Abstract
Benthic cyanobacterial mats are increasingly reported to cover major coral reef areas. Although suggestions have been made that cyanobacterial mats impair coral reef health in multiple ways, information is lacking regarding the distribution, morphotypical variation and bacterial species composition of these microbial mats. As such, this study aimed to (1) Reveal the bacterial community diversity and composition of different mat morphotypes, (2) Identify the most abundant community members and closely related organisms, (3) Assess to what extent, morphotype, colonized substrate (coral or abiotic substrate), depth, and site were significant predictors of bacterial composition. Data were collected on reefs surrounding the island of Koh Tao (Gulf of Thailand). A total of 201 mats from 16 different locations around the island were classified into eight distinct morphotypes. Of these, the bacterial communities of 44 mats, representing colour groups from multiple sites, were characterized using 16S rRNA gene high-throughput sequencing. Our data revealed that Proteobacteria, Cyanobacteria, Bacteriodetes and Planctomycetes were the four most abundant phyla and occurred in all samples. Abundant cyanobacterial zero-radius operational taxonomic units (ZOTUs) were closely related to prokaryotic sequences found in previous studies of coastal mats (98–100%) and were assigned to genera in the order Oscillatoriales, e.g. Hormoscilla, Okeania, and Oscillatoria. Abundant proteobacterial ZOTUs were assigned to orders in the classes Alpha- and Gammaproteobacteria, e.g. Rhodobacterales, Rhizobiales and Alteromonadales. Abundant Bacteriodetes ZOTUs were mainly assigned to the class Bacteroidia and order Cytophagales. Our results showed that mats consist of a diverse and variable bacterial consortium, with mat colour (morphotype), substrate type and geographic location only explaining a small part of the total variation in composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reef ecosystems are well-known for their high levels of biodiversity (Fisher et al. 2015), as well as their economic (Wongthong and Harvey 2014) and ecological importance (Moberg and Folke 1999). Over the past decades, a growing body of evidence has highlighted the influence of both natural and anthropogenic stressors on reef environments (Hughes et al. 2003, 2017). In particular, coral cover decline caused by benthic competition with other species or biofouling organisms has increased (Turicchia et al. 2018; Hudatwi et al. 2021; Allchurch et al. 2022). Marine sponges, for example, have been shown to directly outcompete scleractinian corals across various areas in the Caribbean (Aerts and van Soest 1997; Aerts 1998; Aronson et al. 2002; Loh et al. 2015). Likewise, the cyanobacteriosponge Terpios hoshinota was shown to be responsible for dramatic reductions in coral cover across various locations in the Indo-West Pacific (de Voogd et al. 2013; van der Ent et al. 2016; Yang et al. 2018; Fromont et al. 2019; Aini et al. 2021). The current shifts in community structure in reefs suggest a tendency towards rapid growth and low structural diversity, particularly following mass-mortality events such as those induced by widespread bleaching (Diaz-Pulido and McCook 2002), predator outbreaks (Kayal et al. 2012; Hoeksema et al. 2013) or diseases (Harvell et al. 2007; Montano et al. 2013).
The best documented changes in coral reef composition are the globally occurring coral-algal phase shifts (Gorgula and Connell 2004; McManus and Polsenberg 2004; Sandin et al. 2008; Bahartan et al. 2010; de Bakker et al. 2017), where corals are overgrown or replaced by macro- or turf algae, the latter being densely packed assemblages of short filamentous algae and cyanobacteria. More recently, however, benthic cyanobacterial mats (hereafter referred to as mats) have increasingly been reported to cover large areas of coral reef even to the point of being the dominant benthic substrate in certain areas of the Caribbean, Indian and Pacific Oceans (reviewed by Ford et al. 2018). These specific types of microbial mats often occur on coral rubble or sandy substrates and are suggested to be indicators of recent reef degradation due to their prevalence on recently denuded reef substrate (Sandin et al. 2008; Charpy et al. 2012). Additionally, mats have also been shown to overgrow other benthic denizens, including scleractinian corals and fleshy algae (Ritson-Williams et al. 2005; Puyana and Prato 2013; de Bakker et al. 2017) under certain environmental conditions, such as reduced water quality (iron and phosphorous enrichment), grazing pressure, and/or elevated seawater temperatures (Kuffner and Paul 2001; Bender et al. 2014; Brocke et al. 2015). Mat proliferation on top of corals and algae can also reduce light and oxygen availability (Puyana and Prato 2013).
After colonization, mats alter the functioning of reef ecosystems in various ways, for example, by increasing organic matter production and nitrogen fixation (Cardini et al. 2014; den Haan et al. 2014; Brocke et al. 2018). Increased bioavailable nutrients, in turn, can favour algal as opposed to coral growth. Also, various types of mats have been shown to be toxic to certain coral species (Gantar et al. 2009; Charpy et al. 2010; Sato et al. 2016). For example, the widespread “black band” coral disease is caused by the same microbial consortium found in certain benthic microbial mats (Richardson 1997; Frias-Lopez et al. 2003; Cissell et al. 2022). Other mats have been shown to inhibit coral larvae settlement (Kuffner and Paul 2004; Kuffner et al. 2006), possibly due to secondary metabolites produced by bacterial constituents of the mats. Furthermore, a recent study showed fish that usually feed on corals or algae did not consume cyanobacterial mats (Ford et al. 2021). As such, persistent occurrence of mats over large areas could potentially lead to reductions in fish populations and impair the key ecological functions they fulfil, such as top-down forcing (Ford et al. 2021). In contrast, other observational studies revealed cyanobacterial mats as significant foraging targets of multiple fish species on Indo-Pacific and Caribbean reefs, and suggested cyanobacteria were preferred nutritional targets for several reef grazers (Clements et al. 2017; Cissell et al. 2019). These seemingly contrasting findings might indicate variability in the toxicity of the cyanobacterial mats, leading to heterogeneity in cyanobacterial mat consumption, and as such, top-down forcing (Cissell and McCoy 2022). In freshwater mats of Microcoleus, for example, nitrogen speciation and availability drive the abundance of closely related toxic and nontoxic Microcoleus strains (Tee et al. 2021).
Although microbial mats are widely studied (Bolhuis et al. 2014; Wood et al. 2020), less is known about the specific microbial composition of mats in coral reef systems. Studies that investigated mats in tropical areas have mainly focused on mats which colonized abiotic substrates such as sandy bottoms or did not report on the specific substrate that was colonized (Croce et al. 2021). Only the, visually dominant, cyanobacterial composition of these mats was analysed and showed to vary among different locations; they were dominated by either non-heterocystous (Bauer et al. 2008; Brocke et al. 2018), or a combination of heterocystous and non-heterocystous cyanobacteria (Abed et al. 2003; Charpy et al. 2007). Recently, Cissell and McCoy (2020) reported on the relatively high abundance of other bacterial phyla in addition to cyanobacteria in coral reef microbial mats. In general, microbial mats are known to consist of interdependent layers inhabited by heterotrophic, chemotrophic and phototrophic micro-organisms (Paerl and Pinckney 1996). Their ability to change the dominant metabolic pathway according to nutrient availability, enables them to colonize a wide range of habitats (Paerl and Pinckney 1996). The findings of Cissell and McCoy (2020) suggest that this adaptive strategy might also apply to coral reef cyanobacterial mats. It is important, therefore, to shift focus to the whole bacterial community of mats and not solely focus on the cyanobacterial component.
