Abstract
Cryptobenthic fishes are abundant on coral reefs, and their larvae dominate the ichthyoplankton in near reef waters. However, we have a limited understanding of how pelagic and on-reef processes are linked, especially how late-stage cryptobenthic fish larvae use near reef waters. We therefore used depth-stratified light trap sampling from 2 to 27 m at Lizard Island, Great Barrier Reef. This revealed clear depth variation in late-stage larval fish assemblages. Gobiidae larvae characterised mid-depth (13 m) samples. By contrast, larval Apogonidae were only abundant in shallow samples. Deep samples were typified by (non-target) adult apogonids. Contrary to expectations that poor-swimming cryptobenthic larvae would be flow-sheltering in deeper water, our results suggest that late-stage cryptobenthic larvae use large portions of the water column, although their preferred positions may be taxon-specific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small, cryptobenthic fishes dominate fish communities on coral reefs (Brandl et al. 2018), making up at least 40% of species and up to 50% of all individuals (Ackerman and Bellwood 2000). In addition to their sheer abundance, cryptobenthic fishes can play a pivotal role in reef trophodynamics. Indeed, they may account for over 60% of the consumed fish biomass on coral reefs (Brandl et al. 2019). It has been posited that the mass turnover of these cryptobenthic fishes is critical to the productivity of coral reefs, with the success of this ‘crypto pump’ depending on the constant influx of late-stage pelagic larvae (Brandl et al. 2019; Goatley et al. 2021). However, despite the critical importance of late-stage larvae in linking pelagic and on-reef processes (reviewed in Cowen 2002), our understanding of vertical distributions of late-stage larval cryptobenthic fishes in near reef waters is currently limited.
Despite exceptionally high mortality rates and limited reproductive outputs, the larvae of cryptobenthic fishes vastly outnumber those of larger reef fishes in near-reef (< 10 km) pelagic waters (Brandl et al. 2019). This could be the result of: (a) year-round reproduction and larval survival, which maintains larval abundances regardless of the sampling time (Lefèvre et al. 2016) and/or (b) larvae concentrated in the vicinity of reefs due to limited dispersal, reflecting morphological constraints and poor swimming abilities (Majoris et al. 2019; Goatley et al. 2021; but see Burgess et al. 2022), stronger natal homing or retention (e.g. Bottesch et al. 2016; Mouritsen et al. 2013; Paris et al. 2013; Gerlach et al. 2007) and/or early orientation behaviour (Majoris et al. 2021; Staaterman et al. 2012). A clue to the relative merits of these alternate explanations may lie in how cryptobenthic larvae use near reef waters, especially in relation to their relatively poor swimming abilities (Stobutzki and Bellwood 1997; Fisher 2005; Majoris et al. 2019). If poor swimming ability is a key factor in retaining larvae in the vicinity of reefs, we may expect them to be most abundant in deeper waters, away from stronger currents (cf. Johansen 2014; Armsworth 2001), as seen in subtropical cryptobenthic fishes (Goatley 2021). Indeed, it is well known that late-stage larval fishes may be able to influence their dispersal patterns via behaviour (e.g. Kingsford et al. 2002; Fisher and Bellwood 2003; Bottesch et al. 2016). However, there is a substantial knowledge gap regarding the vertical distribution of cryptobenthic larvae. A knowledge of their depth distributions is key if we are to understand their behaviour.
Previous studies of nearshore larval distributions have recorded variation in vertical distributions using ichthyoplankton tows (Irrison et al. 2010; Leis 1986, 1991) or light traps (Hendriks et al. 2001). Fisher and Bellwood (2002) developed a modified light trap for collecting late-stage larvae from specific depth strata. They found that cryptobenthic taxa (unlike most other taxa) were primarily recovered from the deepest traps, suggesting that settlement-stage cryptobenthic larvae may preferentially occupy or approach reefs in deeper waters, possibly to avoid advection. However, in this previous study the maximum deployment depth was just 16 m and all deployments were on the leeward side of Lizard Island, Great Barrier Reef (GBR). Therefore, there is currently little quantitative data on cryptobenthic larvae below 16 m (Brandl et al. 2018). The goal of this study, therefore, was to use stratified light-trap sampling to investigate the depth distributions of late-stage cryptobenthic reef fish larvae on the deeper, windward, side of Lizard Island.
