014). Despite considerable research effort since the 1960s, the causes of irruptions remain uncertain due to the inability to resolve some critical research questions, hampering the development of corresponding management strategies (Pratchett et al. 2021).
One of the knowledge gaps that remains in CoTS research is the lack of a validated method to determine their age. For example, their size-at-age was found to be highly plastic dependent on food available and population density (Lucas 1984; MacNeil et al. 2017), and thus the differentiation of cohorts based on size-structure has been contentious. On the other hand, the count of visible pigment bands on the spines of CoTS have been considered as reasonably promising size-independent proxies of age (Stump and Lucas 1990). However, this method still needs to be further validated through medium- to long-term mark-recapture studies to elucidate temporal or spatial variation in band formation (Souter 1997; Stump and Lucas 1999). Overall, the inability to age individual CoTS has hindered the understanding of population dynamics and the processes that initiate irruptions. It is, therefore, of interest to search for new approaches for age determination in CoTS.
Telomeres are tandem repeats of short DNA sequence (5′-TxAyGz-3′) and protein complexes found at the ends of eukaryotic chromosomes (Blackburn 1991). The non-coding telomeres act as end caps to prevent inter-chromosomal fusion (van Steensel et al. 1998) and to protect chromosomal DNA from degradation during cell division (Blackburn 2005). At each cell division, telomeres are shortened while chromosomal DNA stays intact, preserving vital information in the genome (Blackburn 1991). While telomere length can be extended and maintained by the ribonucleoprotein enzyme telomerase, activity of this enzyme varies according to cell type, life stage, and species (Blackburn 2005). In somatic tissues, where telomerase activities are typically low, a cell undergoes senescence or apoptosis when its telomeres shorten to a critical limit. Since the first description of telomeres in the 1930s, extensive research effort has been devoted to the understanding of its structure and function. To date, it is widely recognized that telomeres play a central role in cellular ageing (Armanios and Blackburn 2012), while telomere attrition is considered a major hallmark of ageing in human (López-Otín et al. 2013).
As telomeric DNA sequence is widely conserved across eukaryotes (Gomes et al. 2010), studies in the past two decades have drawn on experience gained in human biomedical research and introduced telomere length as a biomarker into the ecological field (reviewed by Louzon et al. 2019). Notably, telomere length has been proposed as a proxy for chronological age in wild animals, based on the fact that age-associated telomere shortening has been found in various taxa such as birds (Haussmann and Vleck 2002), reptiles (Scott et al. 2006), fishes (Hartmann et al. 2009), as well as invertebrates from both protostome (e.g., molluscs; Godwin et al. 2011a) and deuterostome (e.g., echinoderms; Varney et al. 2017) groups. Molecular ageing techniques have been advocated as powerful tools for ecological studies, as they offer versatile alternatives to traditional methods (e.g., repeated sighting of tagged individuals, annual growth bands count on calcified structures, etc.) in obtaining age information from wild populations. Thus far, several studies have demonstrated the potential of using telomere-based technique to classify wild animals into broad age groups (Haussmann et al. 2003; Pauli et al. 2011).
The emergence of telomere length as a biomarker may present a unique opportunity to improve the understanding of CoTS biology and ecology. However, there is considerable work to be done before telomere length can be developed into a reliable ageing tool for marine invertebrates. Despite studies that have demonstrated coarse age indication in some marine invertebrates using telomere-based technique (e.g., sea urchin; Coupé et al. 2019), other research has shown that the negative correlation between age and telomere length cannot be generalized across all species. For example, no age-related changes in telomere length were found in red sea urchin (Strongylocentrotus franciscanus) (Francis et al. 2006) or spiny lobsters (Sagmariasus verreauxi and Jasus edwardsii) (Godwin et al. 2011b). These incongruent findings suggest that telomere dynamics may be species-specific, and thus careful investigations are needed to establish the relationship between telomere length and age in non-model or new study species.
To establish a robust ageing tool, the influence of age-independent factors on telomere dynamics must be considered. For example, recent research has shown that extrinsic factors such as stress and diet can cause oxidative damage to telomeric DNA and thus accelerate telomere attrition rate (Chatelain et al. 2020). In the case of CoTS, it has been established that their growth is highly dependent on diet, where exponential growth has been recorded following the ontogenetic shift of diet from crustose coralline algae (CCA) to coral at 4–12 months post-settlement (Yamaguchi 1974; Wilmes et al. 2020; Neil et al. 2022). However, it has recently been hypothesized that CoTS can prolong their herbivorous phase and delay growth for at least six years while waiting for favorable conditions (e.g., availability of coral) to arise (“hidden army hypothesis”; Deaker et al. 2020). While this hypothesis has important implications in the understanding of CoTS population dynamics, little is known about the physiological consequences of the delay in diet transition, and the proposed trophic flexibility is yet to be tested in natural environment due to a lack of reliable ageing tool. Here we set up treatment groups fed on CCA only, thus mimicking the “hidden army”, to investigate the influence of diet on telomere dynamics in CoTS. In addition, as telomere dynamics can be tissue specific (de Abechuco et al. 2016), it is important to identify the suitable tissue type representing the overall telomere dynamics in CoTS.
