Abstract
There is overwhelming evidence that tropical coral reefs are severely impacted by human induced climate change. Assessing the capability of reef-building corals to expand their tolerance limits to survive projected climate trajectories is critical for their protection and management. Acclimation mechanisms such as developmental plasticity may provide one means by which corals could cope with projected ocean warming and acidification. To assess the potential of preconditioning to enhance thermal tolerance in the coral Pocillopora acuta, colonies were kept under three different scenarios from settlement to 17 months old: present day (0.9 °C-weeks (Degree Heating Weeks), + 0.75 °C annual, 400 ppm pCO2) mid-century (2.5 °C-weeks, + 1.5 °C annual, 685 ppm pCO2) and end of century (5 °C-weeks, + 2 °C annual, 900 ppm pCO2) conditions. Colonies from the present-day scenario were subsequently introduced to the mid-century and end of century conditions for six weeks during summer thermal maxima to examine if preconditioned colonies (reared under these elevated conditions) had a higher physiological performance compared to naive individuals. Symbiodiniaceae density and chlorophyll a concentrations were significantly lower in mid-century and end of century preconditioned groups, and declines in symbiont density were observed over the six-week accumulated heat stress in all treatments. Maximum photosynthetic rate was significantly suppressed in mid-century and end of century preconditioned groups, while minimum saturating irradiances were highest for 2050 pre-exposed individuals with parents originating from specific populations. The results of this study indicate preconditioning to elevated temperature and pCO2 for 17 months did not enhance the physiological performance in P. acuta. However, variations in trait responses and effects on tolerance found among treatment groups provides evidence for differential capacity for phenotypic plasticity among populations which could have valuable applications for future restoration efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is extensive evidence that human induced climate change is significantly impacting natural systems globally (Pachauri and Reisinger 2007). Accumulation of anthropogenic carbon dioxide (CO2) in the earth’s atmosphere is causing global warming due to enhancement of the greenhouse effect (Solomon et al. 2009), as well as increasing the absorption of CO2 into the upper ocean (Doney et al. 2009). Since the industrial revolution, global land and sea temperatures have increased by 1.18 °C and average surface ocean pH has decreased by 0.1 pH units (Hoegh-Guldberg et al. 2014; IPCC 2014, 2021; Lough et al. 2018). Further increases in sea surface temperatures of up to 3.3 °C and decreases as low as 0.42 pH units are predicted by the end of the century under the business as usual Representative Concentration Pathway 8.5 climate scenario (Allen et al. 2018; IPCC 2014, 2022). Greater absorption of CO2 by the ocean reduces oceanic pH and the freely available carbonate ions, making calcification more energetically demanding for corals from bicarbonate (Doney et al. 2009). As these altered conditions are beyond the range that organisms have evolved in, it can result in negative effects to marine species (Harley et al. 2006), because physiological and biological processes are optimised to the environmental conditions normally experienced (Both et al. 2004; Walther et al. 2002).
Tropical ectothermic species are particularly vulnerable to climate change because they have evolved in an environment with a relatively stable temperature and pH, and live close to their physiological upper thermal limits compared to temperate species (Deutsch et al. 2008; Tewksbury et al. 2008; Walther et al. 2002). Coral reefs are considered one of the most sensitive ecosystems to temperature change (Hoegh-Guldberg 1999) as rising water temperature can cause a breakdown in the relationship between the coral host and its intracellular symbiotic algae (Symbiodiniaceae spp.), resulting in a loss of colour known as coral bleaching (Hoegh-Guldberg 1999; Hughes et al. 2003, 2018). If prolonged, coral bleaching can cause significant physiological damage and starvation of the coral host due to the loss of their photosynthetically active Symbiodiniaceae, which can ultimately lead to mass mortality on reefs (Baker et al. 2008; Glynn 1993; Jones et al. 1998). In addition, changes in ocean chemistry can impair the accretion of calcium carbonate skeletons by increasing the cost of production (Spalding et al. 2017), and in extreme cases can limit their formation by calcifying organisms (Anthony et al. 2008; Gazeau et al. 2007; Mollica et al. 2018; Riebesell et al. 2000). As a foundation taxon, scleractinian corals provide essential habitat, food and shelter required by thousands of marine organisms (Cole et al. 2008; Pratchett et al. 2011; Reaka-Kudla 1997), while also providing crucial sources of income, livelihoods and a multitude of other ecosystem services that benefit humans (Moberg and Folke 1999). Given the ecological importance of corals, and the accelerating uncertainty of their future under altered environmental conditions, understanding their capacity to cope with projected climate scenarios is critical to predicting the persistence of reef ecosystems.
Due to the rapid rate of both projected and current environmental change, there is growing concern that genetic adaptation will not be able to keep pace and non-genetic change through phenotypic plasticity is likely to play a critical role in species persistence (Gibert et al. 2019; Morley et al. 2019; Snell-Rood et al. 2018). Phenotypic change can occur in response to variations in environmental conditions throughout an individual’s lifetime (reversible plasticity), during early life experiences (developmental plasticity), or as a result of inherited environmental tolerance limits from previous generations (transgenerational plasticity; Angilletta 2009; Donelson et al. 2018; Mousseau and Fox 1998). Developmental plasticity is likely to play a particularly crucial role in response to a rapidly changing climate, as many organisms possess a sensitive window during early ontogeny (West-Eberhard 1989). During development (pre-conception to young adult), cells have not yet been fully differentiated, and are therefore highly susceptible to environmental influence compared to adult cells (Burton and Metcalfe 2014). These mechanisms can be particularly important in organisms that possess dispersal phases early in life, but are sessile as adults, as it provides a means of responding to environmental variation experienced between generations (Van Kleunen and Fischer 2005). However, the type of plasticity that occurs will depend on the nature and predictability of environmental variation. For example, reversible plasticity is expected be favoured when environmental variation occurs within a generation, development when variation occurs unpredictably across generations, and transgenerational when variation occurs predictably across generations (Herman et al. 2014; Leimar and McNamara 2015; Reed et al. 2010). It is evident that scleractinian corals have a high capacity for within generation plasticity as documented by extensive morphological plasticity to light and wave energy (Anthony and Hoegh-Guldberg 2003a, b; Titlyanov et al. 2001; Todd 2008), however, capacity for thermal plasticity is poorly understood (Padilla and Savedo 2013; Torda et al. 2017).
Exposure to future environmental conditions from early life may trigger plastic responses and help corals cope with elevated oceanic conditions (Burton and Metcalf 2014, Padilla and Savedo 2013). Historic bleaching events provide the opportunity to study the effects of preconditioning and natural selection within natural communities, whereby bleaching severity can be used as an indicator for thermal tolerance. Six mass bleaching events have been documented on the Great Barrier Reef (GBR) over the past two decades (1998, 2002, 2016, 2017, 2020, 2022), with repeated stress reducing bleaching severity during events that closely followed a previous bleaching event, suggesting thermal exposure histories of reef communities may influence thermal plasticity by increasing thermal tolerance or removing the more heat-sensitive genotypes (Hughes et al. 2019a, b; Maynard et al. 2008). To date, several studies have investigated the potential to enhance stress tolerance in corals through experimental exposure (Castillo and Helmuth 2005; Gibbin et al. 2018; Putnam and Gates 2015; but see McRae et al. 2021), with most of these focused on intra-generational responses of adult colonies and using relatively short-term exposure (Bellantuono et al. 2012; Middlebrook et al. 2008; Schoepf et al. 2019, 2022). Within generations, it has been demonstrated that acute exposure (48 h) to heat stress can improve photoprotective mechanisms in corals (Middlebrook et al. 2008) and enhance resistance to thermal stress through physiological plasticity of the host and/or associated symbionts (Bellantuono et al. 2012). More recent work has shown plasticity of Pocillopora damicornis colonies transplanted from the reef slope to the reef flat for 18 months exhibited enhanced heat tolerance when exposed to experimental heat stress compared to their slope-residing counterparts (Marhoefer et al. 2021). However, others have found limited or even negative effects following preconditioning (Dilworth et al. 2021; Martell 2022; Schoepf et al. 2019). In the context of early life exposure, larvae brooded within parent colonies of P. damicornis under increased temperature and ocean acidification resulted in the acclimation of larvae to these elevated conditions once released (Putnam and Gates 2015). Thus, while some of these studies provide preliminary evidence of the capacity for plastic responses in corals, there is still a limited understanding of ontogenetic sensitivity and whether early life exposure or long-term preconditioning is necessary to produce increased tolerance to environmental change.