Materials and methods
Light trap design and deployment
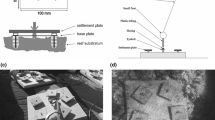
We used modified light traps following Fisher and Bellwood (2002) to sample specific depths in the water column for late-stage (post-flexion, pre-settlement) larval fishes (Fig. 1). Light traps were illuminated using an LED strip light powered by a 15,000 mAh battery bank in a 2 L glass jar (Korken, Ikea), and a Princeton Tec Torrent dive torch; light from the strips and torch lasted for approximately 6 and 8 h, respectively. Three traps were arranged along a single anchor line to sample at 2 m, 13 m, and 27 m and were deployed in water 28–30 m deep (Fig. 1). Sampling was conducted in January/February 2020 at two sites (5 d per site with one light trap array deployment per day), ~ 100 m off the southeastern (windward) reef crest of Lizard Island, a mid-shelf reef on the northern GBR, Australia (Fig. S1).
Conceptual diagram of the depth stratified light traps deployed at Lizard Island. a position of complete sampling array in the water column, b external view of light trap design, and c internal view of light trap. Light trap details follow Fisher and Bellwood (2002)
Light traps were deployed shortly before sunset ~ 5:30 pm and collected the following morning ~ 7:00 am. Non-focal organisms (e.g. pelagic elongate fishes, crustacea) were returned to the sea as soon as possible. Abundant species of interest (e.g. caesionids) were identified, counted, and returned to the sea as soon as possible; a subset was retained, along with all remaining fish larvae, and fixed in buffered 10% seawater formalin for later identification. Retained material was sorted, identified to family and photographed with a scale. The total lengths of fishes were measured using ImageJ, to the nearest 0.1 mm. In species with > 30 individuals per sample, a random subsample (20–30) was selected for measurement.
Statistical analyses
Initially, we undertook multivariate analysis on the full fish assemblage dataset using a permutational multivariate analysis of variance (PERMANOVA) and a canonical analysis of principal coordinates (CAP) ordination to visualise the results (details in Text S1). Based on this initial analysis, the two cryptobenthic fish families most strongly correlated with the multivariate space were the Gobiidae and Apogonidae, warranting a more specific examination. The abundance of each of these two families was treated as the response variable in separate generalised linear mixed effects models. In both cases, depth and site were treated as categorical fixed effects, while the day of deployment was incorporated as a random effect to account for temporal non-independence. Full models with an interaction term were initially fitted, and the most parsimonious model was subsequently identified using the corrected Akaike information criterion (Table S1). The model for Gobiidae was based on a zero-inflated Poisson distribution with a log link, while the Apogonidae model was based on a negative binomial distribution with a log link. Model fits and assumptions were evaluated using simulation-based residual model checking, which were satisfactory in all cases. Pairwise comparisons with Tukey’s adjustment were used to examine within-factor differences. Statistical analyses were conducted in the software R Version 4.0.3 (R Core Team 2020) using the glmmTMB package (Brooks et al. 2017).
Results and discussion
There was clear variation in community composition of fishes in light traps across depth gradients (Table S2). The fish assemblage at the mid-depth was distinct, with separation driven by a relatively high abundance of gobies and monacanthids (Fig. 2). By contrast, the shallow and deep depths were characterised by relatively high apogonid abundance (Fig. 2). Deep samples were also characterised by Caesionidae and Centriscidae and shallow samples were characterised by Pomacentridae and Siganidae (Fig. 2). When considering the cryptobenthic taxa specifically, there was a clear difference in the relative abundance of gobies with depth (Fig. 3a). Indeed, 73.5% of all gobies (2.5 ± 0.97 individuals per sample on average [± SE]) were collected in mid-depth samples; they were less abundant (23.5%; 0.8 ± 0.33) in shallow samples and rare (< 3%; 0.1 ± 0.1) in deep samples (Fig. 3a; GLMM p < 0.05; Tables S3-S6). By contrast, apogonids were largely collected from the shallow (13.1% of individuals; 21 ± 28.93) and deep (86.2%; 138.6 ± 350.22) light traps, with less than 1% (1.2 ± 1.87) collected in the mid-depth (Fig. 3b; GLMM p < 0.05; Tables S3–S6). Therefore, the community composition of fishes in light traps varied considerably across depths with cryptobenthic taxa inhabiting most sections of the water column.