The present study seeks to investigate the potential of telomere length as a biomarker to enhance the understanding of CoTS population irruptions. We set out to adopt and optimize the quantitative PCR (qPCR) protocol developed for humans (Cawthon 2002) to measure telomere length in CoTS for the first time. To explore age-associated telomere dynamics in CoTS, telomere lengths of individuals from four age groups were measured and correlated with potential age-indicator including diameter, spine length, and pigment band count. Effect of diet on telomere dynamics was determined by raising juvenile CoTS under two diet conditions, where one batch shifted diet ontogenetically from CCA to coral, and the other was fed only with CCA since settlement. As a test of tissue effect, telomere lengths measured from pyloric caeca and tube feet were compared.
Methods
Sample collection
Adult Pacific crown-of-thorns seastars (CoTS; Acanthaster cf. solaris) were spawned in December 2020 and 2021 at the Australian Institute of Marine Science (AIMS) National Sea Simulator (SeaSim) following Uthicke et al. (2015). The larvae were settled and raised as described by Balu et al. (2021). The two cohorts were sampled at two distinct time points, constituting three age groups in total (4-, 7-, and 16-month post-settlement; Table 1). To assess the effect of diet on telomere length, seastars were divided into two diet groups: coral and CCA. The coral diet group was fed only with corals once individuals had shifted diet from CCA to coral at 4 months post-settlement. This represents a “typical” situation where CoTS exhibit ontogenetic diet shift while transitioning from juvenile to adult (Wilmes et al. 2020). On the other hand, the CCA diet group was raised entirely on CCA, and was not exposed to corals for the 16 months experimental period, mimicking the “hidden army” hypothesis situation where juveniles exhibit prolonged herbivorous phase (Deaker et al. 2020). As a result, there were five treatment groups of juvenile CoTS in total (Table 1). During sampling, diameters of the seastars were recorded (ranged from 3.5 to 51.9 mm), followed by whole specimen preservation in individual tubes with 95% ethanol. Samples were stored at − 4 °C until DNA extraction.
Adult CoTS specimens were collected from the central section of the Great Barrier Reef by Pacific Marine Group, a CoTS culling operator. The first batch of samples (n = 14) was collected from Wheeler Reef ( − 18.79756, 147.52681) on 20 Apr 2022, and the second batch of samples (n = 15) was collected from Hopkinson Reef ( − 18.54816, 147.19429) on 16 June 2022. The CoTS were transported alive to AIMS and kept at mesocosm until sampling within a week. Although the age of these field samples was unknown and the two batches of samples were presumably composed of different temporal cohorts, it has been established that CoTS larger than 200 mm are likely older than 2 years and are classified as adults (Pratchett et al. 2014). While the growth of CoTS is highly plastic and their juvenile phase could potentially be prolonged under certain conditions (Deaker et al. 2020), it is unlikely for individuals below 2 years old to attain a diameter above 200 mm, due to inherent constraints in growth rates (Wilmes et al. 2020). As such, field collected specimens (diameter ranged from 210 to 380 mm) were grouped together and hereafter referred to as the > 24 months adult group. Tube feet and pyloric caeca tissue was collected from each individual and preserved in in 95% ethanol at − 4 °C until DNA extraction. In addition, the longest aboral spine from four arms were collected for spine length measurement and pigment band count, following Stump and Lucas (1990) and MacNeil et al. (2017).
DNA extraction
Genomic DNA was extracted from CoTS samples using DNeasy Blood and Tissue Kit (Qiagen) according to manufacturer protocol. DNA integrity of all samples was examined by electrophoresis on a 1% agarose gel, while the DNA purity (260/280 absorbance ratio) was assessed using NanoPhotometer N60 (Implen). DNA concentration was measured by Qubit 3.0 Fluorometer (Thermo Fisher Scientific) using a Qubit dsDNA BR (Broad-Range) Assay Kit (Thermo Fisher Scientific), and subsequently standardized to 1 ng/μL before qPCR assays.