This study aimed to better understand the capacity for phenotypic plasticity to future climate change with long-term exposure from early life. To achieve this, individual colonies that were clones of the hermatypic coral Pocillopora acuta were reared from asexual brooded larvae (via parthenogenesis), settled under experimental treatments, and raised for 17 months in three combined temperature and pCO2 scenarios (ambient control, preconditioned 2050, and preconditioned 2100 treatments). After 17 months of exposure, a subset of colonies from the ambient control conditions were transferred into the elevated mid and end of century treatments (termed acute 2050 and acute 2100) to determine whether performance was enhanced in pre-conditioned individuals. A range of physiological traits (Symbiodiniaceae density, chlorophyll a concentration, tissue protein concentration, maximum photosynthetic rate and minimum saturation point) of the coral host and symbiotic algae, Symbiodiniaceae, were explored to holistically assess the tolerance to future climate change, as these metrics are known to be key indicators of bleaching response and overall holobiont health (Anthony and Hoegh-Guldberg 2003a, b; Rodrigues and Grottoli 2007; Roth 2014; Schoepf et al. 2013; Stimson et al. 2002). Total colony size was also measured at the completion of the experiment. Wild colonies of the parent generation were collected from three reefs on the central GBR to begin the experiment with a diverse genetic baseline which allows a better understanding of variation in plastic responses.
Methods
Coral collection
Adult colonies of P. acuta were collected from three reefs on the GBR, Queensland, Australia in July 2017. Located approximately 26–57 km offshore, Coates Reef (17°11′18.60″ S, 146°22′18.48″ E), Feather Reef (17°31′6.74″ S, 146°23′21.84 E) and Rib Reef (18°28′16.39″ S, 146°52′24.96″ E) are all mid-shelf reefs in the central section of the GBR. These three reefs were chosen as knowledge of recent bleaching severity was available from previous in water bleaching surveys during the 2016 and 2017 heat stress events, and annual average and maximum temperatures were similar between reefs, yet located far enough apart to increase the likelihood of selecting unique individuals. Colonies were collected from two to three sites within each reef, with 100 to 1800 m distance between distinct collection sites to decrease the likelihood of selecting clonal colonies. These reefs were all included within the in-water community bleaching surveys conducted by the Australian Institute of Marine Science (AIMS) for the Australian National Bleaching Taskforce response effort in 2016–2017 (Fig. 1). All three reefs experienced low level thermal anomalies ranging from 1.72–2.23 °C-weeks during the summer of 2016 and more severe heat stress ranging from 7.42–8.89 °C-weeks in 2017. Community level bleaching ranged from 16–52%, with low levels of severe bleaching and mortality (2–22%) in 2016. Bleaching severity increased in response to the greater accumulation of Degree Heating weeks (DHW) heat stress to 44–94% community bleached, with 9–58% severely bleached or recently dead in 2017 at these three sites (Cantin et al. 2021). The colonies collected from these locations are considered the survivors following the severe heat stress and bleaching observed during the 2016 and 2017 bleaching events at these reef locations (Cantin et al. 2021; Hughes et al. 2018, 2019a, b).
Experimental setup controls and treatments
Annual temperature profiles were determined using historic National Oceanic and Atmospheric Administration (NOAA) Coral Reef Watch v3.1 climatology of sea surface temperatures for the central GBR. Specifically, sea surface temperatures from 1985–2012 were used to represent the present day historical daily average (ambient treatment). The IPCC AR5 Representative Concentration Pathways (IPCC 2014) were used to set the mid and end of century of pCO2 levels: present day (summer heat stress of 0.9 °C-weeks combined with + 0.75 °C annual average above pre-industrial, 400 ppm pCO2), mid-century (summer heat stress of 2.5 °C-weeks combined with + 1.5 °C annual average above pre-industrial, 685 ppm pCO2), and end of century (summer heat stress of 5 °C-weeks combined with + 2 °C annual average above pre-industrial, 900 ppm pCO2; Table S1; Fig. 2C). Pre-industrial averages for the central GBR were calculated using the HadISST1 data set available from the UK Meteorological Office Hadley Centre (https://www.metoffice.gov.uk/hadobs/hadisst/index.html).
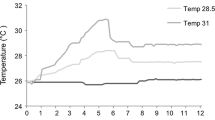
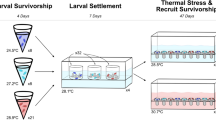
A Experimental timeline: Wild Pocillopora acuta parent colonies were collected from Coates (N = 3), Feather (N = 3) and Rib (N = 2) Reefs in July 2017. Parent colonies released brooded larvae from July–September 2017 and larvae were collected and settled in isolation until October 2017, at which point F1 juveniles were equally spread across the three temperature and pCO2 treatments (present day ambient, 2050 and 2100). F1 corals remained in these treatments until February 2019, when a subset were transferred from ambient into 2050 and 2100 conditions (becoming acute 2050 and 2100, respectively). Vertical lines at T0 (February 2019) represent corals transferred from temperature and pCO2 treatments they were raised under (original treatment), to the experimental treatments for this study. Sample size of each treatment are indicated (n = 24 F1 colonies, representing three replicates from each of the eight original F0 parent colonies in each treatment). B Experimental temperature profiles from January to April 2019 showing the present-day scenario historical average (1985–2012; green line), mid-century 2050 + 1.5 °C scenario (orange line), end of century 2100 + 2.0 °C scenario (red line) compared to the pre-industrial era 1880–2015 using HadISST v1. Black dashed line represents the NOAA Coral Reef Watch v3.1 + 1.0 °C upper thermal limit threshold value of 29.3 °C, the temperature at which heat stress accumulation begins development of daily hotspots that contribute to the final Degree Heating Week (DHW; °C-weeks) accumulation. Time 0 (T0) indicates the transfer of ambient corals into the 2050 and 2100 scenario treatments, T1 indicates the first sample period (week 3 after transfer) and T2 indicates the second sample period (week 6 after transfer). Note: see Fig S2 for the full annual temperature profile for each treatment. C Description of summer thermal and pCO2 maximums for each treatment, where experimental DHW is a representation of the accumulated heat stress within each experimental treatment, and preconditioned DHW represents the difference in heat stress between the treatment of origin and the experimental treatment each group was transferred into
This experiment was conducted within the Australian National Sea Simulator (SeaSim) facility at the AIMS Cape Ferguson site. The experimental aquarium facility is managed by an industrial DCS Process Control System (Siemens SIMATIC PCS 7) running the sea water processing and the LSS; a number of micro-programmable logic controllers (PLC) are integrated with the SCADA software (Siemens SIMATIC WinCC) to control setpoint targets for individual experiments. The mesocosm tanks used for this study were managed by one PLC of the S7-1500 series, networked into the main process control architecture. The control system received feedback from each of the nine separate experimental replicate mesocosms sending continuous pCO2 data from the in-tank seawater CO2 analyser, the temperature transmitter and the PAR sensor to maintain the daily and seasonal profiles for pCO2, temperature and light (Table S1). Values for each of the parameters were logged every 20 s and stored by the Process Historian Server. Data about the manipulate variables (MV0), like the position of the valves controlling the heat exchanger mixing valves or the CO2 dosing valves were also stored.
Temperature control was achieved by passing the system water through a heat exchanger. Two temperature-controlled loops of heated and chilled water at 40 °C and 15 °C, respectively, were mixed by an actuating valve, which was used to control the temperature within the heat exchanger, and in turn, the system water. The mix was adjusted by SCADA based on values measured in the system via water probes (pt100 TC Direct Thermocouple Sensor). CO2 levels were monitored via analysers (Amphenol Telaire) with profiles maintained through SCADA by dosing or removing CO2. Dosing was activated though solenoid valves and a membrane contractor to diffuse CO2 into the system seawater. To remove CO2, an actuating valve opened to expose external blower air through a degassing chamber with system water flowing through, thereby lowing CO2 levels. In addition to diurnal and seasonal changes in temperature and pCO2, corals were also exposed to seasonal variations in solar and lunar cycles using daily sunrise—sunset and moonrise to match natural timing of sunrise-sunset, moonrise-moonset and lunar new moon-full moon cycles with low intensity blue light LED lights.