Community composition of fishes caught in depth stratified light trap samples at Lizard Island. a Multivariate ordination of light trap samples (coloured dots) constrained by depth and based on a Bray–Curtis dissimilarity matrix. b Vectors show how fish families (cryptobenthic families in bold) are correlated with each other and relate to the samples in the ordination space. Vectors that were not strongly correlated with the ordination space are not shown
The abundance of a goby and b apogonid individuals in depth stratified light trap samples from Lizard Island. The coloured points and range indicate the mean predicted value and 95% confidence intervals from generalised linear mixed effects models. c Relative frequency distribution of apogonid sizes in shallow and deep light trap samples
Vertical stratification of reef fish larvae in light traps was first demonstrated by Fisher and Bellwood (2002), who showed family-level preferences in the upper 16 m on the leeward side of Lizard Island. Both Fisher and Bellwood (2002) and Leis (1991), the latter using ichthyoplankton tows, found increasing apogonid larval abundance with depth, which contrasts with the low abundance of apogonids in mid-depth samples herein. Leis (1991) also recorded increasing gobiid larvae with depth, again contrasting with our peak gobiid abundance at mid-depths. These differences may be explained by the different study locations, depth ranges, and capture methods; notably Leis (1991) also collected during the day. Our results extend this previous work and increase the potential range of separation to include depth strata down to 27 m. For our focal groups, the cryptobenthic fishes, there is evidence that all sections of the water column were used but that different families exhibit strong preferences. As noted by Leis (1991), empirical knowledge of the vertical position of larvae is invaluable in terms of understanding the relationships between larval fishes and the environment in which they survive. Vertical distributions can shape everything from potential prey and feeding success to advection and the probability of returning to reefs (Cowen et al. 2002).
Although the vertical distribution of fish larvae has received limited attention in the literature, taxon-specific variation across a depth gradient may be expected for several reasons. Stratification may be partly attributed to available light at a given depth due to larval fishes feeding using visual cues (Job and Bellwood 2000), which are likely to be restricted to the upper 15 m at night, even for late-stage larvae (Job 1999). Depth stratification due to swimming abilities may also be expected, although the patterns observed herein are not consistent with our expectations based on the swimming abilities of cryptobenthic fishes. Indeed, both apogonids (Stobutzki and Bellwood 1997; Fisher 2005) and gobies (Majoris et al. 2019) are relatively poor swimmers and would therefore be expected to avoid currents. For example, Stobutzki & Bellwood (1997) estimated that most apogonids are exhausted after about 20 h at 13.5 cm s−1. In the deployment locations, there are moderate to strong currents running along the reef (on average ~ 8 cm s−1 and up to ~ 23 cm s−1 across the three depths sampled; Text S2; Fig. S2; Table S7). Few apogonids would have been able to maintain their position against these currents for more than a day or two, and any position holding would likely have significant energetic consequences. Moreover, Majoris et al. (2019) found that two species of goby larvae had average swimming speeds of 7.2 and 8.6 cm s−1. Given that the average current speed in our location was ~ 8 cm s−1 at all depths, this suggests that the gobies recorded in our study would have to swim consistently at their average speeds to just keep pace with currents. The presence of gobies in the mid-depth samples is thus unexpected, as is their absence in deep samples, where we expected them to be flow refuging (if this does occur it is probably at a finer scale than examined herein). Their apparent ability to select, use and persist in a range of water depths could be an important factor sustaining their exceptional abundances as both larvae and reef fishes (Brandl et al. 2019; Goatley et al. 2021).