Quantitative PCR assay
Telomere length measurement was conducted using the qPCR method developed by Cawthon (2002). In brief, qPCR reactions were performed, respectively, to quantify telomeric (T) and reference (S; gene that is non-variable in copy number) DNA. Subsequently, the T/S ratio of each sample was calculated, representing the relative telomere length (rTL). Because telomere sequence is conserved among deuterostomes (Gomes et al. 2010), the primers used for the amplification of CoTS telomeric DNA were the same as those used for humans (forward 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′; reverse 5′- GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′) (Epel et al. 2004). Among the candidate reference genes that were examined, 18 s ribosomal RNA (rRNA) demonstrated the most consistent amplification profile as well as clean single-peak melt curves, and thus was selected as reference gene in this study. The suitability of 18 s rRNA as reference gene is also supported by previous research in echinoderm (Ragusa et al. 2013; Dettleff et al. 2020). Primers for 18 s rRNA gene are: forward 5′-ACTCAACACGGGAAACCTCA-3′; reverse 5′-AACCAGACAAATCGCTCCAC-3′.
Quantitative PCR reactions for telomeres and 18 s rRNA were set up on separate 72-well Rotor-Disc (Qiagen) using QIAgility (Qiagen) liquid handling robot. Each reaction consisted of 10 μL of PowerUp SYBR Green Master Mix (Thermo Fisher Scientific), 1000 nM of telomere primers or 500 nM of 18 s rRNA primers (Integrated DNA Technologies), 5 μL of template DNA, and nuclease-free water in a total volume of 20 μL. For experimental samples, the template DNA concentration was 1 ng/μL. The amplification efficiency of each run was evaluated by a standard curve derived from fivefold serial dilution of an arbitrarily assigned reference sample, yielding five concentrations ranging from 0.016 to 10 ng/μL. In addition, each run contained a no template control (substituting nuclease-free water for template DNA) for contamination detection, and a calibrator sample at 1 ng/μL (an identical sample repeated on all runs) to account for inter-run variation. All reactions were performed in technical triplicate using Rotor-Gene Q (Qiagen) with the following conditions: 50 °C for 2 min (UDG activation); 95 °C for 2 min (polymerase activation); 45 cycles of 95 °C for 15 s and 60 °C (for telomeres) or 55 °C (for 18 s rRNA) for 1 min; followed by a melt curve analysis at the end of each run to check for primer dimerization and non-specific amplification.
The quantification cycle (Cq) data were exported for analysis from the Rotor-Gene Q software (Qiagen) after baseline correction. Mean amplification efficiencies (± standard deviation) were 1.03 ± 0.04 for telomere and 1.02 ± 0.05 for 18 s rRNA. Samples were identified as technical outliers and excluded from further analysis if the standard deviation of Cq values between technical triplicate were > 0.2.
The relative telomere length (rTL) of each sample was calculated using the framework by Hellemans et al. (2007). In brief, the mean Cq values across technical triplicate were converted to normalized relative quantities (NRQ) according to Pfaffl (2001):
where E is gene-specific amplification efficiency, and ∆Cq represents the difference between the mean Cq value across all samples within a single run and the mean sample Cq value. Subsequently, inter-run calibration was conducted based on the NRQ of the calibrator sample of each run using formula 13’ and 15’ in Hellemans et al. (2007).
Statistical analyses
Differences in rTL between treatment groups were assessed with Kruskal–Wallis test (Kruskal and Wallis 1952). This non-parametric test was chosen because the assumptions of normality and homogeneity of variance were not met. Post-hoc analysis was conducted using Dunn’s multiple comparisons test with p values adjusted by the Benjamini–Hochberg correction method (Benjamini and Hochberg 1995). Pearson’s correlation test was used to determine whether rTL was correlated between tube feet and pyloric caeca tissue in adult CoTS. To investigate factors affecting rTL in field collected CoTS, a generalized linear mixed model (GLMM) was constructed with the package glmmTMB (Brooks et al. 2017). The model was built with rTL as response variable; diameter, spine length and pigment band count as fixed factors; and reef as random effect to account for different collection locations. The initial model was constructed with all possible two-way interactions, followed by stepwise elimination of non-significant terms and selection of the best fitted model using Akaike information criteria (AIC). The variance inflation factors (VIF) for all predictors in the final model were below 5, indicating no violations of collinearity. For model validations, residual diagnostics were performed using the DHARMa package (Hartig 2017). The level of significance of all tests was set at p < 0.05. All statistical analyses were performed using R version 4.1.1 (R Core Team 2022).