Adult colonies of P. acuta were transferred in July 2017 to the AIMS facility in Townsville, Queensland, where they were maintained under ambient present-day temperature and pCO2 scenario conditions within nine independent replicate flow-through seawater mesocosm systems. From July to September 2017, asexually brooded, and thus genetically identical larvae (parthenogenic larvae produced in the absence of sperm; see Smith et al. 2019 and Nakajima et al. 2018) were collected from adult colonies by isolating colonies within 30 L acrylic chambers with larval collection nets on the outflow from each tank to contain individual larvae cultures. Larvae were collected from the nets each morning and placed in individual, flow-through settlement tanks containing trays of 20 mm diameter conditioned calcium carbonate plugs for 56 d. Larvae were allowed to settle in these trays in isolation and were tracked throughout the experiment to prevent mixing of genotypes and ensure genetic identity. A total of 6532 plugs (most containing multiple larvae) were collected representing 29 F0 colonies (assumed to represent unique genotypes). After settlement, offspring of these F0 colonies were evenly distributed across the three combination temperature and pCO2 treatments (Fig. 2A) and the three replicate aquarium mesocosm systems (hereafter ‘tanks’), which consisted of a deep parent holding tank and two shallow recruit rearing tanks for each treatment. Treatment profiling of future temperature and acidification conditions began in October 2017 following the even distribution of both parent and the offspring of all unique individuals. Corals were fed Artemia (1 nauplii ml−1, raised at AIMS) and macroalgae (2000 cells ml−1) at 4 pm daily.
First generation (F1) corals were raised under the three experimental temperature and acidification scenarios from settlement until 17 months of age (approximately 55 cm2 in size; Fig. S1) when testing for this study was conducted (early 2019). The experimental profiles followed a complete annual cycle of temperature (Fig. S2) and light intensity. During the first summer, preconditioned corals experienced chronic thermal maximum temperature exposure during the normal period of summer heat from January to March 2018 (same thermal profile as pictured in Fig. 2B), combined with future acidification levels. Survival of F1 individuals ranged from 70–100% for ambient, 58–98% for preconditioned 2050, and 58–100% for preconditioned 2100 (Table S2). During the second summer, both the preconditioned corals and a subset of F1 ambient control corals experienced elevated CO2 and thermal conditions. In the case of corals raised under ambient conditions, transfer from the present-day, ambient treatment into the 2050 and 2100 treatment tanks occurred in February 2019 (T0, Fig. 2A, B). This was completed to assess if preconditioned two year-old first generation corals in the 2050 and 2100 scenarios had developed enhanced physiological tolerance compared to the ambient present-day corals. Specifically, 24 F1 coral individuals representing eight F0 colonies from the three replicate ambient tanks (eight F0 colonies per tank) were transferred into replicate mid and end of century tanks, and directly compared to 24 preconditioned coral individuals with the same eight F0 parent colonies in the mid and end of century scenarios (eight F0 colonies from three replicate tanks per treatment to give n = 24 corals per treatment; Fig. 2A). The individuals left within the ambient present-day scenario were used as the control. This created a total of five experimental treatments: ambient-control, acute 2050 (i.e. ambient transferred to 2050), preconditioned 2050, acute 2100 (i.e. ambient transferred to 2100), and preconditioned 2100 (Fig. 2A, C). Each coral individual was sampled and photophysiological health metrics measured at week three and week six during the summer thermal maximum of 2019 in order to assess changes in health with exposure length, as well as differences between peak summer temperature (T1) versus accumulated end of summer stress (T2; Fig. 2B).
Laboratory measurements and data collection
Coral and symbiodiniaceae health metrics
Coral colonies were sampled at week 3 (T1) and week 6 (T2) of the summer experiment (Fig. 2B), following respiration experiments outlined below. Specifically, 0.5–1 cm pieces of colony branches were clipped using bone cutters. Fragments were immediately frozen in liquid nitrogen, and stored at − 80 °C until further processing. After thawing of the fragments, tissue was stripped from each fragment using an air-gun and filtered seawater (Johannes and Wiebe 1970). The resulting tissue homogenate was standardized to an equal volume, and mixed using a homogenizer for 30 s. Three sub-samples of homogenate were then taken for Symbiodiniaceae density, chlorophyll a concentration, and protein concentration, which were kept frozen at − 20 °C in the dark.
To quantify bleaching response, density of Symbiodiniaceae within the homogenate was determined (Fitt et al. 2001; Glynn 1996) using a Neubauer hemocytometer and high-powered microscope. Tissue homogenate was thawed at room-temperature for approximately 10 min, and vortexed for a minimum of 1 min to resuspend the pellet and mix thoroughly. Immediately following, 10 µl of homogenate was pipetted underneath the hemocytometer slide cover slip, and a minimum of two replicate counts were completed per sample. Total number of cells was normalized by surface area of the coral fragment (# cells cm−2; see below).
Chlorophyll a concentration within the Symbiodiniaceae was determined by measuring the absorbance using a BioTek microplate spectrophotometer. Thawed tissue homogenate was centrifuged at 1500 × g for 3 min at 4 °C. The supernatant was discarded to eliminate coral host cell contamination, and the remaining algal pellet was mixed with 95% ethanol using a vortex and sonicator bath to extract chlorophyll from the algal cells. After 20 min of dark incubation, samples were centrifuged at 10,000 × g for 5 min at 4 °C, and 200 µl of the extracted sample was measured using a spectrophotometer. Sample absorbance was measured in triplicate at 632 nm, 649 nm and 665 nm, and the chlorophyll a concentration calculated using the blank corrected absorbance readings from the published equation in Ritchie (2008). Finally, pigment concentration was normalized by the ethanol extract volume and surface area of the branch fragments.
Coral host tissue protein concentration was analyzed using the microassay procedure described by Leuzinger et al. (2003) through use of the BioRad DC Protein Assay Kit II. Specifically, 1.2 ml of thawed coral-symbiont tissue homogenate was centrifuged at 1500 × g for 3 min to pellet the Symbiodiniaceae and the supernatant mixed with 2 M NaOH and incubated at 90 °C for 1 h. Samples were again centrifuged at 1500 × g for 10 min, and the resulting supernatant aliquoted in triplicates were mixed first with a copper tartrate solution, and secondly with a Folin reagent, resulting in a colorimetric assay of protein concentration within the samples. Sample replicates were measured at 750 nm using a BioTek microplate spectrophotometer, along with 8 different concentrations of the BioRad Protein Assay Standard II bovine serum albumin protein standards of a known concentration (0, 0.01, 0.02, 0.08, 0.16, 0.24, 0.36 µg ml−1). Protein concentration of each sample was calculated from the equation of the protein standard curve and normalized by surface area.
Total growth of each coral was measured using three-dimensional modelling. Capturing different angles, 40–80 photographs of each coral individual were taken at the end of the summer (April 2019) following 18 months of growth. A three-dimensional model of each coral was created using Agisoft Metashape Professional (version 1.5.2) from which surface area was calculated.
In order to normalize host and Symbiodiniaceae health metrics, the surface area of sample fragments was determined using the wax dipping method modified from Stimson and Kinzie (1991). Paraffin wax was melted at 80 °C using a beaker submerged in a water bath, and the wax maintained at this temperature throughout the process to ensure consistency in the amount of wax coating each fragment. Cylindrical calibration objects of known surface area were used to create a standard curve, which ranged from 0.8–5 cm2. Each fragment or calibration object was weighed to the nearest 0.0001 g prior to being dipped. Using tweezers, each piece was dipped in wax for approximately 1 s and rotated immediately, to remove excess wax. After a cooling period of approximately 5 min, each object was re-weighed, and the dipping and weighing process repeated. The first dip ensured the porous skeleton was sealed and allowed for an even coating on the second dip. Surface area of each fragment was determined using the standard curve (Fig. S3) from the calibration objects, where the equation of the standard curve was used to calculate the surface area based on the difference in weight between the first and second wax dip.
Photophysiology
Respiration testing was conducted at week 3 and week 6 to measure differences in photophysiology over the summer thermal peaks, and between the 5 treatments. Photosynthetic rate was measured by monitoring changes in dissolved oxygen (O2) concentration within 450 ml acrylic chambers containing individual coral colonies. Chambers were illuminated from above using four LED light panels, and a magnetic stirbar within each chamber was utilized to ensure the seawater was fully mixed, allowing for uniform measurements of O2 concentration within the chambers. Corals were exposed to 10 different light intensities incrementally increasing from 0 to 1000 µmol-photons/m2/s with exposures lasting approximately 500 s. Concentrations of dissolved O2 within the chamber were measured every second using FireSting contactless fiber-optic oxygen sensors. Oxygen probes were manually calibrated prior to the experiments by taking a 0% and 100% calibration value using a sodium sulfite solution (containing no O2) and water–vapor saturated air, respectively. Water temperature was maintained for the duration of the testing by submerging chambers within a temperature-controlled water-bath, with temperatures set at the same treatment temperature as the experimental aquaria. Total dissolved O2 was determined by multiplying O2 concentration and total water volume within the chamber (subtracting the coral colony volume). Finally, rate of O2 production was normalized by colony surface area, which was calculated using three-dimensional photogrammetry methods and computer modeling (Agisoft Metashape Professional, version 1.5.2). Photosynthesis-irradiance curves were then calculated by fitting data to a hyperbolic tangent function modified for respiration (Jassby and Platt 1976), and two associated photophysiological parameters calculated: photosynthetic maximum (Pmax) and minimum irradiance at saturation (Ek).