The present study investigated the abundance, bacterial species composition, and distribution of benthic cyanobacterial mats around the reefs of Koh Tao, Thailand. The reefs around the island consist of biodiverse, dense fringing reefs and offshore submerged granite pinnacles, containing some of the highest coral coverages in the Gulf of Thailand (Yeemin et al. 2006; Phongsuwan et al. 2013; Scott et al. 2017a). The reefs of Koh Tao, however, are threatened by biotic and abiotic factors such as coral predation, mass bleaching and tourism (Hoeksema and Matthews 2011; Lamb et al. 2014; Scott et al. 2017b; Moerland et al. 2019). With the collected data, we aimed to (1) Reveal the bacterial community composition and diversity of different mat morphotypes; (2) Identify the most abundant community members and closely related organisms; (3) Assess to what extent, mat morphotypes, colonized substrate (coral or abiotic substrate), depth, and site were significant predictors of variation in bacterial composition.
Material and methods
Study area and data collection
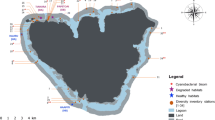
Mat samples were collected from coral reef substrate at depths ranging from 0.5 to 15.2 m during May and June 2018 around the Island of Koh Tao, Gulf of Thailand, Thailand (Fig. 1). Water temperatures varied between 29 and 32 °C. In total, 16 different locations were surveyed around the island (Fig. 1) using the Roving Diving Technique (RDT) (Schmitt et al. 2002; Munro 2005; Hoeksema and Koh 2009). As our goal was to capture the wide diversity of cyanobacterial mats present around the island, the RDT was chosen, as opposed to transect lines, in order to increase the area and environmental gradients covered during one dive. The method enabled us to capture a wide diversity of cyanobacterial mats present around the island. Each detected mat was documented using underwater photographs; colour, depth of occurrence, and the underlying substrate (Tables 1 and 2). Samples were collected from a subset of the observed mats using a small plastic disposable syringe and were subsequently stored in 2-ml Eppendorf tubes containing 96% ethanol. These tubes were later stored (− 20 °C) at Naturalis Biodiversity Center, Leiden, the Netherlands, where DNA was extracted for bacterial community analysis.
DNA extraction and 16S rRNA gene sequencing
Of the collected mats, 44 samples covering different environmental gradients (colour, depth, substrate, geographic location) were selected for prokaryotic community analysis. Samples were dried using a vacuum centrifuge after which DNA extraction was performed using the MoBio Powersoil® DNA isolation kit (MO BIO Laboratories 2016) according to the manufacturer's instructions.
For the 16S rRNA gene amplification, the V4 variable region was amplified by PCR, using the primers illCUs515F 5′-GTGYCAGCMGCCGCGGTAA-3′ (Parada et al. 2016) and new806RB 5′-GGACTACNVGGGTWTCTAAT-3′ (Apprill et al. 2015) with an index on the forward primer to allow multiplexing of samples. A 28 cycle PCR was performed in triplicate using the HotStarTaq Plus Master Mix Kit under the following conditions: 95 °C for 10 min, followed by 28 cycles of 95 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. To check for amplification, products were loaded onto 2% agarose gels (E-gel). Samples were pooled together using qiagility equimolar pooling (Qiagen 2018) based on DNA concentrations measured on the Qiaxcel (Qiagen 2017). The pooled sample was then purified with the use of calibrated Ampure XP beads (Beckman Coulter) to a molar concentration of 38.5 nmol (Agilent Technologies 2009).
To prepare for sequencing, the PGM Hi-Q View OT2 kit was used following the manufacturer’s protocol (Thermofisher Scientific 2017a). The DNA was loaded onto an Ion 318™ Chip v2 BC chip and sequenced using the Ion PGM Hi-Q sequencing kit following the manufacturer’s protocol (Thermofisher Scientific 2017b).
The obtained reads were processed using the OpenStack High-Performance cloud computing environment at Naturalis using Galaxy (Afgan et al. 2018) as a graphic user interface. After removal of the forward and reverse primers with the Cutadapt primer trimmer (Martin 2011), reads were trimmed using PRINSEQ to a length of 250 bp (Schmieder and Edwards 2011). Denoising (error-correction) of reads was performed to identify all correct biological sequences following the UNOISE2 algorithm (α = 2.0, minimally accepted abundance before clustering = 8; Edgar 2016). This algorithm defines centroid sequences by removing chimeras and reads with sequencing errors resulting in ZOTUs (“zero-radius” OTUs) for subsequent identification by BLAST. Total reads, reads per sample and reads maintained after quality are listed in Tables S1 and S2.
Taxonomy was assigned using the SILVA 132 SSUParc database (Quast et al. 2013). First, a nucleotide BLAST (Altschul et al. 1990) was done with 100 hits per ZOTU representative sequence. Hereafter, sequence reads were identified to the taxon of the best hit when the identity percentage and query coverage was above 97%. To be able to assign higher-level taxonomic assignments for ZOTUs without direct hits in the reference database, we used the lowest common ancestor (LCA) tool described in Beentjes et al. (2019). The LCA script was performed on the top 8% hits which met the following conditions: minimum bit-score of 100; minimum identity of 90%; minimum coverage of 97%. ZOTUs with solely hits below 90% identity percentage were classified as “not identified”.
For the cyanobacterial ZOTUs, the SILVA taxonomic assignment did not show consistent results, e.g. several genera and families were assigned to varying orders and classes. Correct nomenclature of cyanobacteria has long been a topic of discussion (Oren and Ventura 2017), which has resulted in inconsistencies in taxonomic assignment. To obtain consistent classification of our cyanobacterial ZOTUs, we blasted the representative sequences of the cyanobacterial ZOTUs using NCBI nucleotide blast (NCBI 2021). Subsequently, we verified taxonomic classification using the taxonomy of Algae Base (Guiry and Guiry 2020). Best blast hits with identity coverages < 90% (21 ZOTUs) did not replace the acquired SILVA taxonomy. The DNA sequences generated in this study can be downloaded from the National Center for Biotechnology Information (NCBI 2021) Sequence Read Archive (SRA): PRJNA752973. Sample metadata are listed in Tables S3 and S4.
Statistical analysis
A data matrix containing ZOTU counts per sample was imported into R using the read.csv function. ZOTUs not identified at the phylum level and above, chloroplasts and mitochondria were removed prior to statistical analysis. The resultant reduced data table was rarefied to 10,900 sequences per sample, which excluded three low-read samples. A Hellinger dissimilarity/distance matrix was constructed using the ZOTU counts table as input. The Hellinger (Rao 1995) distance is recommended to analyse species abundance data as it corrects for the double 0 problem when assessing similarities among samples (Legendre and Gallagher 2001; Ramette 2007). Moreover, in a comparative study of various distance matrixes (Chord, Chi-square, Hellinger, Bray–Curtis), it gave low weights to rare species, was monotonically related to the geographic distance along a model gradient and reached an asymptote for sites with no species in common. It also produced little ‘horseshoe effect’ or inward folding of sites at opposite ends of the gradient, in ordinations (Legendre and Gallagher 2001). The Hellinger matrix was constructed by first transforming the ZOTU table with the decostand function in vegan (Oksanen et al. 2019) with the method argument set to ‘Hellinger’. With this transformation, the ZOTU table was adjusted such that subsequent analyses preserved the chosen distance among objects (samples in this case). The Hellinger distance matrix was subsequently created with the vegdist() function in vegan using the Hellinger-transformed ZOTU table as input and the method argument set to ‘euclidean’.