Results
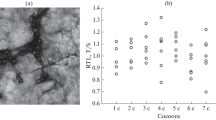
To investigate telomere dynamics in CoTS, rTL were compared among four age groups. The median rTL value was the highest in the 4-month juvenile group at 4.23, and the lowest in the 16-month CCA diet group at 2.04. Significant differences in rTL among groups were detected by Kruskal–Wallis test (X2 = 30.495, df = 5, p < 0.001). Pairwise comparisons revealed that apart from the 16-month CCA diet group, the juveniles exhibited significantly longer rTL than the adults (presumed > 24 months), while the rTL among other juvenile groups were not significantly different from each other (Dunn’s multiple comparisons test; Benjamini–Hochberg adjusted p, significance level = 0.05) (Fig. 1). Overall, there was large within-age class variation, with some adults showing similar rTL as the juveniles. For example, the rTL of the adult group ranged from 0.11 to 5.35, while the rTL of the 4-month group ranged from 2.10 to 8.51. With regards to diet treatment, the 16-month CCA diet group showed significantly lower median rTL (2.04) compared to the 16-month coral diet group (3.34) (p = 0.02); while no significant differences were found between the 7-month CCA and coral diet group. A significant positive correlation was found between the rTL measured from adult tube feet and pyloric caeca tissue (Pearson’s r = 0.73, p < 0.05) (Fig. 2). Finally, there were no significant relationships between field collected adult CoTS rTL and the diameter of the seastar, the length of the spines, or the count of pigment bands on spines (GLMM; Table 2, Fig. S1).
Relative telomere length (rTL) in crown-of-thorns seastar (CoTS; Acanthaster cf. solaris) by age (months after settlement) and diet. Boxes represent interquartile range of the distribution; horizontal lines within the boxes represent median; vertical extending lines represent maximum and minimum values within 1.5 interquartile range; each dot represents a sample. Different letters indicate statistically significant differences between groups (Kruskal–Wallis test followed by Dunn’s multiple comparisons, Benjamini–Hochberg adjusted p < 0.05). CCA = crustose coralline algae
Discussion
The present study is the first report of telomere measurement in the Pacific crown-of-thorns seastar (CoTS; Acanthaster cf. solaris). We demonstrated that the qPCR method allowed CoTS relative telomere length (rTL) to be measured in a high-throughput manner, providing a cost- and time-efficient approach for future research in CoTS telomere biology. As an exploratory study, our findings offer important insight into the potential factors influencing telomere dynamics in CoTS, while raising the possibility that telomere length could be a relevant biomarker to enhance the understanding of the population ecology of CoTS. More specifically, knowledge of the age structure of CoTS will help resolve persistent controversy regarding the putative cause(s) of population irruptions (Pratchett et al. 2017). The ability to age CoTS is for example, critical for testing whether juvenile seastar can defer ontogenetic shifts to withstand periods of resource limitation (Deaker et al. 2020).
Age-related telomere dynamics
Our results indicate that adult CoTS in general exhibit shorter rTL than juveniles, which is the first evidence of age-related telomere attrition in CoTS. These findings are in line with the general pattern observed in vertebrates (reviewed by Remot et al. 2021), while adding to the few examples of age-related telomere attrition in marine invertebrates (e.g., seastar and sea urchin; Varney et al. 2017; Coupé et al. 2019). Our observation contributes to the understanding of the relationship between age and telomere length in marine invertebrates, where inconsistent results have been reported from a limited number of existing studies. However, with juvenile and adult CoTS sourced from different growing environments (aquaria vs. field), these findings must be interpreted with caution, as the age-rTL relationship could be obscured by extrinsic factors (see below).
Our data illustrate that juvenile CoTS (< 16 months) fed on their natural diet (i.e., CCA followed by coral) can retain their telomere length, likely through energy demanding repair mechanisms. Previous research has established that telomere dynamics of a species is closely associated with the activity of telomerase, the enzyme that is involved in the repair and maintenance of telomeres (Blackburn 2005). For example, continuous telomerase expression has been reported in invertebrates with regeneration capacity (e.g., sea urchin; Francis et al. 2006) and/or indeterminate growth (e.g., lobster; Klapper et al. 1998), resulting in constant telomere restoration and thus a lack of age-associated shortening pattern. Whilst telomerase activities were not measured directly in this study, the telomere dynamics observed here appear to suggest that the telomerase expression pattern in CoTS is more similar to those observed in vertebrates: being stable during early development, followed by downregulation in later life stages (Gomes et al. 2010) or under stressful situations such as sub-optimal diet (see below). This is a compelling area for further research as telomerase activity is closely linked to the process of senescence, which is poorly understood in CoTS. In this context, future research should be undertaken to measure telomerase activity in CoTS using assay such as the telomeric repeat amplification protocol (TRAP; Kim et al. 1994).
