Abstract
Ricordea yuma (Ricordeidae WATZL, 1922) is a tropical corallimorpharian found throughout the Indo-Pacific region. Individuals of this species are often found in bright colours making them popularly sought-after within the marine ornamental trade. Despite their popularity in marine aquaria, little is known about the sexual reproductive biology of this species and therefore it's capacity to maintain and renew genetically diverse populations in the wild. This note provides evidence that this species is gonochoric and engages in broadcast spawning during the austral summer. While further research is required to discern the size and/or age of sexual maturity, duration of the gametogenic cycle, and the precise periodicity of spawning, the information provided here is an important first in step elucidating the sexual reproductive biology of this species. Understanding the general biology of R. yuma will allow for a better understanding of this species’ life history, and enhance the monitoring and management of populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corallimorpharia currently comprises 48 accepted species and is a relatively small order within Anthozoa (phylum: Cnidaria), when compared to Scleractinia (hard corals; 1679 species) and Actiniaria (sea anemones; 1176 species) (WoRMS 2023). Despite being noncalcareous, corallimorpharians are taxonomically more closely related to hard corals than sea anemones (Lin et al. 2016) and are consequently commonly referred to as false or mushroom corals. Corallimorpharians inhabit a diverse range of habitats such as shallow tropical waters to deep temperate seas (Fautin et al. 2009). Owing to the bright coloration of many corallimorpharian species, coupled with their tolerance to a range of environmental conditions, species such as Ricordea sp. are popular amongst marine aquarium hobbyists (Fenner 2015; Torres-Pratts et al. 2011; Wallace and Crowther 2019).
Ricordea yuma (Fig. 1) and R. florida are the sole members of the family Ricordeidae (WATZL, 1922) (WoRMS 2023). Polyps of both these species may be found as solitary individuals or in colonial aggregations (Muhando et al. 2002; Parr 2019; Torres-Pratts et al. 2011) in shallow (< 54 m) tropical waters (den Hartog 1980). They are each found in distinct regions, with R. yuma distributed throughout the Indo-Pacific region and R. florida in the Atlantic Ocean (Parr 2019; Torres-Pratts et al. 2011). Polyps within this family are characterised as being large fleshy polyps, with stocky bodies and flat oral discs entirely covered with short tentacles (den Hartog 1980). As with many other scleractinian and actiniarian species, Ricordea sp. are photosymbiotic and have a close mutualistic relationship with micro-dinoflagellates within the family Symbiodiniaceae (Lin et al. 2019). Similarly, both species have been shown to expel their symbionts and undergo ‘bleaching’ when exposed to stressful environmental conditions (Lin et al. 2019; Parr 2019). As such, like other photosymbiotic cnidarians, Ricordea sp. may be susceptible to the threats of climate change.
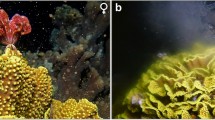
Ricordea yuma polyps (A) from a captive clonal population that has established asexually through methods such as (inset) pedal laceration, and (B) from genetically distinct colonies, identified by their unique colorations, collected from the wild. Scale bars are approximately: A, 4 cm; B, 1 cm; and inset, 1 cm
As with many other anthozoans, corallimorpharian species may engage in both sexual and asexual reproduction (Chadwick‐Furman et al. 2000; Chen et al. 1995a, 1995b; Holts and Beauchamp 1993; Parr 2019). While several asexual strategies, including pedal laceration, marginal budding, inverse budding, longitudinal fission, two-mouth fission, and tentacle autotomy, have been documented within Corallimorpharia (Chadwick‐Furman et al. 2000; Chen et al. 1995a; den Hartog 1980; Kaposi et al. 2022; Lin et al. 2013), the sexual reproductive biology of these species is poorly understood. This is despite the significance of sexual reproduction in a species’ ability to maintain and renew populations and promote genetic diversity (Harrison 2011).
Descriptions of sexual reproduction currently only exist for four corallimorpharian species; Corynactis californica, Rhodactis rhodostoma, Rhodactis indosinensis, and Ricordea florida (Chadwick‐Furman et al. 2000; Chen et al. 1995a, 1995b; Holts and Beauchamp 1993; Parr 2019) (Table 1). Subsequently, these species have been reported to be either gonochoric or sequential hermaphrodites, participating in broadcast spawning, whereby gametes are fertilised externally in the water column (Chadwick‐Furman et al. 2000; Chen et al. 1995a; Holts and Beauchamp 1993). In contrast, anthozoans may also be simultaneous hermaphrodites and/or undergo internal fertilisation, viviparous brooding, and release developed planula larvae (Harrison and Wallace 1990). Indeed, a mix of strategies may also occur between species of the same family (Harrison and Wallace 1990). As such, it is premature to make broad conclusions as to the sexual biology of all corallimorpharians, based on the current evidence alone.