Statistical analysis
In order to statistically analyze differences in each of the five physiological health metrics, generalized linear mixed effects models were run for three fixed factors, along with their combined interactions: treatment, sample period (week 3 or 6) and reef, and two random factors: coral individual nested within F0 colony. All data were tested for homogeneity of variance and normality prior to conducting any analyses, with the use of Levene and Shapiro–Wilk tests. Symbiodiniaceae density and chlorophyll a concentration did not pass these tests (P < 0.05), and were transformed using a cube-root transformation to ensure assumptions were met. Photosynthetic maximum and protein concentration were unable to fit a normal distribution upon transformation, and these were analyzed using a log Gamma distribution within the function. As an additional holobiont response metric, total growth of each coral at 18 months of age was also compared between the treatment groups. Mean surface area was analyzed using the same generalized linear mixed effect model as above (assumptions of normality and homoscedasticity met following a cube-root transformation), with sample period removed as a fixed factor as growth was only measured at a single time point (fixed factors: treatment and reef, random factor: F0 colony). To analyze interactions from all glmmTMB models, an Anova was used which reports a Wald chi-square test statistic (X2). Analyses that resulted in a significant difference in physiological traits were further analyzed using an emmeans pairwise comparison with a Tukey adjustment to reveal differences among groups. All statistical analyses were conducted using R version 1.1.442 (packages: glmmTMB, car, bbmle).
Results
Symbiodiniaceae characteristics
Mean Symbiodiniaceae density had a significant negative response to elevated temperature and pCO2 (X2(4) = 27.536, P < 0.001, Table 1; Fig. 3). Symbiont densities were lowest in preconditioned 2050 and 2100 corals, with significant declines compared to ambient control (post-hoc P = 0.002, P < 0.001, respectively). Symbiodiniaceae density also varied by the combined interaction of sample period and parental reef (X2(2) = 7.050, P = 0.030, Table 1). This interaction was driven by significant declines in symbionts from week 3 to week 6 for Coates (post-hoc P < 0.001) and Rib Reef (post-hoc P < 0.001), but not Feather Reef (post-hoc P = 0.089). Symbiodiniaceae density was also significantly affected by exposure period, with a decline from an average of 7.03 (± 0.36 SE) × 105 cells cm−2 to 4.59 (± 0.32 SE) × 105 cells cm−2, representing a 34% loss in Symbiodiniaceae from week 3 to week 6 (X2(1) = 56.682, P < 0.001, Fig. 3).
Symbiodiniaceae density (mean ± standard error) for Pocillopora acuta colonies originating from three different parental reefs (N = 9 colonies for Coates and Feather Reef, N = 6 for Rib Reef) at week 3 and week 6 sample points, exposed to ambient control, mid-century (acute 2050, and preconditioned 2050) and end of century (acute 2100, and preconditioned 2100) temperature and pCO2 treatments (n = 24 coral individuals per treatment)
Chlorophyll a concentration was lower in all four of the elevated temperature and pCO2 treatments (acute and preconditioned) by 4–52% compared to the ambient control (4.79 μg cm−2 ± 0.37 SE) (X2(4) = 60.517, P < 0.001; Fig. 4; Table 1). Preconditioned treatments had the lowest concentration of all groups, with a significant 33% decrease for mid-century (post-hoc P = 0.004) and 52% decrease for the end of century treatments compared to ambient control (post-hoc P < 0.001). Parental reef had a significant influence on chlorophyll a concentrations ((X2(2) = 9.525, P < 0.009; Fig. 4; Table 1), with Rib Reef maintaining a significantly higher concentration compared to both Coates and Feather Reef (post-hoc P = 0.014, P = 0.022, respectively). Concentrations also varied by the combined interaction of reef and length of exposure, with Feather Reef showing a significant increase from week 3 to 6 (post-hoc P < 0.001) compared to Coates and Rib Reef (post-hoc P = 0.979, P = 0.975, respectively). Finally, chlorophyll a concentration had a positive relationship with length of exposure (X2(1) = 10.820, P = 0.001, Table 1), as pigment concentration increased on average by 17% from 3.40 μg cm−2 (± 0.18 SE) to 4.08 μg cm−2 (± 0.22 SE) from week 3 to week 6.
Chlorophyll a concentration for Pocillopora acuta colonies originating from three different parental reefs (N = 9 colonies for Coates and Feather Reef, N = 6 for Rib Reef) at week 3 and week 6 sample points, exposed to ambient control, mid-century (acute 2050, and preconditioned 2050) and end of century (acute 2100, and preconditioned 2100) temperature and pCO2 treatments (n = 24 coral individuals per treatment)
Host physiology
Tissue protein concentration did not vary significantly between treatment groups (X2(4) = 5.006, P = 0.287, Table 1), ranging from 407.86 μg cm−2 (± 21.22 SE) in the 2100 preconditioned group, to 480.31 μg cm−2 (± 39.43 SE) in the acute 2050 treatment (Fig. 5). Protein concentration was only influenced by the parental origin reef (X2(2) = 7.212, P = 0.027, Table 1), where colonies with parents from Rib Reef had a mean protein concentration 26% higher than individuals with parents from Coates Reef (post-hoc P = 0.023) and 19% higher than Feather Reef (post-hoc P = 0.141, Fig. 5). Protein concentration was not affected by length of exposure, with no significant difference found between week 3 and week 6 (X2(1) = 0.200, P = 0.655, Fig. 5), and no significant interactions were found between treatment, sample period or parent reef (Table 1).
Tissue protein concentration (mean ± standard error) for Pocillopora acuta colonies originating from three different parental reefs (N = 9 colonies for Coates and Feather Reef, N = 6 for Rib Reef) at week 3 and week 6 sample points, exposed to ambient control, mid-century (acute 2050, and preconditioned 2050) and end of century (acute 2100, and preconditioned 2100) temperature and pCO2 treatments (n = 24 coral individuals per treatment)
Total growth varied among treatments (X2(4) = 22.164, P < 0.001, Table S3), ranging from a mean of 42.8 (± 8.3 SE) cm2 in the preconditioned 2100 group to 74.6 (± 6.2 SE) cm2 in preconditioned 2050 groups. Preconditioned 2050 individuals were found to be significantly larger than ambient control (post-hoc P = 0.026), acute 2050 (post-hoc P = 0.039) and preconditioned 2100 individuals (post-hoc P < 0.001, Fig. S4). Total growth did not vary by reef (X2(2) = 2.014, P = 0.365) or the combined interaction of treatment and reef (X2(8) = 10.132, P = 0.256, Table S3).
Photophysiology
Photosynthetic capacity varied significantly among temperature and pCO2 treatments (X2(4) = 18.194, P = 0.001, Table 1), with preconditioned 2100 individuals having a significantly lower photosynthetic maximum compared to ambient control individuals (post-hoc P = 0.023). Additionally, preconditioned 2050 and 2100 individuals had a significantly lower maximum compared to their acute 2050 and 2100 counterparts (post-hoc P = 0.040, P = 0.019, respectively, Fig. 6). Photosynthetic maximum was also significantly influenced by the combined interaction of treatment and sample period (X2(4) = 17.990, P = 0.001), indicating not all treatments performed similarly over time. This was primarily driven by an elevated capacity in ambient control corals in week 3 versus 6 (post-hoc P = 0.002). Photosynthetic maximum had a significant negative response to exposure time, whereby there was a reduction in photosynthetic capacity from week 3 (2.58 ± 0.13) compared to week 6 (2.22 ± 0.085) (X2(1) = 8.492, P = 0.004, Table 1).