Effects of diet on telomere dynamics
We found that among the 16-month-old juvenile CoTS, individuals that were fed on CCA diet (the situation suggested by the “hidden army hypothesis”) exhibited significantly shorter average rTL than their counterparts that were allowed diet transition to coral at 4-month post-settlement (the “typical” situation). Previous research has proposed that telomere dynamics are likely influenced by a combination of factors (Haussmann and Marchetto 2010). Our results suggest that diet could be one of such factors that influences rTL in CoTS. It is well established in human research that nutritional status is positively associated with telomere length (Paul 2011), while a similar pattern has also been observed in animals such as bird and fish (Badás et al. 2015; McLennan et al. 2021). It is proposed that sub-optimal diet is linked to a deficiency of dietary antioxidant intake, leading to a weakening in defence against oxidative damage to telomere DNA (Paul 2011). In addition, malnourished animals might not have excess energy to produce telomerase, compromising the ability to repair and maintain telomere length. Energy shortage of CCA-fed juveniles is supported by the fact that their growth was stunted, indicating that they have no “scope for growth” in their energy budget. Hence, it could conceivably be hypothesized that the shorter rTL observed here might be associated with inadequate levels of nutrients or energy in the CCA diet for CoTS that were supposed to have transitioned to coral. Further research might explore whether this damage can be reverted if CoTS switch to a coral diet later in life, and whether these energy-deficit individuals would undergo sexual maturation and be able to reproduce.
Our results, while preliminary, suggest that even though juvenile CoTS are seemingly able to survive on CCA diet for a prolonged period (Deaker et al. 2020), the delay in diet transition might have imposed oxidative stress and undermined their body conditions, which is manifested as accelerated telomere attrition. However, it is important to note that generalizability of our results could be limited by the scale of the study, and further investigation is required to prove our hypothesis. For example, measuring the rTL of juvenile-sized CoTS collected from the field would be an essential next step to validate our findings in situ.
Effects of tissue type on telomere dynamics
Significant positive correlation was found between rTL measured from tube feet and pyloric caeca tissue. While it is likely that the cell proliferative activity of pyloric caeca is higher than that of tube feet due to the continuous growth and replacement of digestive epithelium (Van der Plas et al. 1983), previous research found that telomere dynamics across somatic tissues in an individual is in general synchronized, regardless of proliferative status (Daniali et al. 2013). The same observation has been made in various taxa including invertebrates such as oyster (Godwin et al. 2011a; Debes et al. 2016; Power et al. 2021). On the other hand, while regeneration history of our samples is unknown, it has been demonstrated that telomere lengths do not differ between regenerated and non-regenerated arm tissues in the seastar Asterias rubens (Hernroth et al. 2010). Overall, our results broadly corroborate earlier findings which suggest no tissue effect in general, supporting the use of tube feet rTL as a proxy for overall telomere dynamics in CoTS. These findings are of interest to future sample collection, as tube feet tissue is easily accessible and convenient to collect from adult CoTS without requiring dissection.
Other age-independent factors
Contrary to expectations, this study did not find a significant relationship between the rTL of adult CoTS and the diameter of the seastar, the length of the spines, or the count of pigment bands on spines. While these physical measurements and features have not been fully validated as age proxies in CoTS, there is general consensus that they could, to some degree, be used to distinguish broad age classes, especially within the same population (Pratchett et al. 2014). As such, our results might point to the possibility that rTL in CoTS are considerably influenced by additional factors than age. An example of such factors is environmental stress, which has been demonstrated to cause oxidative damage to telomeric DNA and thus accelerate telomere attrition rate (Von Zglinicki 2002; Monaghan 2014). It is well established that CoTS development and growth are strongly influenced by environmental conditions such as food availability and temperature (Lucas 1984; Kamya et al. 2014; MacNeil et al. 2017). It is therefore reasonable to hypothesize that exposure histories to unfavorable conditions may be reflected in the rTL of CoTS, obscuring the age-related telomere attrition pattern. There are, nonetheless, other possible explanations that are worth further investigation, such as reproductive history, pathogen infection, and competition (reviewed by Chatelain et al. 2020).
As our adult seastar samples were collected from the wild where individuals’ growth histories were unclear and presumably complicated, it was intractable to disentangle the effects of various factors on rTL. Furthermore, the largest individual included in this study was 380 mm in diameter, while CoTS can grow to > 600 mm in the wild (Stump 1996). As such, future studies with samples across a wider size range, ideally from more controlled environment such as mesocosm experiments will be needed if a full picture of age-rTL association is to be developed.