Despite their popularity in marine aquaria, little is known about the sexual reproductive biology of Ricordea yuma. This study aimed to address this knowledge gap and provide valuable information on the reproductive system and strategy implemented by this species, as well as provide preliminary evidence for size of maturity and reproductive timing.
Methods
Routine fragmentation (via bisection) of Ricordea yuma polyps from an established captive clonal population (Fig. 1a) held at the James Cook University (JCU), Cairns (Australia) (hereafter referred to as the ‘JCU population’) in October 2020 revealed that some of these polyps had started to exhibit distinct white features amongst their mesentery filaments that had not been present earlier in the year. As austral spring coincides with when many anthozoans on the Great Barrier Reef (GBR) begin to show signs of fecundity and sexual maturity (Babcock et al. 1986), it was hypothesised that these polyps may be maturing in preparation for spawning. As the JCU population comprised entirely of genetically identical, asexually derived polyps, additional specimens from distinct colonies (n = 13), distinguished by their unique coloration (Fig. 1b) and spatial separation, were freshly harvested from local reefs around Cairns during October and November 2020 to increase genetic diversity, allowing for this hypothesis to be investigated.
All polyps were maintained in a large 60,000-L system with salinity 35 ± 1‰ and pH 8–8.5. The temperature is maintained at 27 ± 1 °C, but may reach 28.5 °C during November through to January (austral summer).
For the determination of sex, twenty-five polyps (12 from the JCU population and 13 freshly harvested from the wild) were bisected in October–November of 2020 (austral spring), by cutting down through the mouth, in the centre of the oral disc. In doing so, the internal features of the polyps were exposed whereby the actinopharynx, mesentery filaments, and gonads could be easily identified. Polyps for which gonads were identified were also measured for approximate size (diameter of the oral disc), and a portion of gonad was removed. The presence of gametes within the gonads was confirmed under a Zeiss inverted compound microscope (10–100 × magnification). The resulting halves of each polyp were placed back into the tank to regenerate, a process which takes approximately 14 days (Vroom 2016). Similarly, some of these polyps were randomly resampled in February 2021 to determine if they still displayed signs of sexual maturity.
To determine the behaviour of gamete release (slow, vigorous, or passive), mode (internal or external fertilisation), and timing (day and time) of spawning within R. yuma, observations of polyps were made in December 2020. Specifically, signs of ‘setting’ (eggs visible in the actinopharynx), the direct release of eggs or sperm clouds, increased water opacity (indicative of sperm), and the presence of eggs on the surface or in the water column, were checked for.
Polyps were placed into individual glass holding baths within the main system. Observations were made in 15-min intervals between 1730 and 0000 h (AEST), each night for eight nights (1–8 days after the full moon (DAFM) in November; 30/11/20). These times were selected as they coincide with when the majority of scleractinian species on the GBR are reported to spawn (Babcock et al. 1986). The time of sunset for this period was approximately 1839–1843 h (AEST). A metal halide UV lamp set on a 12 h:12 h light cycle (0600–1800 h) was suspended above the system. A red LED headtorch was used for all inspections so as to not disrupt the biological circadian rhythm of the polyps (Kaniewska et al. 2015). Similarly, attempts were made to block out all other potential sources of artificial light, such as security sensor lights.
From the 9th DAFM, polyps were maintained in a 235-L observation tank, connected to the main aquarium system with a flow-through exchange rate of approximately 150 L per hour. To prevent the loss of gametes, the water outlet was protected with a 125-µm mesh filter. The mesh filter, surface, and water column were checked each morning at approximately 0600 h, and periodically every 2–3 h thereafter throughout the day, for evidence of spawning.