Photosynthetic maximum (mean ± standard error) for Pocillopora acuta colonies originating from three different parental reefs (N = 9 colonies for Coates and Feather Reef, N = 6 for Rib Reef) at week 3 and week 6 sample points, exposed to ambient control, mid-century (acute 2050, and preconditioned 2050) and end of century (acute 2100, and preconditioned 2100) temperature and pCO2 treatments (n = 24 coral individuals per treatment)
Minimum light intensity at saturation (Ek) was significantly influenced by elevated temperature and pCO2 treatments (X2(4) = 33.302, P < 0.001), where preconditioned 2050 individuals had a significantly higher saturating irradiance compared to all other treatment groups, Table 1). A significant interaction between treatment and reef was found (X2(8) = 15.545, P = 0.049, Table 1). While individuals from Rib and Feather Reef had a similar Ek across treatments, corals with parents collected from Coates Reef had a significantly higher Ek in the preconditioned 2050 treatment compared to acute 2100 and ambient control corals from the same reef (post-hoc, P = 0.007, P = 0.003, Fig. 7). The response of Ek was also affected by exposure time and the combined interaction of treatment and length of exposure (X2(4) = 21.058, P ≤ 0.001, Table 1). Minimum light intensity at saturation increased from week 3 to week 6 for all treatment groups with the exception of the ambient control, and was significant for preconditioned 2050 individuals (post-hoc P < 0.001), which resulted in an overall significant increase from week 3 to week 6 for all groups combined (X2(1) = 20.350, P < 0.001), Fig. 7).
Minimum light intensity at saturation (mean ± standard error) for Pocillopora acuta colonies originating from three different parental reefs (N = 9 colonies for Coates and Feather Reef, N = 6 for Rib Reef) at week 3 and week 6 sample points, exposed to ambient control, mid-century (acute 2050, and preconditioned 2050) and end of century (acute 2100, and preconditioned 2100) temperature and pCO2 treatments (n = 24 coral individuals per treatment)
Discussion
Given the accelerating rate of coral reef decline globally, intervention approaches that can enhance the climate resilience and survival of reef-building corals are coming to the forefront of discussion and implementation through restoration efforts worldwide (Hoegh-Guldberg et al. 2014; van Oppen et al. 2015). The potential of preconditioning as a means of inducing acclimation in corals has been relatively unexplored to date, with a limited number of studies (but see Henley et al. 2022) assessing the effects of long-term pre-exposure from early life (multiple years). The present research observed limited evidence of enhanced resistance to future climate conditions through preconditioning as F1 colonies developed for 17 months under mid and end of century temperature and acidification conditions did not enhance bleaching tolerance. Overall, we observed negative effects to the coral-algal symbiosis in preconditioned and acute corals, but this did not result in impacts to the energetic status of the coral host as tissue protein remained comparable to the ambient control individuals (Fig. 8). While some differences in the physiological response over time were observed between natal reefs (Symbiodiniaceae density and chlorophyll a), only minimum light at saturation (Ek) was found to differ with reef and climate change treatments. Considered together, these findings indicate that coral colonies from reefs in relatively close proximity may have differential capacity for coping with climate change.
Summary of host and symbiont physiological changes in acute and preconditioned treatments compared to ambient control at week 6. Arrow direction indicates increase (upwards) or decrease (downwards) in trait response, arrow size indicates magnitude of response (neutral, small, moderate or large) and greyed arrows represent treatments which were not significant from the control or acute counterparts
The reduced performance in preconditioned corals compared to ambient naive corals, and either reduced or similar performance to their matched acute treatment, indicates that within-generation preconditioning was unable to mitigate the stress response to future climatic conditions. Declines in Symbiodiniaceae density, chlorophyll a concentrations and gross photosynthetic rate (Pmax) indicate long-term exposure to both mid and end of century treatments did not enhance performance limits within P. acuta. The observed bleaching response in preconditioned individuals was characterised by the combined loss of Symbiodiniaceae cell density and degradation of chlorophyll a pigments, which has been extensively documented in response to thermal anomalies (Baker et al. 2008; Fitt et al. 2001; Hoegh-Guldberg 1999; Lesser 1997) and other environmental stressors including CO2 (Egana and DiSalvo 1982; Lesser 1996). As the dominant pigment directly responsible for light absorption, reduced chlorophyll a (in combination with a decline in Symbiodiniaceae) likely in part explains the lower photosynthetic rates also observed for preconditioned individuals under mid and end of century conditions (Fig. 8). Surprisingly, photoacclimation through the regulation of maximum rate of photosynthesis and saturating irradiance did not occur, yet this has been seen in response to variations in light and temperature in a variety of coral species ex-situ, as well as Turbinaria mesenterina in the natural environment (Anthony and Hoegh-Guldberg 2003a, b; Nitschke et al. 2018; Roth 2014). It is possible that the thermal anomalies experienced in the summers of 2016 and 2017 acted as natural preconditioning events, priming these corals to reach their upper physiological limits. Alternatively, the observed difference between acute and preconditioned groups may be a result of the former experiencing less stressful conditions leading up to the start of the experiment. Ambient corals could have had greater capacity to withstand thermal stress and acidification due to starting in a state of lower physiological stress compared to their elevated counterparts, as was hypothesised by Huffmyer et al. (2021).
Breaking down the conditions under which plasticity will occur for corals is important for predicting their future persistence and the outcomes of intervention approaches. The lack of improved performance in these preconditioned P. acuta colonies could be due to our exposure timing from post-settlement missing a critical window (Putnam et al. 2020). Alternatively, since plasticity is expected to be costly (Angilletta 2009), and due to the limited environmental change that would usually occur within and across generations for this species, longer and even cross-generational exposure may be required to induce phenotypic plasticity (Putnam 2021; Putnam et al. 2020). Some of our results perhaps even indicate that preconditioning accentuated the effects to photosynthesis compared to the acute counterparts, including reductions in chlorophyll a concentration (acute only 11% compared to 44% in preconditioned). However, this could alternatively be due to preconditioned corals experiencing slightly more heat-stress due to the transplantation of acute corals occurring just after the greatest peak in summer temperature. This is supported by the trend that acute exposure and preconditioned responses were more similar in week 6 compared to week 3 when testing for gross photosynthetic rate and minimum light at saturation.
The length of heat exposure had a severe impact on physiological health for all groups. Symbiodiniaceae density declined significantly between peak temperature and end of summer heat accumulation (week 3 versus 6), a direct indication of further bleaching under prolonged exposure. These results are congruent with previous research, where seasonal declines in symbiont density over warmer months have indicated that bleaching events need to be interpreted as a continuum, rather than a single event immediately following peak temperature (Fitt et al. 2000; Suggett and Smith 2011; Warner et al. 2002). Additionally, photosynthetic performance (Pmax and Ek) was impacted by length of exposure for particular treatment groups, with minimum saturating irradiance increasing in the preconditioned 2050 group from week 3 to 6. While this improvement in performance under mid-century conditions may suggest chronic exposure had a positive impact, this response was not mirrored by photosynthetic maximum or retained in the end of century preconditioned group, indicating that in this circumstance preconditioning will likely not result in shifts in the capacity for these corals to combat future climate conditions. Interestingly, the loss of Symbiodiniaceae cells observed at the end of summer accumulated heat stress was accompanied by slight increases in chlorophyll a concentration. This phenomenon has been observed in previous work (D’Croz and Maté 2004; Jones 1997), and may be the result of higher nutrient availability due to reductions in density, or the loss of pigment-reduced Symbiodiniaceae from the surface tissue, leaving “dark-adapted” symbionts remaining deeper (Le Tissier and Brown 1996). Despite this, pronounced declines in chlorophyll a concentration for the chronically stressed preconditioned groups and naive acute individuals under both peak summer heat and accumulated thermal stress were consistent with the negative trends observed in symbiont cell density, characteristic of bleaching responses. While it is important to assess the effects of chronic heat stress versus short-term thermal pulses to understand the response of corals to future climate change, these stressors likely elicit very different molecular and cellular responses within the holobiont (Bowler 2005). As a result, these differences in exposure may have played a part in the results found from week 3 to week 6 in this study, highlighting a need to further elucidate the underlying mechanisms associated with coral acclimatization.
Generally, we observed limited differences in the photophysiological responses of 2050 and 2100 treatments indicating that both conditions were stressful. Symbiodiniaceae density and Pmax at the end of this experiment were comparable between mid and end of century treatments, suggesting that exposure to moderate heat stress up to 2.5 °C-weeks, and the accompanying elevated pCO2, was severe enough to elicit a bleaching response similar to that of 5 °C-weeks and 900 ppm pCO2. Similar performances were found in a recent study which examined the additive effect of marine heatwaves and ocean acidification on coral physiological attributes. While acidification was minor in its overall impact on coral survival relative to temperature, the model showed a decrease in photosynthesis and coral survival under the intermediate emission scenario for the mid and late-century compared to when heat stress was considered in isolation (Klein et al. 2022). This work supports our theory that despite differences in the magnitude of heat stress and acidification between 2050 and 2100 conditions, these corals may have reached a threshold of concurrent effects which diminished any possible plastic response. As such, there is limited evidence that with this approach corals will fare much better in mid-century compared to end of century scenarios.