Telomere length as biomarker for age determination in CoTS
Our results suggest that rTL could potentially be used as a tool to distinguish CoTS into broad age groups or life stages, though individual variation constrains the ability to resolve specific cohorts. This conclusion is in line with that of Dunshea et al. (2011), who found overall loose relationships between age and rTL across a range of invertebrates. To improve the utility of telomere length as an ageing tool, further research accounting for the influence of extrinsic factors will need to be undertaken. While a broad-scale ageing tool is nonetheless valuable and relevant for CoTS research, considerably more work is needed to tighten the age-rTL relationship in CoTS before such tool can be developed.
When comparing rTL to conventional ageing methods such as size-at-age, it appears that both approaches might be susceptible to the influence of extrinsic factors, particularly food availability. Consequently, like size-at-age, the effectiveness of using rTL as an ageing tool may be constrained if these extrinsic factors are not accounted for. However, it is worth noting that rTL presents a unique advantage in directly assessing the physiological conditions of CoTS. Therefore, there is abundant ground for future research to explore rTL as biomarker for stress or health status in CoTS (Haussmann and Marchetto 2010). For example, our results indicated that sub-optimal diet could be a potential stress factor accelerating rTL attrition in juvenile CoTS. If this pattern holds true in situ, rTL can be used as a tool to test the aquarium-based “hidden army hypothesis”, confirming whether CoTS exhibit prolonged delay in diet transition to coral in natural settings (Deaker et al. 2020). Furthermore, through measurement of rTL from CoTS populations experiencing varying stages of outbreak, we might be able to better understand the process of population collapse at the end of the outbreak cycle. For example, rTL might reflect stress related to starvation and over-crowding at the end of outbreaks, which is a yet-to-be-tested mechanism leading to population collapse. Nonetheless, due to the variable nature of telomeres, extensive field sampling as well as carefully controlled experiments will be required before rTL can be used as a relevant biomarker for CoTS management.
Conclusions
Here we established the first evidence of age-related telomere attrition in CoTS. However, telomere dynamics in CoTS are likely influenced by various age-unrelated factors such as diet and environmental stressors, limiting the use of telomere length as a precise chronological age indicator in this species. Overall, this study contributes to the understanding of telomere dynamics in marine invertebrates, while laying the groundwork for future research into rTL as a molecular biomarker for CoTS management. This is a compelling area for further investigation, given the potential of rTL to provide valuable biological information in a relatively low-cost and high-throughput manner.
References
Armanios M, Blackburn EH (2012) The telomere syndromes. Nature Reviews Genetics 13:693–704
Badás E, Martínez J, de Aguilar R, Cachafeiro J, Miranda F, Figuerola J, Merino S (2015) Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J Evol Biol 28:896–905
Balu V, Messmer V, Logan M, Hayashida-Boyles AL, Uthicke S (2021) Is predation of juvenile crown-of-thorns seastars dependent on age, size, or diet? Coral Reefs 40:641
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57:289–300
Blackburn EH (1991) Structure and Function of Telomeres. Nature (London) 350:569–573
Blackburn EH (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579:859–862
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9:378–400
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47–e47
Chatelain M, Drobniak SM, Szulkin M (2020) The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol Lett 23:381–398
Chesher RH (1969) Destruction of Pacific corals by the sea star Acanthaster planci. Science 165:280–283
Coupé S, Couvray S, Lechable M, Gaillard S, Dalvise NP (2019) Telomere Length as a Biomarker for Monitoring Wild Populations of the Sea Urchin Paracentrotus lividus. J Shellfish Res 38
Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai KK, Granick M, Aviv A (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nature communications 4:1–7
de Abechuco EL, Hartmann N, Soto M, Díez G (2016) Assessing the variability of telomere length measures by means of Telomeric Restriction Fragments (TRF) in different tissues of cod Gadus morhua. Gene Reports 5:117–125