Variation in prokaryotic composition among biotopes was visualised with Principal Coordinates Analysis (PCO) using the cmdscale function in R with the Hellinger distance matrix as input (the Bray–Curtis matrix gave very similar results). Variation among colour morph (morphs with at least three replicates: green–brown, red and purple), substrate (biotic versus abiotic), site (eastern vs. western site of the island) and depth (grouped into 0–5, 5–10, and 11–15 m depth ranges) were tested for significance using the adonis2() function in vegan. In the adonis analysis, the hellinger matrix of mat composition was the response variable with colour morph, substrate, depth or site as independent variables (permutations set to 999). To control for interdependence among variables, we restricted permutations to only occur within substrate, when testing for variation among colour morphs. Likewise, we restricted permutations to occur within colour morph, when testing the effect of substrate. Additionally, differences in depth distribution among substrates and colour morphs were analysed using the function Gtest() in the R package DescTools. The results gave a non-significant difference in depth distribution between substrate (G = 0.91836, Chi-square df = 2, p = 0.6318) and colour morphs G = 5.2033, Chi-square df = 4, p = 0.2671). Subsequently, the association between bacterial dissimilarity (using the Hellinger distance matrix) and geographic distance/depth difference was tested for significance with the mantel function in the R package vegan (Oksanen et al. 2013). Detailed descriptions of the functions used here can be found in R (e.g. ?cmdscale) and online in the reference manuals (e.g. http://cran.r-project.org/web/packages/vegan/index.html; assessed 21 September 2021).
In addition to PCO, we used unconstrained, hierarchical clustering to identify strongly supported clusters with the pvclust package (Suzuki and Shimodaira 2013). The pvclust package provides validation procedures to test the uncertainty of a classification (Borcard et al. 2018), namely, calculating the bootstrap probability (BP) and the approximately unbiased P values (AU) based on multiscale bootstrap resampling. High AU values indicate that a given cluster is strongly (e.g. AU ≥ 80) or significantly (AU ≥ 95) supported by the data. In pvclust, a hierarchical clustering dendrogram was produced using the pvclust() function with the method.dist argument set to "euclidean" and the method.hclust set to "ward.D2" (Ward 1963). Input for the function consisted of the Hellinger-transformed ZOTU table. Ward’s method (Ward 1963) minimises within-group sum of squares. With the above procedure, we were interested in assessing to what extent mat morphotype formed strongly supported clusters. The simper() function in vegan was used to identify significantly discriminating ZOTUs between pairs of morphotypes based on the square root transformed ZOTU table and 999 permutations. The discriminating ZOTUs contribute the most to differences between morphotypes.
Closely related organisms to the most abundant ZOTUs were identified using the NCBI BLAST command line “blastn” tool with the -db argument set to nt. In the blast alignment, default parameter settings were used: Expect threshold (e-value) = 0.05, word size = 28, maximum target sequences = 100, and Match/Mismatch scores = 1,-2. To detect a core community, our ZOTU table, taxonomy data and metadata were loaded into a phyloseq object using the function phyloseq() of the R package phyloseq. The created phyloseq object was loaded into the function core_members() from the R package microbiome, with the arguments set to: detection = 0, prevalence = 95/100. A detection threshold equal to 0 includes all the nonzero occurrences.
Results
Morphotypes cyanobacterial mats and distribution
A total number of 201 mats were observed at 16 different locations around the island (Table S3). Eight differently coloured mats were distinguished on nine different substrate types including ten different coral genera (Tables 1 and 2; Fig. 2). Acropora and Pocillopora were the most frequently colonized coral genera (observed at six locations), followed by corals of the genus Porites (three locations) and Diploastrea (two locations). Mats differed in terms of structure; green, brown, green–brown, purple, orange and red mats consisted of thin slimy layers. Black-grey mats, in turn, were more filamentous and lilac mats consisted of small patches, both slimy and filamentous. Of all mats, purple (31%), green–brown (20%) and red (25%) were the most frequently observed morphotypes and occurred at all survey depths (from the reef edge up to 15 m depth) at 6, 9, 10 and 12 different locations, respectively (Tables 1, 2, S3).
Underwater photographs of the various benthic cyanobacterial mats observed around the Island of Koh Tao: (a) brown mat on benthos, (b) orange mat on benthos, (c) purple mat on Fungiidae spp., (d) red mat on Pocillopora sp., (e) greenbrown mat on coral rubble, (f) green mat on benthos, (g) lila mat on a vermetid gastropod, Ceraesignum maximum, (h) greyblack mat on green algae and benthos
Overall bacterial composition
Forty-four mat samples were analysed using 16S rRNA gene sequencing (Table S4). After quality control and rarefaction, the dataset in the present study consisted of 446,900 sequence reads binned into 13,023 ZOTUs. These were assigned to 41 phyla, 83 classes, and 192 orders. The most abundant phyla were Proteobacteria (182,248 sequences; 6028 ZOTUs), Cyanobacteria (101,297 sequences; 251 ZOTUs), Bacteroidetes (77,027 sequences; 2639 ZOTUs), Planctomycetes (35,240 sequences; 1717 ZOTUs), and Verrucomicrobia (20,213 sequences; 535 ZOTUs) (Fig. 3). The most abundant classes were Cyanophyceae (94,365 sequences; 212 ZOTUs), Gammaproteobacteria (88,573 sequences; 2374 ZOTUs), Bacteroidia (74,472 sequences; 2487 ZOTUs), Alphaproteobacteria (70,902 sequences; 1899 ZOTUs), and Deltaproteobacteria (20,753 sequences; 1658 ZOTUs). The most abundant orders were Oscillatoriales (91,129 sequences; 92 ZOTUs), Cytophagales (45,700 sequences; 1017 ZOTUs), Rhodobacterales (24,530 sequences; 455 ZOTUs), Alteromonadales (23,025 sequences; 293 ZOTUs), and Cellvibrionales (17,806 sequences; 554 ZOTUs).
Relative abundance of the 10 most abundant bacterial phyla (a), orders (b), and classes (c) of the whole dataset. Boxplots represent the first (25%) and the third (75%) quartile (lower and upper hinge) and the median with a 95% confidence interval (the notch). Whiskers display 1.5 × the interquartile range. Outliers are presented as dots. Full names of the taxa abbreviated in the figure from left to right: Phyla: Proteobacteria, Cyanobacteria, Bacteriodetes, Planctomycetes, Verrucomicrobia, Chloroflexi, Acidobacteria, Actinobacteria, Fusobacteria, and Lentisphaerae. Classes: Cyanophyceae, Gammaproteobacteria, Bacteroidia, Alphaproteobacteria, Deltaproteobacteria, Verrucomicrobiae, Phycispaerae, vadinHA49, Unassigned, and Anaerolineae. Orders: Oscillatoriales, Cytophagales, Unassigned, Rhodobacterales, Alteromonadales, Cellvibrionales, Flavobacteriales, Opitutales, unknown.order, and Vibrionales
Bacterial mat community structure and diversity
The samples exhibited pronounced variation in terms of the relative abundance of phyla, classes and orders (Fig. 3). For example, the percentage of sequences assigned to the Proteobacteria varied from 12.8 to 70.1% among samples, while cyanobacterial relative abundance varied from 0.77 to 67.3%. Richness varied from a low of 633 to a high of 3631 ZOTUs per sample, while evenness varied from 0.38 to 0.90 per sample. There was also considerable variation within colour morphs. For example, samples of the red colour morph included specimens with the lowest and highest richness values (Fig. S1).