Interestingly, the distribution of apogonids could also hold the key to the rarity of gobies in deep samples. All apogonids in the shallow samples were late-stage larvae (Finn and Kingsford 1996) < 20 mm total length, with the vast majority ~ 10 mm (Fig. 3c). By contrast, apogonids from deep samples were up to 56.4 mm, with 56% of measured individuals > 20 mm (Fig. 3c). These are reflective of post-settlement adult apogonids (Ishihara and Tachihara 2011). Adult apogonids roam over open off-reef locations at night (Marnane and Bellwood 2002) and may have been attracted to light traps due to either the light or to prey attracted to the light. In this respect, the high abundance of adult apogonids could have also affected the distribution of larval fishes in deep light traps, as apogonids are known to prey on ichthyoplankton (Marnane and Bellwood 2002). Therefore, the rarity of gobies in deep samples could be because: (a) gobies were abundant in deep samples but were preyed on by apogonids and were therefore undetected (Fig. 3a) or (b) gobies actively avoided deep areas (possibly because of potential predation by apogonids). Although, it would be surprising if option (b) explained the rarity of gobies in deep samples because deeper areas are closer to the benthos and the topographic complexity it provides. Such complexity can be utilised by fishes to shelter from strong currents, as has previously been found for temperate and sub-tropical cryptobenthic taxa (Goatley et al. 2021). Clearly, future studies that tease apart the trade-offs cryptobenthic larval fishes make between factors such as predation and sheltering from currents, is warranted across depth strata.
Overall, the larvae of cryptobenthic fishes exhibited an unexpected capacity to occupy moderate-high flow locations (on average ~ 8 cm s−1) in the water column off the exposed face of a mid-shelf coral reef. Their weak swimming abilities may belie a remarkable capacity to use the water column. Whether this reflects feeding movement (which would be permitted based on the visual acuity of these species [Job and Bellwood 2000]) or enhanced swimming movement prior to settlement (cf. Fisher and Bellwood 2002) remains to be determined. Regardless, cryptobenthic fishes continue to reveal their exceptional capabilities, especially during their highly successful larval phase.
Data availability
The data underpinning the results of this study are publicly available on Figshare (https://doi.org/10.6084/m9.figshare.22180210.v1).
References
Ackerman JL, Bellwood DR (2000) Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar Ecol Prog Ser 206:227–237
Armsworth PR (2001) Directed motion in the sea: efficient swimming by reef fish larvae. J Theor Biol 210:81–91
Bottesch M, Gerlach G, Halbach M, Bally A, Kingsford MJ, Mouritsen H (2016) A magnetic compass that might help coral reef fish larvae return to their natal reef. Curr Biol 26:R1266–R1267
Brandl SJ, Goatley CHR, Bellwood DR, Tornabene L (2018) The hidden half: ecology and evolution of cryptobenthic fishes on coral reefs. Biol Rev 93:1846–1873
Brandl SJ, Tornabene L, Goatley CHR, Casey JM, Morais RA, Côté IM, Baldwin CC, Parravicini V, Schiettekatte NMD, Bellwood DR (2019) Demographic dynamics of the smallest marine vertebrates fuel coral-reef ecosystem functioning. Science 364:1189–1192
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Burgess SC, Bode M, Leis JM, Mason LB (2022) Individual variation in marine larval‐fish swimming speed and the emergence of dispersal kernels. Oikos 2022:e08896
Cowen RK (2002) Larval dispersal and retention and consequences for population connectivity. In: Sale PF (ed) Coral Reef Fishes Diversity and Dynamics in a Complex Ecosystem. Academic Press, San Diego, pp 149–170
Finn MD, Kingsford MJ (1996) Two-phase recruitment of apogonids (pisces) on the great barrier reef. Mar Freshw Res 47:423–432
Fisher R (2005) Swimming speeds of larval coral reef fishes: impacts on self-recruitment and dispersal. Mar Ecol Prog Ser 285:223–232
Fisher R, Bellwood DR (2002) A light trap design for stratum-specific sampling of reef fish larvae. J Exp Mar Bio Ecol 269:27–37