Deaker DJ, Aguera A, Lin HA, Lawson C, Budden C, Dworjanyn SA, Mos B, Byrne M (2020) The hidden army: corallivorous crown-of-thorns seastars can spend years as herbivorous juveniles. Biol Lett 16:20190849
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109:17995–17999
Debes PV, Visse M, Panda B, Ilmonen P, Vasemägi A (2016) Is telomere length a molecular marker of past thermal stress in wild fish? Mol Ecol 25:5412–5424
Dettleff P, Villagra M, González J, Fuentes M, Estrada JM, Valenzuela C, Molina A, Valdés JA (2020) Effect of bacterial LPS, poly I: C and temperature on the immune response of coelomocytes in short term cultures of red sea urchin (Loxechinus albus). Fish Shellfish Immunol 107:187–193
Dunshea G, Duffield D, Gales N, Hindell M, Wells RS, Jarman SN (2011) Telomeres as age markers in vertebrate molecular ecology. Mol Ecol Resour 11:225–235
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM (2004) Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences 101:17312–17315
Francis N, Gregg T, Owen R, Ebert T, Bodnar A (2006) Lack of age-associated telomere shortening in long- and short-lived species of sea urchins. FEBS Lett 580:4713–4717
Godwin R, Brown I, Montgomery S, Frusher S, Green T, Ovenden J (2011a) Telomere dynamics in the Sydney rock oyster (Saccostrea glomerata): an investigation into the effects of age, tissue type, location and time of sampling. Mar Biol 159:77–86
Godwin RM, Frusher S, Montgomery SS, Ovenden J (2011b) Telomere length analysis in crustacean species: Metapenaeus macleayi, Sagmariasus verreauxi, and Jasus edwardsii. ICES J Mar Sci 68:2053–2058
Gomes NM, Shay JW, Wright WE (2010) Telomere biology in Metazoa. FEBS Lett 584:3741–3751
Hartig F (2017) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Package Version 01:5
Hartmann N, Reichwald K, Lechel A, Graf M, Kirschner J, Dorn A, Terzibasi E, Wellner J, Platzer M, Rudolph KL, Cellerino A, Englert C (2009) Telomeres shorten while Tert expression increases during ageing of the short-lived fish Nothobranchius furzeri. Mechanisms of ageing and development 130:290–296
Haussmann MF, Vleck CM (2002) Telomere length provides a new technique for aging animals. Oecologia 130:325–328
Haussmann MF, Vleck CM, Nisbet IC (2003) Calibrating the telomere clock in common terns, Sterna hirundo. Experimental Gerontology 38:787–789
Haussmann M, Marchetto N (2010) Telomeres: linking stress and survival, ecology and evolution. Current Zoology. Current Zoology
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Hernroth B, Farahani F, Brunborg G, Dupont S, Dejmek A, Skold HN (2010) Possibility of mixed progenitor cells in sea star arm regeneration. J Exp Zool B Mol Dev Evol 314:457–468
Kamya PZ, Dworjanyn SA, Hardy N, Mos B, Uthicke S, Byrne M (2014) Larvae of the coral eating crown-of-thorns starfish, Acanthaster planci in a warmer-high CO 2 ocean. Global Change Biol 20:3365–3376
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Klapper W, Kühne K, Singh KK, Heidorn K, Parwaresch R, Krupp G (1998) Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett 439:143–146
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. Journal of the American statistical Association 47:583–621
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Louzon M, Coeurdassier M, Gimbert F, Pauget B, de Vaufleury A (2019) Telomere dynamic in humans and animals: Review and perspectives in environmental toxicology. Environ Int 131:105025
Lucas JS (1984) Growth, maturation and effects of diet in Acanthaster planci (L.) (Asteroidea) and hybrids reared in the laboratory. J Exp Mar Biol Ecol 79:129–147
MacNeil M, Chong-Seng K, Pratchett D, Thompson C, Messmer V, Pratchett M (2017) Age and Growth of An Outbreaking Acanthaster cf. solaris Population within the Great Barrier Reef. Divers 9
McLennan D, Auer SK, McKelvey S, McKelvey L, Anderson G, Boner W, Duprez JS, Metcalfe NB (2021) Habitat restoration weakens negative environmental effects on telomere dynamics. Mol Ecol
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217:57–66
Nakamura M, Okaji K, Higa Y, Yamakawa E, Mitarai S (2014) Spatial and temporal population dynamics of the crown-of-thorns starfish, Acanthaster planci, over a 24-year period along the central west coast of Okinawa Island, Japan. Mar Biol 161:2521–2530
Neil RC, Gomez Cabrera M, Uthicke S (2022) Juvenile age and available coral species modulate transition probability from herbivory to corallivory in Acanthaster cf. solaris (Crown-of-Thorns Seastar). Coral Reefs:1–6