However, some differences were seen between corals in the preconditioned 2050 and 2100 treatments. Coral individuals in the preconditioned 2050 treatment had the highest growth rate of all groups, suggesting that the annual temperature profile in the mid-century treatment may have been closer to their thermal optimum. Indeed, it is well-documented that corals have seasonal shifts in growth rates, often with reduced growth during the cooler months (Anderson et al. 2017; Bak et al. 2009; Barnes and Lough 1989; Lough and Barnes 2000). As the mid-century group experienced a + 1.5 °C increase above average (present-day) temperature throughout the year, warmer conditions during winter months may have provided a boost in growth for these corals. However, when considered alongside the declines in the other physiological metrics, this difference in growth is likely driven by responses to the ambient conditions, rather than increased plasticity due to preconditioning. Differences in Ek and chlorophyll a between preconditioned and 2050 and 2100 treatments suggests additional stress occurred in the end of century treatment. This would match a recent study examining changes in physiological performance of Pocillopora damicornis under elevated temperature and pCO2 (Sun et al. 2022). Without further investigation it is difficult to determine whether the observed shifts in Ek were photoprotective to minimize photodamage (Baek et al. 2022). However, as preconditioned 2100 had the poorest performance of all groups, it likely indicates that any carbon or temperature-driven enhancement in photosynthesis was negated as climate change continued, potentially due to photodamage delivered through chronic exposure to end of century conditions.
Even between adjacent reefs, differences in energetic status and population level stress tolerance can be found. Throughout the experiment, corals originating from Rib Reef parents had the highest tissue protein content compared to Coates and Feather Reef offspring. Corals from Coates Reef parents exhibited one of the lowest minimum light at saturation (Ek) values at ambient control conditions, but had the highest Ek of all reefs following exposure to long-term (preconditioned) mid-century conditions. Coates and Rib Reef offspring had the poorest response in Symbiodiniaceae health (symbiont density and chlorophyll a) with exposure time. In contrast, juvenile corals raised from Feather Reef parents exhibited limited reduction in symbiont health over time (week 3 to 6). Previous work on the thermal performance of two coral species across latitudinal gradients of the GBR has shown geographical variation in thermal performance at both the symbiont and holobiont level, revealing that regional specialization in thermal acclimatization is not uncommon (Jurriaans and Hoogenboom 2019). Furthermore, as we know that regional differences in Symbiodiniaceae composition have been observed in P. acuta (Botté et al. 2022), it is possible patterns in the physiological responses observed in this study could be linked to differences in dominant symbiont clades from parental reefs.
Historical conditions experienced by individuals and populations can shape future plastic and adaptive responses. As all three reef communities in this study succumbed to moderate levels of bleaching in 2016 and severe bleaching during the more extreme heat stress event for this central section of the GBR in 2017 (comparable to the degree heating weeks temperature stress experienced during the summer months of this study, but without the combined interaction of pCO2), parent colonies collected in the following winter experienced similar levels of thermal stress preconditioning in situ (maximum of 1.7–2.2 °C-weeks in 2016 and 5.2 (Rib Reef), 7.2 (Feather Reef) and 7.4 °C-weeks (Coates Reef) in 2017 (Cantin et al. 2021). However, despite their proximity and concurrence in recent heat stress, Coates Reef offspring tended to exhibit greater tolerance to future conditions while Feather Reef was more severely impacted, suggesting that even reefs in close proximity may possess significant differences in their ability to cope with climate change. Although we acknowledge this is a relatively small sample size, our results indicate only 7–24% of the variation in the response metrics can be attributed to the F0 coral colony, suggesting much of the observed response is explained by the fixed experimental factors (treatment, time period and reef). Nonetheless, it is likely that some of our reef effect is due to gene × environment interactions. Gene × environment interactions are common in corals (Drury and Lirman 2021; Million et al. 2022, Todd et al. 2004) and as such, increased replication would likely be needed to elucidate the level of genetic effect in this study.
While we observed individual physiological effects, total tissue protein concentrations within the coral host remained relatively uniform across treatments and time. When considered together with suppressed photosynthetic activity, declines in tissue biomass would have been expected, as the observed photoinhibition and bleaching responses would result in the direct loss of fixed carbon translocated to the coral host. In order to compensate for this deficit, catabolic breakdown of energy reserves (including proteins, lipids and carbohydrates) in the host tissue would have been anticipated to maintain metabolic requirements (Rodrigues and Grottoli 2007). As this was not observed for tissue protein, it may be an indication that pre-exposure enhanced stress tolerance within the coral host, despite the bleaching response. However, declines in tissue protein would have been expected in acute 2050 and 2100 treatments in this scenario. Alternatively, heterotrophic feeding can promote energy storage, under both healthy and stressed conditions, and marked increases in feeding have been observed as a phenotypic response to bleaching and acidification (Camp et al. 2017; Drenkard et al. 2013; Grottoli et al. 2006). Therefore, it is possible that changes in behaviour compensated for the loss of symbiotic photosynthates in order to maintain tissue composition under elevated conditions, as all treatments were consistently fed equally.
Understanding the capacity for within generation acclimation in corals is crucial for accurately predicting the response and persistence of reef ecosystems into the future (Pandolfi et al. 2011). Despite unprecedented rates of reef degradation in recent decades, assessing the potential for long-term preconditioning to facilitate acclimation in reef-building coral has so far been overlooked. This experiment is one of the first to date to evaluate the effects of long-term preconditioning on reef-building corals. The results of this study demonstrate that preconditioning did not confer improved thermal tolerance within a generation, as bleaching responses were comparable to non-preconditioned groups at elevated heat stress. Despite bleaching prevalence, host tissue protein remained healthy, suggesting preconditioned individuals were not metabolically compromised and emphasizing the need to advance our understanding of the differential acclimatization responses of the coral host and its symbionts. This research has also demonstrated that preconditioning is a useful tool for identifying unique individuals or populations that are more tolerant, which will have valuable applications for future restoration approaches. Future studies should examine the impacts of long-term preconditioning across multiple life stages and generations, including the potential for developmental plasticity during pre-settlement stages, as well as coral species with distinct life history strategies to address gaps in knowledge of the potential for chronic conditioning to not only enhance but also maintain tolerance. A better understanding of the direction and magnitude of these mechanisms will be undoubtedly valuable given the inevitable challenges facing coral reefs.
References
Allen MR, Dube OP, Solecki W, Aragón-Durand F, Cramer W, Humphreys S, Kainuma M, Kala J, Mahowald N, Mulugetta Y, Perez R, Wairiu M, Zickfeld K (2018) Chapter 1: Framing and Context. In: Global Warming of 1.5°C. An IPCC Special Report [Masson-Delmotte V, Zhai P, Pörtner HO, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (Eds)]. Cambridge, United Kingdom: Cambridge University Press, and New York, NY, USA
Anderson KD, Cantin NE, Heron SF, Pisapia C, Pratchett MS (2017) Variation in growth rates of branching corals along Australia’s Great Barrier Reef. Sci Rep 7(1):2920
Angilletta MJ Jr (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford, UK
Anthony KRN, Hoegh-Guldberg O (2003a) Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Functional Ecology 246–259.
Anthony KRN, Hoegh-Guldberg O (2003b) Kinetics of photoacclimation in corals. Oecologia 134(1):23–31. https://doi.org/10.1007/s00442-002-1095-1
Anthony KR, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci 105(45):17442–17446. https://doi.org/10.1073/pnas.0804478105
Baek JW, Lee JS, Kim SH, Lee T, Jung SW, Lee WC, Kim KT, An SU (2022) Effects of irradiance and temperature on the photosynthesis of the crustose coralline algae Pneophyllum fragile (Corallinales, Rhodophyta) in the coastal waters of Korea. Journal of Marine Science and Engineering 10(7):851
Bak RP, Nieuwland G, Meesters EH (2009) Coral growth rates revisited after 31 years: what is causing lower extension rates in Acropora palmata? Bull Mar Sci 84(3):287–294
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80(4):435–471. https://doi.org/10.1016/j.ecss.2008.09.003
Barnes DJ, Lough JM (1989) The nature of skeletal density banding in scleractinian corals: fine banding and seasonal patterns. J Exp Mar Biol Ecol 126(2):119–134
Bellantuono AJ, Hoegh-Guldberg O, Rodriguez-Lanetty M (2012) Resistance to thermal stress in corals without changes in symbiont composition. Proceedings of the Royal Society b: Biological Sciences 279(1731):1100–1107. https://doi.org/10.1098/rspb.2011.1780
Both C, Artemyev AV, Blaauw B, Cowie RJ, Dekhuijzen AJ, Eeva T, Enemar A, Gustafsson L, Ivankina EV, Järvinen A, Metcalfe NB (2004) Large–scale geographical variation confirms that climate change causes birds to lay earlier. Proceedings of the Royal Society of London. Series B: Biological Sciences 271(1549): 1657–1662. https://doi.org/10.1098/rspb.2004.2770
Botté ES, Cantin NE, Mocellin VJ, O’Brien PA, Rocker MM, Frade PR, Webster NS (2022) Reef location has a greater impact than coral bleaching severity on the microbiome of Pocillopora acuta. Coral Reefs 1–17
Bowler K (2005) Acclimation, heat shock and hardening. J Therm Biol 30(2):125–130. https://doi.org/10.1016/j.jtherbio.2004.09.001
Burton T, Metcalfe NB (2014) Can environmental conditions experienced in early life influence future generations? Proceedings of the Royal Society B: Biological Sciences 281(1785). https://doi.org/10.1098/rspb.2014.0311
Camp EF, Nitschke MR, Rodolfo-Metalpa R, Houlbreque F, Gardner SG, Smith DJ, Zampighi M, Suggett DJ (2017) Reef-building corals thrive within hot, acidified and deoxygenated waters. Sci Rep 7(1):1–9. https://doi.org/10.1038/s41598-017-02383-y
Cantin NE, Klein-Salas E, Frade P (2021) Spatial variability in coral bleaching severity and mortality during the 2016 and 2017 Great Barrier Reef coral bleaching events. Report to the National Environmental Science Program. Reef and Rainforest Research Centre Limited, Cairns.