Overall, no significant differences (p < 0.05) were detected among the three most abundant colour morphs (nred = 14, npurple = 17, ngreen–brown = 7) when considering cyanobacterial ZOTUs (adonis: F2, 35 = 1.27; p = 0.156; R2 = 0.068). However, significant differences were detected when considering the whole bacterial communities (adonis: F2, 35 = 1.18; p = 0.039; R2 = 0.063, Fig. 4). Colour morph, however, only explained a small amount of the variation in composition in our dataset (~ 6%) and the PCO analysis, furthermore, revealed considerable overlap among clusters of green–brown, red, and purple mats along the first two axes of variation (Fig. 4).
Ordination showing the first two axes of the principal coordinates analysis (PCO) of ZOTU composition. Light grey symbols represent operational taxonomic unit (ZOTU) scores with the symbol size proportional to their abundance (number of sequence reads). Coloured symbols represent samples labeled by the colour morphs of the mats
We also tested to what extent substrate type (nlive = 20 and nnon-living = 18), depth (nshallow = 3, ninter = 23, ndeep = 12) and sampling location (neastern = 16 and nwestern = 22) influenced bacterial composition. There was a significant effect of sampling location (adonis: F1, 36 = 1.17; p = 0.026; R2 = 0.031), whereas we observed a non-significant effect of depth (adonis: F2, 35 = 0.97; p = 0.408; R2 = 0.055) and substrate (adonis: F1, 36 = 1.12; p = 0.203; R2 = 0.030) on bacterial composition. These factors, however, only explained a small amount of the variation in the dataset (e.g. substrate and sampling location: ~ 3% each). There were no statistically supported interactions. Also, no statistical support was found for the hypothesis that mat bacterial community composition varied among mats recorded on different coral species (including only specimens sampled from live coral colonies; adonis: F3, 14 = 1.03; p = 0.346; R2 = 0.181). Additionally, there was a non-significant association between community dissimilarity and the distance among sample locations (Mantel r: 0.074; p = 0.221) or depth (Mantel r: 0.090; p = 0.162; Fig. 5).
To further analyse if there was any clustering of our samples based on their associated bacterial community, a hierarchical clustering analysis was performed (Fig. S2). Strongly supported clusters are indicated by blue nodes (0.90 < AU < 0.95) and significantly supported clusters by red nodes (AU > 0.95). Our data displayed one strongly supported cluster, which consisted of 17 samples of varying colour morphs. Within this larger cluster, two green–brown samples formed a significant cluster. The data showed one other significant cluster, which consisted of one green–brown and one purple sample. The rest of the data did not reveal any significant clustering.
Most abundant bacterial members
The 40 most abundant ZOTUs are presented (Fig. 6, Table S5), including their taxonomic assignment and their closest relatives in GenBank. The seven most abundant ZOTUs were assigned to Cyanobacteria, of which six were closely related to different genera of the order Oscillatoriales (98–100% sequence similarity). The most abundant ZOTU (ZOTU 1) had 100% sequence similarity to a benthic cyanobacterial organism classified as Blennothrix cantharidosma (note: BLAST hit shows the homotypic synonym name Hydrocoleum cantharidosmum; Table S5) collected from coral reefs in French Polynesia. The second and fourth most abundant ZOTUs (ZOTU 3 and ZOTU 10) both had high sequence similarity to an organism identified as Oscillatoria sp., which was collected from coral reefs in French Polynesia (> 99%). ZOTU 6, the third most abundant ZOTU, was a discriminating ZOTU between purple and red mats (simper, p = 0.017, Table S6) and had 100% sequence similarity to an organism identified as Okeania collected from a cyanobacterial mat in a coastal area of Japan. One abundant cyanobacterial ZOTU (ZOTU 16, genus Hormoscilla) had high sequence similarity to an organism obtained from a coral (Dipsastraea sp.) suffering from black band disease, collected in the Red Sea (99.2%). It was a discriminating ZOTU between red and green–brown mats and was particularly abundant in one green–brown mat sample (simper, p = 0.001; Table S6, Fig. 6). Other cyanobacterial ZOTUs had high sequence similarities to organisms of the genera Planktothricoides, Lyngbya, and Caldora (all order Oscillatoriales, 98–99%), which were obtained from various marine habitats, e.g. a mangrove system, fish and an intertidal microbial mat. In addition to the cyanobacteria, the mats consisted of several abundant proteobacterial ZOTUs assigned, using the Silva database, to the classes Alpha- and Gammaproteobacteria, and ZOTUs assigned to the class Bacteroidia (Fig. 6, Table S5). Of the 40 most abundant ZOTUs, ZOTUs 4 (unassigned cyanobacteria), 16, 80 (assigned to Hormoscilla), 50 (assigned to Persicobacter), 58 (assigned to Vibrio), and 81 (assigned to the family Phycispaeraceae) significantly discriminated between red and green–brown mats, whereas ZOTUs 6 (assigned to Okeania), 7 (assigned to Ruegeria) and 51 (assigned to the family Cyclobacteriaceae) significantly discriminated between purple and red mats (simper, p < 0.05; Table S6). None of the 40 most abundant ZOTUs significantly discriminated between purple and green–brown mats (simper, p > 0.05; Table S6).
Relative abundance of the 40 most abundant ZOTUs. The size of the symbol is proportional to the relative abundance of the specific ZOTU. Colours display the corresponding bacterial phyla. Y-axis represents ZOTUs labelled with their assigned genus and number. X-axis displays samples. Samples are ordered according to colour morph: G: greenbrown; L: lila, O: orange, P: purple, R: red. Numbers represent individual sample numbers. Core ZOTUs are labelled as “*”
Besides the variation detected among our mat bacterial communities, we found 22 ZOTUs that occurred in 95% of the samples (Table S7), of which 11 were among the 40 most abundant ZOTUs (Fig. 6: core ZOTUs labelled as “*”). Of these ZOTUs, 18 were assigned to varying families and orders of three proteobacterial classes (Table S7). 3 ZOTUs were assigned to the family Cyclobacteriaceae and 1 ZOTU was assigned to the family Crocinitomicaceae (Bacteriodetes).
4. Discussion
In this study, we provide observational data on the occurrence and bacterial diversity of cyanobacterial mats in a tropical coral reef ecosystem. Our findings showed that there are a variety of mat morphotypes across varying substrates, including live corals. The bacterial community analysis revealed mats with variable diversity and structure. Of the total number of reads detected in our dataset, 22.6% was assigned to Cyanobacteria, whereas 40.8% was assigned to Proteobacteria, 17.2% to Bacteroides, and 7.9% to Planctomycetes. Among mats, we detected a core community composed of several Proteobacteria and Bacteriodetes, but also observed pronounced variation in bacterial composition. The variation was only weakly related to the measured environmental variables (colour, depth, geographical location and substrate).