When eggs were observed on the surface or in the water column, they were collected with a pasture pipette. Over the entire study, a subset of these eggs (approximately 30 eggs) was taken and fixed in 4% formalin for later size measurements, and the remainder (approximately 40 eggs) were inspected with a Zeiss inverted compound microscope for signs of fertilisation and embryonic development (e.g. cleavage and cellular division). To this end, inspections of eggs showed no sign of larval development (e.g. gastrulation and/or elongation) and were thus confirmed not to be brooded larvae. The eggs were then transferred to 50 ml of seawater and monitored for four hours, at which point signs of fertilisation should be apparent (Babcock et al. 1986). Of the eggs that were preserved, 18 were photographed with a camera mounted onto a Zeiss Axio Imager.M1 microscope and measured for diameter with Zeiss Zen Software.
Results and discussion
Anthozoans may be either gonochoric, exhibiting distinct sexes, or hermaphroditic, whereby polyps possess both male and female gametes (Harrison 2011; Kahng et al. 2011; Ryland 1997). Gonads were observed in polyps from six of the fourteen colonies that were sampled, of which polyps from three colonies contained ovaries and three contained spermaries. Gonads were not found in polyps from the remaining eight colonies, and the presence of both male and female features in a single polyp was not observed. The gonads observed in R. yuma were similar to those previously described for the corallimorpharian Rhodactis indosinensis (Chen et al. 1995a, 1995b). Females were characterised by the presence of orange eggs that were distributed throughout the mesentery filaments in bundled clusters, seemingly encapsulated by a thin membrane (Fig. 2a and b). Conversely, male polyps were identified by the presence of distinct white spermaries amongst the mesentery filaments (Fig. 2c and d). Similarly, the occurrence of separate sexes has also been confirmed in R. florida (Parr 2019). Altogether, our observations suggest that R. yuma is a gonochoric species.
Several gonochoric anthozoan species may undergo sequential hermaphroditism, transitioning from one sex to another, or exhibit sexual dimorphism (Harrison and Wallace 1990). The sex of 10 of 12 polyps from the JCU population was successfully determined and identified to be male. These male polyps ranged from approximately 6-12cm diameter, while the sex of polyps smaller than this could not be determined. Similarly, each of the male and female polyps that were identified from specimens collected from the wild also fell within this size range. This is in contrast with R. florida; however, where male polyps were significantly smaller than females (Parr 2019). This size differentiation suggests that R. florida may either display sexual dimorphism or undergo protandry (Parr 2019), with females, therefore, representing larger size classes. Sequential hermaphroditism has also been reported in other corallimorpharian species (Chadwick‐Furman et al. 2000; Chen et al. 1995a; Holts and Beauchamp 1993).
Owing to the distinct lack of females identified within the JCU population (despite the large range in sizes sampled), and females harvested from the wild being similarly sized as the JCU males, R. yuma appear to be gonochoric and do not undergo sex change or exhibit sexual dimorphism as reported in R. florida and other corallimorphs (Chadwick‐Furman et al. 2000; Chen et al. 1995a, 1995b; Holts and Beauchamp 1993; Parr 2019. Further research and a larger sample size may be required before a definitive assessment as to the sexual status of R. yuma can be made.
In marine broadcast-spawning species, including anthozoans, an important determinant of successful reproduction is the ability for individuals in a population to release gametes synchronously, often within minutes from one another, thereby increasing the likelihood of external gamete fertilisation (Randall et al. 2020). Patterns in a range of environmental factors such as sea temperature, and lunar, tidal, and light cycles contribute to this synchrony (Babcock et al. 1986; Randall et al. 2020). While the precise time of spawning is often species and region specific (Babcock et al. 1986), many species will engage in broadcast spawning within a shared temporal window. On the Great Barrier Reef (GBR), the syncronised spawning of multiple species occurs during the warmer months of the late austral spring–summer, often between October and December, depending on the year (Babcock et al. 1986).
Observations conducted during December 2020 and early January 2021 (austral summer) revealed that R. yuma is likely a broadcast-spawning species, passively releasing gametes in the water column for external fertilisation. This is in agreeance with a report that shows that R. florida also participates in broadcast spawning during the summer months (June and July) in the Florida Keys (Parr 2019). A small number of eggs, sometimes as little as one, were released at irregular intervals over the course of a month, beginning 16 days after the full moon (DAFM) in November through to 14 DAFM in December. In total, approximately 70 eggs were observed from ten spawning events, whereby the greatest number (approximately 40) were found in December, 22 DAFM. While females spawned the majority of these eggs at night, they also released a small number of eggs during the day, as early as 1430 h (AEST). None of the females were ever observed releasing large amounts of eggs, as is typically the case during major spawning event. Similarly, males showed no signs of spawning.