Fisher R, Bellwood DR (2003) Undisturbed swimming behaviour and nocturnal activity of coral reef fish larvae. Mar Ecol Prog Ser 263:177–188
Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V (2007) Smelling home can prevent dispersal of reef fish larvae. Proc Natl Acad Sci 104:858–863
Goatley CHR, Brandl SJ, Wroe S, Bellwood DR (2021) Simple larvae sustain the world’s smallest marine vertebrates. Coral Reefs 40:75–82
Hendriks IE, Wilson D, Meekan M (2001) Vertical distributions of late stage larval fishes in the nearshore waters of the San Blas Archipelago, Caribbean Panama. Coral Reefs 20:77–84
Irrison J, Paris CB, Guigand C, Planes S (2010) Vertical distribution and ontogenetic “migration” in coral reef fish larvae. Limnol Oceanogr 55:909–910
Ishihara T, Tachihara K (2011) Pelagic larval duration and settlement size of apogonidae, labridae, scaridae, and tripterygiidae species in a coral lagoon of Okinawa Island, southern Japan. Pacific Sci 65:87–93
Job SD (1999) The functional visual capabilities of coral reef fish larvae. James Cook University, Townsville
Job SD, Bellwood DR (2000) Light sensitivity in larval fishes: implications for vertical zonation in the pelagic zone. Limnol Oceanogr 45:362–371
Johansen JL (2014) Quantifying water flow within aquatic ecosystems using load cell sensors: a profile of currents experienced by coral reef organisms around Lizard Island, Great Barrier Reef. Australia Plos One 9:e83240
Kingsford MJ, Leis JM, Shanks A, Lindeman KC, Morgan SG, Pineda J (2002) Sensory environments, larval abilities and local self-recruitment. Bull Mar Sci 70:309–340
Lefèvre CD, Nash KL, González-Cabello A, Bellwood DR (2016) Consequences of extreme life history traits on population persistence: do short-lived gobies face demographic bottlenecks? Coral Reefs 35:399–409
Leis JM (1986) Vertical and horizontal distribution of fish larvae near coral reefs at Lizard Island, Great Barrier Reef. Mar Biol 90:505–516
Leis JM (1991) Vertical distribution of fish larvae in the Great Barrier Reef Lagoon, Australia. Mar Biol 109:157–166
Majoris JE, Catalano KA, Scolaro D, Atema J, Buston PM (2019) Ontogeny of larval swimming abilities in three species of coral reef fishes and a hypothesis for their impact on the spatial scale of dispersal. Mar Biol 166:59
Majoris JE, Foretich MA, Hu Y, Nickles KR, Persia CLD, Chaput R, Schlatter E, Webb JF, Paris CB, Buston PM (2021) An integrative investigation of sensory organ development and orientation behavior throughout the larval phase of a coral reef fish. Sci Rep 11:12377
Marnane MJ, Bellwood DR (2002) Diet and nocturnal foraging in cardinalfishes (Apogonidae) at one tree reef, Great Barrier Reef, Australia. Mar Ecol Prog Ser 231:261–268
Mouritsen H, Atema J, Kingsford MJ, Gerlach G (2013) Sun compass orientation helps coral reef fish larvae return ot their natal reef. PLoS ONE 8:e66039
Paris CB, Atema J, Irisson J-O, Kingsford M, Gerlach G, Guigand CM (2013) Reef odor: a wake up call for navigation in reef fish larvae. PLoS ONE 8:e72808
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Staaterman E, Paris CB, Helgers J (2012) Orientation behavior in fish larvae: a missing piece to Hjort’s critical period hypothesis. J Theor Biol 304:188–196
Stobutzki IC, Bellwood DR (1997) Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar Ecol Prog Ser 149:35–41
Acknowledgements
We thank M. Mihalitsis and Lizard Island Research Station Staff for field support; T. Miskiewicz for assistance with larval fish identification; S. Swan and A. Oakley-Cogan for logistical support; and two reviewers for insightful and constructive comments.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by the Australian Research Council (DRB; grant numbers CE140100020 and FL190100062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
This research was conducted in accordance with James Cook University Animal Ethics approval, number A2529.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Douglas, S.R.L., Tebbett, S.B., Choukroun, S. et al. Depth stratified light trap sampling reveals variation in the depth distribution of late-stage cryptobenthic reef fish larvae. Coral Reefs 42, 507–512 (2023). https://doi.org/10.1007/s00338-023-02363-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02363-x