Paul L (2011) Diet, nutrition and telomere length. The Journal of nutritional biochemistry 22:895–901
Pauli JN, Whiteman JP, Marcot BG, McClean TM, Ben-David M (2011) DNA-based approach to aging martens (Martes americana and M. caurina). J Mammal 92:500–510
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45–e45
Power ML, Power S, Bertelsen MF, Jones G, Teeling EC (2021) Wing: A suitable nonlethal tissue type for repeatable and rapid telomere length estimates in bats. Mol Ecol Resour 21:421–432
Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman H (2014) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanography and Marine Biology 52:133–200
Pratchett MS, Caballes CF, Wilmes JC, Matthews S, Mellin C, Sweatman HP, Nadler LE, Brodie J, Thompson CA, Hoey J, Bos AR (2017) Thirty years of research on crown-of-thorns starfish (1986–2016): scientific advances and emerging opportunities. Diversity 9(4):41
Pratchett MS, Caballes CF, Cvitanovic C, Raymundo ML, Babcock RC, Bonin MC, Bozec Y-M, Burn D, Byrne M, Castro-Sanguino C, Chen CCM, Condie SA, Cowan Z-L, Deaker DJ, Desbiens A, Devantier LM, Doherty PJ, Doll PC, Doyle JR, Dworjanyn SA, Fabricius KE, Haywood MDE, Hock K, Hoggett AK, Høj L, Keesing JK, Kenchington RA, Lang BJ, Ling SD, Matthews SA, McCallum HI, Mellin C, Mos B, Motti CA, Mumby PJ, Stump RJW, Uthicke S, Vail L, Wolfe K, Wilson SK (2021) Knowledge Gaps in the Biology, Ecology, and Management of the Pacific Crown-of-Thorns Sea Star, Acanthaster sp., on Australia’s Great Barrier Reef. The Biological Bulletin: 241(3):330–346
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ragusa MA, Costa S, Gianguzza M, Roccheri MC, Gianguzza F (2013) Effects of cadmium exposure on sea urchin development assessed by SSH and RT-qPCR: Metallothionein genes and their differential induction. Mol Biol Rep 40:2157–2167
Remot F, Ronget V, Froy H, Rey B, Gaillard JM, Nussey DH, Lemaitre JF (2021) Decline in telomere length with increasing age across nonhuman vertebrates: A meta-analysis. Mol Ecol
Scott NM, Haussmann MF, Elsey RM, Trosclair Iii PL, Vleck CM (2006) Telomere Length Shortens with Body Length in Alligator mississippiensis. Southeast Nat 5:685–692
Souter DW, Cameron, A. M., & Endean, R. (1997) Implications of sublethal predation, autotomy and regeneration: Pigment bands on their spines can not be used to determine the ages of adult specimens of the corallivore acanthaster planci.
Stump RJW, Lucas JS (1999) Age estimation and patterns of growth in Acanthaster planci: a reply to Souter et al. (1997). Marine and Freshwater Research 50
Stump R, Lucas J (1990) Linear growth in spines from Acanthaster planci (L.) involving growth lines and periodic pigment bands. Coral Reefs 9:149–154
Stump R (1996) An investigation of the methods to describe the population dynamics of Acanthaster planci (L.) around Lizard Island, northern Cairns Section. Rep
Uthicke S, Logan M, Liddy M, Francis D, Hardy N, Lamare M (2015) Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci Rep 5:8402–8402
Van der Plas A, Brands F, Voogt P (1983) An autoradiographic investigation of the proliferative activity of pyloric caeca and gondas of Asterias rubens. J Morphol 177:51–58
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 Protects Human Telomeres from End-to-End Fusions. Cell (Cambridge) 92:401–413
Varney RM, Pomory CM, Janosik AM (2017) Telomere elongation and telomerase expression in regenerating arms of the starfish Luidia clathrata (Asteroidea: Echinodermata). Mar Biol 164
Von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wilmes JC, Hoey AS, Pratchett MS (2020) Contrasting size and fate of juvenile crown-of-thorns starfish linked to ontogenetic diet shifts. Proc Biol Sci 287:20201052
Yamaguchi M (1974) Growth of juvenile Acanthaster planci (L.) in the laboratory. Pac Sci 28:123–138
Acknowledgements
Funding for this research was provided by the Australian Institute of Marine Science and AIMS@JCU, a collaborative scheme between the Australian Institute of Marine Science and James Cook University. We would like to thank Dr. Maria Gomez-Cabrera (K-le) for her help in rearing and sampling the crown-of-thorns seastars. We are also grateful to Jason Doyle for methodological advice and support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwong, S.L.T., Villacorta-Rath, C., Pratchett, M. et al. Telomere dynamics in the Pacific crown-of-thorns seastar (Acanthaster cf. solaris): effect of age, diet, and tissue type. Coral Reefs 42, 977–985 (2023). https://doi.org/10.1007/s00338-023-02405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02405-4