Variation and similarity in mat community structure and composition
There was pronounced variation in bacterial diversity and community structure among mat communities. Several of our sampled mats were dominated by one or two cyanobacterial ZOTUs, whereas others consisted of a multitude of bacterial members at lower relative abundances. Other studies have likewise confirmed high bacterial variation in mat communities (Cleary et al. 2019). Certain mats seem to be dominated by a single cyanobacterial species (Brocke et al. 2018), where others consisted of a consortium of bacterial members (Cleary et al. 2019; Cissell and McCoy 2020).
Although not statistically significant, we observed a trend where, up to 4 km distance, mat community dissimilarity increased with greater geographic distance (Fig. 5). Increased dissimilarity with distance (distance-decay relationship) could indicate that the variation was driven by limited species dispersal or spatial heterogeneity in the environment. From 4 to 7 km distance, however, dissimilarity between mats decreased again, suggesting the relative importance of spatial heterogeneity and dispersal limitation decreased in comparison to local drivers of change. For example, the successional patterns of the mats during their development may have been an important driver of community variation. Cyanobacterial mats are known to shift in bacterial composition and structure over time, where early-colonizing assemblages of photo-autotrophic, nitrogen fixing bacteria create a niche for heterotrophic bacteria (e.g. Proteobacteria and Bacteriodetes) (Brislawn et al. 2019). Such a trend may have masked the effects of other factors on the bacterial community composition of the mats (e.g. substrate, depth, or geographic distance).
Moreover, the colour of the mat only explained 6% of variation in composition, whereas cyanobacterial reads did not differ significantly at all among colour morphs. These results indicate that classification based on morphotype, a method used in several field guides (Littler and Littler 2000; Humann and Deloach 2013) cannot be used to make any assumption about the cyanobacterial composition of benthic cyanobacterial mats.
Up until now, studies on marine (cyano)bacterial mats did not include core community analyses, possibly due to the often-limited number of mats investigated (Cardoso et al. 2017; Cleary et al. 2019; Cissell and McCoy 2020). Here, next to the observed variation among mats, we could detect a core community of 22 ZOTUs, meaning these ZOTUs were present in 95% of the samples. Interestingly, there were no cyanobacterial ZOTUs among the core members but all were either classified as Alpha-, Gamma- and Deltaproteobacteria or Bacterodia. Blast results revealed 100% sequence similarity of several abundant core members, classified as Ruegeria (ZOTU 7), Fabibacter (ZOTU 18), Congregibacter (ZOTU 43) and Marinovum (ZOTU 57), to organisms previously found in seawater. In congruence, other studies reported on high abundances of these taxa in mats (Cardoso et al. 2019; Cleary et al. 2019) and seawater (Sonnenschein et al. 2017; Cleary et al. 2019).
Auto- and heterotrophic bacteria in diverse mat communities
As previously observed in mats sampled in other coral reef systems in the Pacific, the Indian Ocean, and Caribbean Sea (Abed et al. 2003; Thacker and Paul 2004; Charpy et al. 2010; Brocke et al. 2018; Ford et al. 2021), our results revealed high abundances of cyanobacterial ZOTUs of the order Oscillatoriales and the genera Blennothrix, Hormoscilla, Oscillatoria, Okeania, Planktothricoides, and Lyngbya. Interestingly, ZOTU 1, the most abundant and widespread ZOTU in our data and assigned to the genus Blennothrix, had 100% sequence similarity to Blennothrix cantharidosma. Strains of this genus have been shown to be abundant and important benthic dinitrogen fixers in mats collected from reefs of the southwestern Indian and northwestern Pacific ocean (Charpy et al. 2010). It should be noted that although cyanobacteria were not the most dominant group in terms of sequence reads, filamentous cyanobacterial cells are typically far larger than most bacterial cells of the groups recorded in the present study (Palińska et al. 2015). Cyanobacteria may, thus, still represent a dominant portion of the total bacterial biomass. The distinction between biomass versus the relative abundance of sequencing reads might be especially relevant regarding reef trophic ecology. Bacterial species that dominate in terms of biomass likely have a greater impact on the nutritional status of the mat, which in turn, influences fish grazing (top-down control) (Cissell and McCoy 2022).
In terms of sequence abundance, however, cyanobacteria are only one component of a diverse microbial consortium (Wong et al. 2015; Cissell and McCoy 2020). Within this diverse consortium, Alphaproteobacteria, Gammaproteobacteria and Bacteroidia were consistently abundant members. Bacteria of these classes have been shown to be abundant in mat communities in previous studies (Wong et al. 2015; Cissell and McCoy 2020). The consistent occurrence of these clades suggests a putative, diverse phototrophic community, as the light-capturing pigment proteorhodopsin has been found to be ubiquitous among members of these clades (Rusch et al. 2007), whereas anoxigenic phototrophy (AAP), in which Bacteriochlorophyll serves as the light-harvesting protein, has repeatedly been found in Rhodobacterales and Rhodospirrilales members (reviewed by Koblížek, 2015). Unlike cyanobacterial phototrophy, the latter two mechanisms are thought to be photoheterotrophic processes, where light provides ATP, but the carbon source is organic matter (Fuhrman et al. 2008). As mats were collected in the shallow euphotic zone of the reef, it is not surprising that phototrophy might be a dominant metabolic pathway in the mat communities.
In addition to the above, Rhodobacterales and various Bacteriodetes members are known to actively degrade high molecular weight macromolecules, e.g. extracellular polymeric substances, and cyanobacterial-derived metabolites (Cottrell and Kirchman 2000). A potentially aerobic heterotrophic taxon that was abundant in our dataset was the order Phycispaerae (phylum Planctomycetes). Members of this order are ubiquitous in marine environments, and are usually associated with photoautotrophs (algal and cyanobacterial blooms) (Wiegand et al. 2018). It would be interesting to explore the functional diversity of cyanobacterial mats using, for instance, metagenomic shotgun sequencing and metabolomics to ascertain to what extent the results of our compositional analysis are reflected in functional profiles and metabolite composition.
Mat biofouling and toxicity
Mats were most frequently observed on abiotic substrates. This trend has previously been detected (Golubic et al. 2010) and could be explained by coral defence mechanisms. Koh (1997) examined antimicrobial activity among scleractinian corals and revealed that extracts of corals inhibited growth of varying prokaryotes, in particular a strain of Synechococcus sp. Also, coral mucous production, previously shown to be a protective mechanism against sedimentation in several species of the genus Porites (Bessell-Browne et al. 2017), could possibly protect from other types of biofouling.