Similarly to other broadcast-spawning anthozoans (Bocharova 2016), R. yuma females released the eggs through the mouth. Eggs were released individually and were positively buoyant. Most eggs were orange/pink in colour and had an average diameter of 596 µm (± SD 34). On one occasion, eggs were seen to have a green–blue hue. Variable egg colour has also been reported in some scleractinian species (Babcock et al. 1986). Inspections of the eggs revealed no signs of fertilisation or embryonic development. Additionally, when left for observation, all eggs disintegrated within a few hours.
By February, there was no evidence of mature gonads in any Ricordea yuma polyp. Further research is required to determine whether the spawning event was simply missed, or whether collection, dissection, or unintentional interference by artificial lights, placed undue stress on the polyps, leading to the reabsorption of gametes (Rossin et al. 2019). Other anthozoan species, including a male Heteractis magnifica (sea anemone), have been successfully spawned within this facility, however. Similarly, such aquarium-based spawning events have coincided with the natural mass spawning that occurred on the GBR during that time. To this end, future studies seeking to discern the precise timing of spawning in this species may benefit by refraining from bisecting polyps in the months and weeks leading up to the predicted spawning period, increasing protection from artificial lights, increasing the window of sampling observations, and if possible, implement video surveillance.
The biology of the popular corallimorpharian Ricordea yuma is poorly understood. This is the first study documenting the sexual reproduction of this species. Using both histological analyses and spawning observations, we provide evidence that this species is gonochoric and reproduces via broadcast spawning. Several key aspects of this species’ reproductive biology, however, remain unanswered, including minimum size or age at sexual maturity, onset and duration of gametogenesis, as well as timing of reproduction. Nonetheless, the information presented here is a valuable first step towards better understanding this species’ life history. Additionally, this information may also contribute towards enabling the captive breeding of this species for the ornamental trade or for potential restocking of wild populations if required.
References
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90(3):379–394. https://doi.org/10.1007/BF00428562
Bocharova E (2016) Reproduction of sea anemones and other hexacorals. In Goffredo, S., Dubinsky, Z. (eds.) The Cnidaria, Past, Pres Future (pp. 239–248). https://doi.org/10.1007/978-3-319-31305-4_15
Chadwick-Furman NE, Spiegel M, Nir I (2000) Sexual reproduction in the tropical corallimorpharian Rhodactis rhodostoma. Invertebr Biol 119(4):361–369. https://doi.org/10.1111/j.1744-7410.2000.tb00104.x
Chen CLA, Chen CP, Chen IM (1995a) Sexual and asexual reproduction of the tropical corallimorpharian Rhodactis (= Discosoma) indosinensis (Cnidaria: Corallimorpharia) in Taiwan. Zool Stud 34(1):29–40
Chen CLA, Chen CP, Chen IM (1995b) Spatial variability of size and sex in the tropical corallimorpharian Rhodactis (= Discosoma) indosinensis (Cnidaria: Corallimorpharia) in Taiwan. Zool Stud 34(2):82–87
den Hartog JC (1980) Caribbean shallow water Corallimorpharia. Zoologische Verhandelingen 176(1):1–83
Fautin DG, Guinotte JM, Orr JC (2009) Comparative depth distribution of corallimorpharians and scleractinians (Cnidaria: Anthozoa). Mar Ecol Prog Ser 397:63–70. https://doi.org/10.3354/meps08271
Fenner R (2015) “Magical Maine Mushrooms”: The Order Corallimorpharia. UltraMarine Magazine 55:30
Harrison PL (2011) Sexual reproduction of scleractinian corals. In Dubinsky Z, Stambler N (Eds.) Coral Reefs: an ecosystem in transition (pp. 59–85)
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In Dubinsky Z (Eds.), Coral Reefs (pp. 133–206)
Holts LJ, Beauchamp KA (1993) Sexual reproduction in the corallimorpharian sea anemone corynactis californica in a central California kelp forest. Mar Biol Inter J Life Oceans Coast Water 116(1):129–136. https://doi.org/10.1007/BF00350740