Castillo KD, Helmuth BST (2005) Influence of thermal history on the response of Montastraea annularis to short-term temperature exposure. Mar Biol 148(2):261–270. https://doi.org/10.1007/s00227-005-0046-x
Cole AJ, Pratchett MS, Jones GP (2008) Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 9(3):286–307. https://doi.org/10.1111/j.1467-2979.2008.00290.x
D’Croz L, Maté JL (2004) Experimental responses to elevated water temperature in genotypes of the reef coral Pocillopora damicornis from upwelling and non-upwelling environments in Panama. Coral Reefs 23(4):473–483. https://doi.org/10.1007/s00338-004-0397-7
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences USA 105:6668–6672. https://doi.org/10.1073/pnas.0709472105
Dilworth J, Caruso C, Kahkejian VA, Baker AC, Drury C (2021) Host genotype and stable differences in algal symbiont communities explain patterns of thermal stress response of Montipora capitata following thermal pre-exposure and across multiple bleaching events. Coral Reefs 40(1):151–163
Donelson JM, Salinas S, Munday PL, Shama LN (2018) Transgenerational plasticity and climate change experiments: where do we go from here? Glob Change Biol 24(1):13–34
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192. https://doi.org/10.1146/annurev.marine.010908.163834
Drenkard EJ, Cohen AL, McCorkle DC, de Putron SJ, Starczak VR, Zicht AE (2013) Calcification by juvenile corals under heterotrophy and elevated CO2. Coral Reefs 32(3):727–735. https://doi.org/10.1007/s00338-013-1021-5
Drury C, Lirman D (2021) Genotype by environment interactions in coral bleaching. Proc R Soc B 288(1946):20210177
Egana AC, DiSalvo LH (1982) Mass expulsion of zooxanthellae by Easter Island corals. Pacific Science 36(1).
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr 45(3):677–685. https://doi.org/10.4319/lo.2000.45.3.0677
Fitt WK, Brown BE, Warner ME, Dunne RP (2001) Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20(1):51–65. https://doi.org/10.1007/s003380100146
Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, Heip CH (2007) Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters 34(7). https://doi.org/10.1029/2006GL028554
Gibbin EM, Krueger T, Putnam HM, Barott KL, Bodin J, Gates RD, Meibom A (2018) Short-term thermal acclimation modifies the metabolic condition of the coral holobiont. Front Mar Sci 5:10. https://doi.org/10.3389/fmars.2018.00010
Gibert P, Debat V, Ghalambor CK (2019) Phenotypic plasticity, global change, and the speed of adaptive evolution. Current Opinion in Insect Science 35:34–40
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12(1):1–17. https://doi.org/10.1007/BF00303779
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol 2(6):495–509
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440(7088):1186. https://doi.org/10.1038/nature04565
Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9(2):228–241. https://doi.org/10.1111/j.1461-0248.2005.00871.x
Henley EM, Bouwmeester J, Jury CP, Toonen RJ, Quinn M, Lager CV, Hagedorn M (2022) Growth and survival among Hawaiian corals outplanted from tanks to an ocean nursery are driven by individual genotype and species differences rather than preconditioning to thermal stress. PeerJ 10:e13112
Herman JJ, Spencer HG, Donohue K, Sultan SE (2014) How stable ‘should’ epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution 68(3):632–643
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50(8):839–866. https://doi.org/10.1071/MF99078
Hoegh-Guldberg O, Cai R, Poloczanska ES, Brewer PG, Sundby S, Hilmi K, Fabry VJ, Jung S (2014) The Ocean. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (Eds)]. Cambridge, United Kingdom: Cambridge University Press, and New York, NY, USA, pp. 1655–1731.
Huffmyer AS, Johnson CJ, Epps AM, Lemus JD, Gates RD (2021) Feeding and thermal conditioning enhance coral temperature tolerance in juvenile Pocillopora acuta. Royal Society Open Science 8(5):210644
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JB, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi M, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301(5635):929–933. https://doi.org/10.1126/science.1085046
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018) Global warming transforms coral reef assemblages. Nature 556(7702):492. https://doi.org/10.1038/s41586-018-0041-2
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M, Kerswell A, Madin JS, Mieog A, Paley AS, Pratchett MS, Torda G, Woods RM (2019a) Global warming impairs stock–recruitment dynamics of corals. Nature 568(7752):387. https://doi.org/10.1038/s41586-019-1081-y
Hughes TP, Kerry JT, Connolly SR, Baird AH, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Jacobson M, Liu G, Pratchett MS, Skirving W, Torda G (2019b) Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat Clim Chang 9(1):40–43
IPCC. (2014). In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the IPCC. [Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (Eds.)]. Cambridge, United Kingdom: Cambridge University Press, and New York, NY, USA
IPCC. (2021). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (Eds.)]. Cambridge University Press. In Press
IPCC. (2022). Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (Eds.)]. Cambridge University Press. In Press.
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21(4):540–547. https://doi.org/10.4319/lo.1976.21.4.0540
Johannes RE, Wiebe WJ (1970) Method for determination of coral tissue biomass and composition 1. Limnol Oceanogr 15(5):822–824
Jones RJ (1997) Changes in zooxanthellar densities and chlorophyll concentrations in corals during and after a bleaching event. Mar Ecol Prog Ser 158:51–59
Jones RJ, Hoegh-Guldberg O, Larkum AW, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant, Cell Environ 21(12):1219–1230. https://doi.org/10.1046/j.1365-3040.1998.00345.x
Jurriaans S, Hoogenboom MO (2019) Thermal performance of scleractinian corals along a latitudinal gradient on the Great Barrier Reef. Philos Trans R Soc B 374(1778):20180546
Klein SG, Geraldi NR, Anton A, Schmidt-Roach S, Ziegler M, Cziesielski MJ, Martin C, Rädecker N, Frölicher TL, Mumby PJ, Pandolfi JM, Suggett DJ, Voolstra CR, Aranda M, Duarte CM (2022) Projecting coral responses to intensifying marine heatwaves under ocean acidification. Glob Change Biol 28(5):1753–1765
Le Tissier MDA, Brown BE (1996) Dynamics of solar bleaching in the intertidal reef coral Goniastrea aspera at Ko Phuket, Thailand. Mar Ecol Prog Ser 136:235–244. https://doi.org/10.3354/meps136235
Leimar O, McNamara JM (2015) The evolution of transgenerational integration of information in heterogeneous environments. Am Nat 185(3):E55–E69
Lesser MP (1996) Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol Oceanogr 41(2):271–283. https://doi.org/10.4319/lo.1996.41.2.0271
Lesser MP (1997) Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16(3):187–192. https://doi.org/10.1007/s003380050073
Leuzinger S, Anthony K, Willis BL (2003) Reproductive energy investment in corals: scaling with module size. Oecologia 136(4):524–531
Lough JM, Barnes DJ (2000) Environmental controls on growth of the massive coral Porites. J Exp Mar Biol Ecol 245(2):225–243
Lough JM, Anderson KD, Hughes TP (2018) Increasing thermal stress for tropical coral reefs: 1871–2017. Sci Rep 8(1):1–8
Marhoefer SR, Zenger K R, Strugnell JM, Logan M, van Oppen MJ, Kenkel CD, Bay L K (2021) Signatures of Adaptation and Acclimatization to Reef Flat and Slope Habitats in the Coral Pocillopora damicornis. Frontiers in Marine Science 1194. https://doi.org/10.3389/fmars.2021.704709
Martell HA (2022) Thermal priming and bleaching hormesis in the staghorn coral, Acropora cervicornis (Lamarck 1812). Journal of Experimental Marine Biology and Ecology 151820.