Interestingly, and likewise found in several other studies (Nagle and Gerwick 1995; Ritson-Williams et al. 2005; Puyana and Prato 2013), mat overgrowth was also observed on living tissue of several coral taxa. In these cases, the insufficiency of the coral defence mechanism could be explained by the possible toxic nature of the mats. For instance, the cyanotoxin microcystin is associated with the widely known black band disease and has been shown to impair the structural integrity of coral tissue, which causes extrusion of zooxanthellae from their normal location (Gantar et al. 2009; Richardson et al. 2009). Among the relatively abundant ZOTUs in our dataset, ZOTU 16, classified as Hormoscilla, showed 99% sequence similarity to an organism associated with black band disease of a scleractinian coral (Dipsastraea sp.). ZOTU 3, the second most abundant ZOTU in our dataset, was classified as Oscillatoria; several strains of this genus are known to produce microcystin (reviewed by Kaebernick and Neilan 2001). Furthermore, several other genera that have been identified in mats are known to be able to produce toxins, e.g. Blennothrix (formerly named as Hydrocoleum; Laurent et al. 2008; Méjean et al. 2010), Planktothrioides (Guidi-Rontani et al. 2014) and Lyngbya (Osborne et al. 2001). This may show the potential of cyanotoxin production, however, it does not indicate whether the species in the mats do produce toxins as cyanotoxin production is strain specific (e.g. Kurmayer et al. 2004; Kardinaal et al. 2007). In addition to potentially harmful cyanobacteria, ZOTU 14 and ZOTU 58, which were both part of the core community of the mats, were assigned to the genus Vibrio with 100% sequence similarity to Vibrio harveyi and Vibrio neptunius, respectively. Both of these species are known pathogens of fish and invertebrates (Prado et al. 2005; Austin and Zhang 2006). Moreover, Vibrio harveyi has repeatedly been associated with coral diseases causing tissue necrosis, e.g. White and Yellow band diseases (Cervino et al. 2008; Luna et al. 2010).
An improved understanding of cyanobacterial mat ecology will help to guide endeavours to improve coral reef health, particularly given that future climate conditions have been predicted to enhance mat proliferation at the expense of coral growth. Our results revealed widespread abundance, high taxonomic diversity and biofouling activity of different mat morphotypes in an Indo-pacific coral reef. Our findings emphasise a holistic approach to studying coral reefs in general and benthic (cyano)bacterial mats in particular, which should include assessments of the whole prokaryotic community.
Data availability
The DNA sequences generated in this study can be downloaded from the National Center for Biotechnology Information (NCBI 2021) Sequence Read Archive (SRA): PRJNA752973. Sample metadata are listed in Table S3 and S4.
References
Abed RMM, Golubic S, Garcia-Pichel F, Camoin GF, Sprachta S (2003) Characterization of microbialite-forming cyanobacteria in a tropical lagoon: Tikehau Atoll, Tuamotu, French Polynesia. J Phycol 93:862–873
Aerts LAM (1998) Sponge/coral interactions in Caribbean reefs: analysis of overgrowth patterns in relation to species identity and cover. Mar Ecol Prog Ser 175:241–249
Aerts LAM, van Soest RWM (1997) Quantification of sponge/coral interactions in a physically stressed reef community, NE Colombia. Mar Ecol Prog Ser 148:125–134
Afgan E, Baker D, Batut B, Van Den Beek M, Bouvier D, Ech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544
Agilent Technologies (2009) Agilent High Sensitivity DNA Kit Guide
Aini SN, Tang SL, Yamashiro H (2021) Monthly progression rates of the coral-killing sponge Terpios hoshinota in Sesoko Island, Okinawa, Japan. Coral Reefs 40:973–981
Allchurch A, Mehrotra R, Carmody H, Monchanin C, Scott CM (2022) Competition and epibiosis by the sponge Pseudoceratina purpurea (Carter, 1880) on scleractinian corals at a tourism hotspot in the Gulf of Thailand. Reg Stud Mar Sci 49:102131
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Apprill A, Mcnally S, Parsons R, Weber L (2015) Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Microb Ecol 75:29–137
Aronson RB, Precht WF, Toscano MA, Koltes KH (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar Biol 141:435–447
Austin B, Zhang XH (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 2:231–245
Bahartan K, Zibdah M, Ahmed Y, Israel A, Brickner I, Abelson A (2010) Macroalgae in the coral reefs of Eilat (Gulf of Aqaba, Red Sea) as a possible indicator of reef degradation. Mar Pollut Bull 60:759–764
Bauer K, Díez B, Lugomela C, Seppälä S, Borg AJ, Bergman B (2008) Variability in benthic diazotrophy and cyanobacterial diversity in a tropical intertidal lagoon. FEMS Microbiol Ecol 63:205–221
Beentjes KK, Speksnijder AGCL, Schilthuizen M, Hoogeveen M, Pastoor R, van der Hoorn BB (2019) Increased performance of DNA metabarcoding of macroinvertebrates by taxonomic sorting. PLoS ONE 14:e0226527
Bender D, Diaz-Pulido G, Dove S (2014) Warming and acidification promote cyanobacterial dominance in turf algal assemblages. Mar Ecol Prog Ser 517:271–284
Bessell-Browne P, Fisher R, Duckworth A, Jones R (2017) Mucous sheet production in Porites: an effective bioindicator of sediment related pressures. Ecol Indic 77:276–285
Bolhuis H, Cretoiu MS, Stal LJ (2014) Molecular ecology of microbial mats. FEMS Microbiol Ecol 90:335–350
Borcard D, Gillet F, Legendre P (2018) Numerical ecology with R, sec. edition
Brislawn CJ, Graham EB, Dana K, Ihardt P, Fansler SJ, Chrisler WB, Cliff JB, Stegen JC, Moran JJ, Bernstein HC (2019) Forfeiting the priority effect: turnover defines biofilm community succession. ISME J 13:1865–1877
Brocke HJ, Polerecky L, De Beer D, Weber M, Claudet J, Nugues MM (2015) Organic matter degradation drives benthic cyanobacterial mat abundance on Caribbean coral reefs. PLoS ONE 10:e0125445
Brocke HJ, Piltz B, Herz N, Abed RMM, Palinska KA, John U, den Haan J, de Beer D, Nugues MM (2018) Nitrogen fixation and diversity of benthic cyanobacterial mats on coral reefs in Curaçao. Coral Reefs 37:861–874
Cardini U, Bednarz VN, Foster RA, Wild C (2014) Benthic N2 fixation in coral reefs and the potential effects of human-induced environmental change. Ecol Evol 4:1706–1727
Cardoso DC, Sandionigi A, Cretoiu MS, Casiraghi M, Stal L, Bolhuis H (2017) Comparison of the active and resident community of a coastal microbial mat. Sci Rep 7:2969
Cardoso DC, Cretoiu MS, Stal LJ, Bolhuis H (2019) Seasonal development of a coastal microbial mat. Sci Rep 9:9035
Cervino JM, Thompson FL, Gomez-Gil B, Lorence EA, Goreau TJ, Hayes RL, Winiarski-Cervino KB, Smith GW, Hughen K, Bartels E (2008) The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J Appl Microbiol 105:1658–1671
Charpy L, Alliod R, Rodier M, Golubic S (2007) Benthic nitrogen fixation in the SW New Caledonia lagoon. Aquat Microb Ecol 47:73–81