Kahng SE, Benayahu Y, Lasker HR (2011) Sexual reproduction in octocorals. Mar Ecol Prog Ser 443(2006):265–283. https://doi.org/10.3354/meps09414
Kaniewska P, Alon S, Karako-Lampert S, Hoegh-Guldberg O, Levy O (2015) Signaling cascades and the importance of moonlight in coral broadcast mass spawning. Elife 4:1–14. https://doi.org/10.7554/elife.09991
Kaposi KL, Courtney RL, Seymour JE (2022) Tentacle autotomy: An additional mode of asexual reproduction in Ricordea yuma (Cnidaria, Anthozoa, Corallimorpharia). Mem Queensland Mus Nat 63
Lin MF, Chen CA, Miller DJ (2013) Asexual reproduction by marginal budding in the tropical corallimorpharian, Ricordea yuma (Corallimorpharia; Ricordeidae). Galaxea, Journal of Coral Reef Studies 15(2):41–42. https://doi.org/10.3755/galaxea.15.41
Lin MF, Takahashi S, Forêt S, Davy SK, Miller DJ (2019) Transcriptomic analyses highlight the likely metabolic consequences of colonization of a cnidarian host by native or non-native Symbiodinium species. Biology Open 8(3):1–11. https://doi.org/10.1242/bio.038281
Lin MF, Chou WH, Kitahara MV, Chen CLA, Miller DJ, Forêt S (2016) Corallimorpharians are not “naked corals”: Insights into relationships between Scleractinia and Corallimorpharia from phylogenomic analyses. PeerJ 2016(10):1–16. https://doi.org/10.7717/peerj.2463
Muhando CA, Kuguru BL, Wagner GM, Mbije NE, Öhman MC (2002) Environmental effects on the distribution of corallimorpharians in Tanzania. AMBIO J Hum Environ 31(7):558–561. https://doi.org/10.1579/0044-7447-31.7.558
Parr, ND (2019) Environmental tolerance and reproduction of Florida false corals Ricordea florida (Anthozoa: Corallimorpharia): Implications for ornamental fisheries management. PhD. thesis, Auburn University.
Randall CJ, Negri AP, Quigley KM, Foster T, Ricardo GF, Webster NS, Bay LK, Harrison PL, Babcock RC, Heyward AJ (2020) Sexual production of corals for reef restoration in the Anthropocene. Mar Ecol Prog Ser 635:203–232
Rossin AM, Waller RG, Stone RP (2019) The effects of in-vitro pH decrease on the gametogenesis of the red tree coral, Primnoa pacifica. Plos ONE 14(4):1–17. https://doi.org/10.1371/journal.pone.0203976
Ryland JS (1997) Reproduction in Zoanthidea (Anthozoa: Hexacorallia). Invertebr Reprod Dev 31(1–3):177–188. https://doi.org/10.1080/07924259.1997.9672575
Torres-Pratts H, Lado-Insua T, Rhyne AL, Rodríguez-Matos L, Schizas NV (2011) Two distinct, geographically overlapping lineages of the corallimorpharian Ricordea florida (Cnidaria: Hexacorallia: Ricordeidae). Coral Reefs 30:391–396. https://doi.org/10.1007/s00338-010-0709-z
Vroom P (2016) Regeneration in Corallimorpharia. Msc. Thesis, The University of Guelph
Wallace CC, Crowther AL (2019) Hexacorals 1: Sea anemones and anemone-like animals (Actiniaria, Zoantharia, Corallimorpharia, Ceriantharia and Antipatharia). In Hutchings P, Kingsford M, Hoegh-Guldberg O. (Eds.), The Great Barrier Reef: Biol Environ Manage (pp. 257–266)
WoRMS Editorial Board (2023) World Register of Marine Species. https://doi.org/10.14284/170
Acknowledgements
Kaposi K.L. was supported by an Australian Postgraduate Award provided by the Australian Government and by the Joyce and George Vaughan Bequest Scholarship awarded by James Cook University. We thank S. Turner as well as other staff and volunteers of the EduQuarium for their expertise in marine invertebrate husbandry, making it possible to study these animals in captivity. We greatly appreciate the assistance of C. Cousland at Great Barrier Reef Aquatics and all of the staff at Cairns Marine with specimen collection. We are also grateful to P. Harrison and C. Randall for their mentorship and intellectual discussions relevant to this study. Lasty, we would like to sincerely thank the reviewers for their comments and feedback which helped in improving this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaposi, K.L., Courtney, R.L. & Seymour, J.E. A note on the sexual reproductive biology of Ricordea yuma (Corallimorpharia). Coral Reefs 42, 755–760 (2023). https://doi.org/10.1007/s00338-023-02382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02382-8