Maynard JA, Anthony KR, Marshall PA, Masiri I (2008) Major bleaching events can lead to increased thermal tolerance in corals. Mar Biol 155(2):173–182. https://doi.org/10.1007/s00227-008-1015-y
McRae CJ, Huang WB, Fan TY, Côté IM (2021) Effects of thermal conditioning on the performance of Pocillopora acuta adult coral colonies and their offspring. Coral Reefs 40(5):1491–1503
Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211(7):1050–1056. https://doi.org/10.1242/jeb.013284
Million WC, Ruggeri M, O’Donnell S, Bartels E, Conn T, Krediet CJ, Kenkel CD (2022) Evidence for adaptive morphological plasticity in the Caribbean coral, Acropora cervicornis. Proc Natl Acad Sci 119(49):e2203925119
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29(2):215–233. https://doi.org/10.1016/S0921-8009(99)00009-9
Mollica NR, Guo W, Cohen AL, Huang KF, Foster GL, Donald HK, Solow AR (2018) Ocean acidification affects coral growth by reducing skeletal density. Proc Natl Acad Sci 115(8):1754–1759. https://doi.org/10.1073/pnas.1712806115
Morley SA, Peck LS, Sunday JM, Heiser S, Bates AE (2019) Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob Ecol Biogeogr 28(7):1018–1037
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13(10):403–407. https://doi.org/10.1016/S0169-5347(98)01472-4
Nakajima Y, Chuang PS, Ueda N, Mitarai S (2018) First Evidence of Asexual Recruitment of Pocillopora Acuta in Okinawa Island Using Genotypic Identification PeerJ 6:e5915
Nitschke MR, Gardner SG, Goyen S, Fujise L, Camp EF, Ralph PJ, Suggett DJ (2018) Utility of photochemical traits as diagnostics of thermal tolerance amongst great barrier reef corals. Front Mar Sci 5:45
Pachauri RK, Reisinger A (2007) Synthesis report. Fifth Assessment Report of the Intergovernmental Panel on Climate Change 151–165.
Padilla DK, Savedo MM (2013) A systematic review of phenotypic plasticity in marine invertebrate and plant systems. Advances in Marine Biology Vol 65 (ed. Lesser M), pp. 67–94. Academic Press, London, UK. https://doi.org/10.1016/B978-0-12-410498-3.00002-1
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333(6041):418–422. https://doi.org/10.1126/science.1204794
Pratchett MS, Hoey AS, Wilson SK, Messmer V, Graham NA (2011) Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 3(3):424–452. https://doi.org/10.3390/d3030424
Putnam HM (2021) Avenues of reef-building coral acclimatization in response to rapid environmental change. Journal of Experimental Biology 224.
Putnam HM, Gates RD (2015) Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J Exp Biol 218(15):2365–2372. https://doi.org/10.1242/jeb.123018
Putnam HM, Ritson-Williams R, Cruz JA, Davidson JM, Gates RD (2020) Environmentally-induced parental or developmental conditioning influences coral offspring ecological performance. Sci Rep 10(1):1–14. https://doi.org/10.1038/s41598-020-70605-x
Reaka-Kudla ML (1997) The global biodiversity of coral reefs: a comparison with rain forests. Biodiversity II: understanding and protecting our biological resources (pp. 83–108) Washington, D.C.: Joseph Henry Press.
Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT (2010) Phenotypic plasticity and population viability: the importance of environmental predictability. Proceedings of the Royal Society b: Biological Sciences 277(1699):3391–3400
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407(6802):364–367. https://doi.org/10.1038/35030078
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46(1):115–126. https://doi.org/10.1007/s11099-008-0019-7
Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr 52(5):1874–1882. https://doi.org/10.4319/lo.2007.52.5.1874
Roth MS (2014) The engine of the reef: photobiology of the coral–algal symbiosis. Front Microbiol 5:422. https://doi.org/10.3389/fmicb.2014.00422
Schoepf V, Grottoli AG, Warner ME, Cai WJ, Melman TF, Hoadley KD, Baumann JH (2013) Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8(10):e75049
Schoepf V, Carrion SA, Pfeifer SM, Naugle M, Dugal L, Bruyn J, McCulloch MT (2019) Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat Commun 10(1):1–10
Schoepf V, Sanderson H, Larcombe E (2022) Coral heat tolerance under variable temperatures: Effects of different variability regimes and past environmental history vs. current exposure. Limnology and Oceanography 67(2): 404–418.
Smith HA, Moya A, Cantin NE, Van Oppen MJ, Torda G (2019) Observations of simultaneous sperm release and larval planulation suggest reproductive assurance in the coral Pocillopora acuta. Front Mar Sci 6:362
Snell-Rood EC, Kobiela ME, Sikkink KL, Shephard AM (2018) Mechanisms of plastic rescue in novel environments. Annu Rev Ecol Evol Syst 49:331–354. https://doi.org/10.1146/annurev-ecolsys-110617-062622
Solomon S, Plattner GK, Knutti R, Friedlingstein P (2009) Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci 106(6):1704–1709. https://doi.org/10.1073/pnas.0812721106
Spalding C, Finnegan S, Fischer WW (2017) Energetic costs of calcification under ocean acidification. Global Biogeochem Cycles 31(5):866–877. https://doi.org/10.1002/2016GB005597
Stimson J, Kinzie RA (1991) The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol 153:63–74. https://doi.org/10.1016/S0022-0981(05)80006-1
Stimson J, Sakai K, Sembali H (2002) Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21:409–421
Suggett DJ, Smith DJ (2011) Interpreting the sign of coral bleaching as friend vs. foe. Global Change Biology 17(1) 45–55. https://doi.org/10.1111/j.1365-2486.2009.02155.x
Sun Y, Jiang L, Gong S, Diaz-Pulido G, Yuan X, Tong H, Huang L, Zhou G, Zhang Y, Huang H (2022) Changes in physiological performance and protein expression in the larvae of the coral Pocillopora damicornis and their symbionts in response to elevated temperature and acidification. Sci Total Environ 807:151251
Tewksbury JJ, Huey RB, Deutsch CA (2008) Ecology—putting the heat on tropical animals. Science 320:1296–1297. https://doi.org/10.1126/science.1159328
Titlyanov EA, Titlyanova TV, Yamazato K, van Woesik R (2001) Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J Exp Mar Biol Ecol 263(2):211–225
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83(3):315–337
Todd PA, Ladle RJ, Lewin-Koh NJI, Chou LM (2004) Genotype× environment interactions in transplanted clones of the massive corals Favia speciosa and Diploastrea heliopora. Mar Ecol Prog Ser 271:167–182
Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, Foret S, Matz M, Miller DJ, Moya A, Putnam HM, Ravasi T, van Oppen MJH, Thurber RV, Vidal-Dupiol J, Voolstra CR, Watson S, Whitelaw E, Willis BL, Munday PL, (2017) Rapid adaptive responses to climate change in corals. Nat Clim Chang 7(9):627. https://doi.org/10.1038/nclimate3374
Van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166(1):49–60. https://doi.org/10.1111/j.1469-8137.2004.01296.x
van Oppen MJ, Oliver JK, Putnam HM, Gates RD (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci 112(8):2307–2313. https://doi.org/10.1073/pnas.1422301112
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416(6879):389. https://doi.org/10.1038/416389a
Warner M, Chilcoat G, McFarland F, Fitt W (2002) Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar Biol 141(1):31–38. https://doi.org/10.1007/s00227-002-0807-8
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst 20(1):249–278. https://doi.org/10.1146/annurev.es.20.110189.001341
Acknowledgements
We would like to thank the Paul G. Allen Family Foundation for providing funding for this project. MJHvO acknowledges Australian Research Council Laureate Fellowship FL180100036. Thank you also to Luke Morris for his laboratory assistance and to Murray Logan and Jake Crosby for their statistics knowledge and technical assistance, along with experimental support from the Australian Institute of Marine Science National Sea Simulator team.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roper, C.D., Donelson, J.M., Ferguson, S. et al. Long-term preconditioning of the coral Pocillopora acuta does not restore performance in future ocean conditions. Coral Reefs 42, 1079–1096 (2023). https://doi.org/10.1007/s00338-023-02401-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02401-8