Charpy L, Palinska KA, Casareto B, Langlade MJ, Suzuki Y, Abed RMM, Golubic S (2010) Dinitrogen-fixing cyanobacteria in microbial mats of two shallow coral reef ecosystems. Microb Ecol 59:174–186
Charpy L, Palinska KA, Abed RMM, Langlade MJ, Golubic S (2012) Factors influencing microbial mat composition, distribution and dinitrogen fixation in three western Indian Ocean coral reefs. Eur J Phycol 47:51–66
Cissell EC, McCoy SJ (2020) Shotgun metagenomic sequencing reveals the full taxonomic, trophic, and functional diversity of a coral reef benthic cyanobacterial mat from Bonaire, Caribbean Netherlands. Sci Total Environ 755:142719
Cissell EC, McCoy SJ (2022) Marine cyanobacteria in the anthropocene: Are top-down paradigms robust to climate change? Clim Chang Ecol 3:100057
Cissell EC, Manning JC, McCoy SJ (2019) Consumption of benthic cyanobacterial mats on a Caribbean coral reef. Sci Rep 9:12693
Cissell EC, Eckrich CE, McCoy SJ (2022) Cyanobacterial mats as benthic reservoirs and vectors for coral black band disease pathogens. Ecol Appl 32(6):e2692. https://doi.org/10.1002/eap.2692
Cleary DFR, Polónia ARM, Huang YM, Putchakarn S, Gomes NCM, de Voogd NJ (2019) A comparison of prokaryote communities inhabiting sponges, bacterial mats, sediment and seawater in Southeast Asian coral reefs. FEMS Microbiol Ecol 95:fiz169
Clements KD, German DP, Piché J, Tribollet A, Choat JH (2017) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc 120:729–751
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the Cytophaga-flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66:1692–1697
Croce ME, Gauna MC, Fernández C, Poza AM, Parodi ER (2021) Biology and ecology of the benthic algae. In: Fiori SM, Pratolongo PD (eds) The Bahía Blanca Estuary. Springer, Cham, pp 113–151
de Bakker DM, van Duyl FC, Bak RPM, Nugues MM, Nieuwland G, Meesters EH (2017) 40 Years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 36:355–367
de Voogd NJ, Cleary DFR, Dekker F (2013) The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 32:755
den Haan J, Visser PM, Ganase AE, Gooren EE, Stal LJ, van Duyl FC, Vermeij MJA, Huisman J (2014) Nitrogen fixation rates in algal turf communities of a degraded versus less degraded coral reef. Coral Reefs 33:1003–1015
Diaz-Pulido G, McCook LJ (2002) The fate of bleached corals: patterns and dynamics of algal recruitment. Mar Ecol Prog Ser 232:115–128
Edgar RC (2016) UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 081257. https://doi.org/10.1101/081257
Fisher R, O’Leary RA, Low-Choy S, Mengersen K, Knowlton N, Brainard RE, Caley MJ (2015) Species richness on coral reefs and the pursuit of convergent global estimates. Curr Biol 25:500–505
Ford AK, Bejarano S, Nugues MM, Visser PM, Albert S, Ferse SCA (2018) Reefs under siege—the rise, putative drivers, and consequences of benthic cyanobacterial mats. Front Mar Sci 5:18
Ford AK, Visser PM, van Herk MJ, Jongepier E, Bonito V (2021) First insights into the impacts of benthic cyanobacterial mats on fish herbivory functions on a nearshore coral reef. Sci Rep 11:7147
Frias-Lopez J, Bonheyo GT, Jin Q, Fouke BW (2003) Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Appl Environ Microbiol 69:2409–2413
Fromont J, Richards ZT, Wilson NG (2019) First report of the coral-killing sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A new threat to Kimberley coral reefs? Diversity 11:184
Fuhrman JA, Schwalbach MS, Stingl U (2008) Proteorhodopsins: an array of physiological roles? Nat Rev Microbiol 6:488–494
Gantar M, Sekar R, Richardson LL (2009) Cyanotoxins from black band disease of corals and from other Coral Reef environments. Microb Ecol 58:856–864
Golubic S, Abed RMM, Palińska K, Pauillac S, Chinain M, Laurent D (2010) Marine toxic cyanobacteria: diversity, environmental responses and hazards. Toxicon 56:836–841
Gorgula SK, Connell SD (2004) Expansive covers of turf-forming algae on human-dominated coast: the relative effects of increasing nutrient and sediment loads. Mar Biol 145:613–619
Guidi-Rontani C, Jean MR, Gonzalez-Rizzo S, Bolte-Kluge S, Gros O (2014) Description of new filamentous toxic Cyanobacteria (Oscillatoriales) colonizing the sulfidic periphyton mat in marine mangroves. FEMS Microbiol Lett 359:173–181
Guiry MD, Guiry GM (2020) AlgaeBase. https://www.algaebase.org
Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 20:172–195
Hoeksema BW, Koh EG (2009) Depauperation of the mushroom coral fauna (Fungiidae) of Singapore (1860s–2006) in changing reef conditions. Raffles Bull Zool Suppl 22:91–101
Hoeksema BW, Matthews JL (2011) Contrasting bleaching patterns in mushroom coral assemblages at Koh Tao, Gulf of Thailand. Coral Reefs 30:95
Hoeksema BW, Scott C, True JD (2013) Dietary shift in corallivorous Drupella snails following a major bleaching event at Koh Tao, Gulf of Thailand. Coral Reefs 32:423–428
Hudatwi M, Syari IA, Utami E, Nugraha MA, Akhrianti I, Pamungkas A (2021) Diversity of benthic organisms on artificial reef structure. IOP Conf Ser Earth Environ Sci 926:012033
Hughes T, Baird A, Bellwood D, Card M, Connolly S, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson J, Kleypas J, Lough J, Marshall P, Nyström M, Palumbi S, Pandolfi J, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Hughes T, Barnes M, Bellwood D, Cinner J, Cumming G, Jackson J, Kleypas J, Van De Leemput I, Lough J, Morrison T, Palumbi S, Van Nes E, Scheffer M (2017) Coral reefs in the Anthropocene. Nature 546:82–90
Humann P, Deloach N (2013) Reef coral identification: Florida, Caribbean. Bahamas. Third Edition. New World Publications, Jacksonville, Florida
Kaebernick M, Neilan BA (2001) Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol Ecol 35:1–9
Kardinaal W, Edwin A, Janse I, Kamst-van Agterveld M, Meima M, Snoek J, Mur LR, Huisman J, Zwart G, Visser PM (2007) Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquat Microb Ecol 48:1–12
Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M (2012) Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7:e47363
Koblížek M (2015) Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev 39:854–870
Koh EGL (1997) Do scleractinian corals engage in chemical warfare against microbes? J Chem Ecol 23:379–398
Kuffner IB, Paul VJ (2001) Effects of nitrate, phosphate and iron on the growth of macroalgae and benthic cyanobacteria from Cocos Lagoon, Guam. Mar Ecol Prog Ser 222:63–72
Kuffner IB, Paul VJ (2004) Effects of the benthic cyanobacterium Lyngbya majuscula on larval recruitment of the reef corals Acropora surculosa and Pocillopora damicornis. Coral Reefs 23:455–458
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Kurmayer R, Christiansen G, Fastner J, Börner T (2004) Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environ Microbiol 6:831–841
Lamb JB, True JD, Piromvaragorn S, Willis BL (2014) Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol Conserv 178:88–96
Laurent D, Kerbrat AS, Darius HT, Girard E, Golubic S, Benoit E, Sauviat MP, Chinain M, Molgo J, Pauillac S (2008) Are cyanobacteria involved in Ciguatera Fish Poisoning-like outbreaks in New Caledonia? Harmful Algae 7:827–838
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Littler DS, Littler MM (2000) Caribbean reef plants. An identification guide to the reef plants of the Caribbean, Bahamas, Florida and Gulf of Mexico. Offshore Graphics. Inc., Washington, DC, 542. Offshore Graphics, Washington
Loh TL, McMurray SE, Henkel TP, Vicente J, Pawlik JR (2015) Indirect effects of overfishing on Caribbean reefs: Sponges overgrow reef-building corals. PeerJ 3:e901
Luna GM, Bongiorni L, Gili C, Biavasco F, Danovaro R (2010) Vibrio harveyi as a causative agent of the White Syndrome in tropical stony corals. Environ Microbiol Rep 2:120–127
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17(1):10–12
McManus JW, Polsenberg JF (2004) Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr 60:263–279
Méjean A, Peyraud-Thomas C, Kerbrat AS, Golubic S, Pauillac S, Chinain M, Laurent D (2010) First identification of the neurotoxin homoanatoxin-a from mats of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly linked to giant clam poisoning in New Caledonia. Toxicon 56:829–835
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233
Moerland MS, Scott CM, Hoeksema BW (2019) Prey selection of corallivorous muricids at Koh Tao (Gulf of Thailand) four years after a major coral bleaching event. Contrib Zool 85:291–309
Montano S, Strona G, Seveso D, Galli P (2013) Prevalence, host range, and spatial distribution of black band disease in the Maldivian Archipelago. Dis Aquat Organ 105:65–74
Munro C (2005) Diving systems. In: Eleftheriou A, McIntyre A (eds) Methods for the study of marine benthos, 3rd edn. Blackwell Science, Oxford, pp 112–159
Nagle DG, Gerwick WH (1995) Nakienones A-C and nakitriol, new cytotoxic cyclic C11metabolites from an Okinawan cyanobacterial (Synechocystis sp.) overgrowth of coral. Tetrahedron Lett 36:849–852
NCBI (2021) NCBI Nucleotide Basic Local Alignment Search Tool. https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome. Accessed on 21–12–2020
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB (2013) Package vegan. R Packag ver
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Oren A, Ventura S (2017) The current status of cyanobacterial nomenclature under the “prokaryotic” and the “botanical” code. Antonie Van Leeuwenhoek. Int J Gen Mol Microbiol 110:1257–1269
Osborne NJ, Webb PM, Shaw GR (2001) The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int 27:381–392
Paerl HW, Pinckney JL (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol 31:225–247
Palińska KA, Abed RMM, Charpy L, Langlade MJ, Beltrán-Magos Y, Golubic S (2015) Morphological, genetic and physiological characterization of Hydrocoleum, the most common benthic cyanobacterium in tropical oceans. Eur J Phycol 50:139–154
Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol 18:1403–1414
Phongsuwan N, Chankong A, Yamarunpatthana C, Chansang H, Boonprakob R, Petchkumnerd P, Thongtham N, Paokantha S, Chanmethakul T, Panchaiyapoom P, Bundit OA (2013) Status and changing patterns on coral reefs in Thailand during the last two decades. Deep Res Part II Topol Stud Oceanogr 96:19–24
Prado S, Romalde JL, Montes J, Barja JL (2005) Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. first description of Vibrio neptunius as an oyster pathogen. Dis Aquat Organ 67:209–215
Puyana M, Prato J (2013) Overgrowth of reef organisms by benthic cyanobacteria in the Colombian Caribbean. Rev Mutis 3:58–60
Qiagen (2017) QIAxcel advanced user manual
Qiagen (2018) QIAgilityTM user manual
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160
Rao CR (1995) A review of canonical coordinates and an alternative to correspondence analysis using Hellinger distance. Qüestii 19:23–63
Richardson LL (1997) Occurrence of the black band disease cyanobacterium on healthy corals of the Florida keys. Bull Mar Sci 61:485–490
Richardson LL, Miller AW, Broderick E, Kaczmarsky L, Gantar M, Stanić D, Sekar R (2009) Sulfide, microcystin, and the etiology of black band disease. Dis Aquat Organ 87:79–90
Ritson-Williams R, Paul VJ, Bonito V (2005) Marine benthic cyanobacteria overgrow coral reef organisms. Coral Reefs 24:629
Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S, Wu D, Eisen JA, Hoffman JM, Remington K, Beeson K, Tran B, Smith H, Baden-Tillson H, Stewart C, Thorpe J, Freeman J, Andrews-Pfannkoch C, Venter JE, Li K, Kravitz S, Heidelberg JF, Utterback T, Rogers YH, Falcón LI, Souza V, Bonilla-Rosso G, Eguiarte LE, Karl DM, Sathyendranath S, Platt T, Bermingham E, Gallardo V, Tamayo-Castillo G, Ferrari MR, Strausberg RL, Nealson K, Friedman R, Frazier M, Venter JC (2007) The Sorcerer II global ocean sampling expedition: Northwest Atlantic through eastern tropical Pacific. PLoS Biol 5:e77
Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM, Konotchick T, Malay M, Maragos JE, Obura D, Pantos O, Paulay G, Richie M, Rohwer F, Schroeder RE, Walsh S, Jackson JBC, Knowlton N, Sala E (2008) Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3:e1548
Sato Y, Civiello M, Bell SC, Willis BL, Bourne DG (2016) Integrated approach to understanding the onset and pathogenesis of black band disease in corals. Environ Microbiol 18:752–765
Schmieder R, Edwards R (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864
Schmitt EF, Sluka RD, Sullivan-Sealey KM (2002) Evaluating the use of roving diver and transect surveys to assess the coral reef fish assemblage off southeastern Hispaniola. Coral Reefs 21:216–223
Scott C, Mehrotra R, Cabral M, Arunrugstichai S (2017a) Changes in hard coral abundance and composition on Koh Tao, Thailand, 2006–2014. Coast Ecosyst 4:26–38
Scott CM, Mehrotra R, Hein MY, Moerland MS, Hoeksema BW (2017b) Population dynamics of corallivores (Drupella and Acanthaster) on coral reefs of Koh Tao, a diving destination in the Gulf of Thailand. Raffles Bull Zool 65:68–79
Sonnenschein EC, Nielsen KF, D’Alvise P, Porsby CH, Melchiorsen J, Heilmann J, Kalatzis PG, López-Pérez M, Bunk B, Spröer C, Middelboe M, Gram L (2017) Global occurrence and heterogeneity of the Roseobacter-clade species Ruegeria mobilis. ISME J 11:569–583
Suzuki R, Shimodaira H (2013) pvclust : an R package for hierarchical clustering with p-values. Bioinformatics
Tee HS, Wood SA, Bouma-Gregson K et al (2021) Genome streamlining, plasticity, and metabolic versatility distinguish co-occurring toxic and nontoxic cyanobacterial strains of Microcoleus. Mbio 12:e0223521
Thacker RW, Paul VJ (2004) Morphological, chemical, and genetic diversity of tropical marine cyanobacteria Lyngbya spp. and Symploca spp. (Oscillatoriales). Appl Environ Microbiol 70:3305–3312
Thermofisher Scientific (2017a) Ion PGM TM Hi - Q TM View OT2 User guide
Thermofisher Scientific (2017b) Ion PGMTM Hi-QTM View Sequencing Kit USER GUIDE
Turicchia E, Hoeksema BW, Ponti M (2018) The coral-killing sponge Chalinula nematifera as a common substrate generalist in Komodo National Park, Indonesia. Mar Biol Res 14:827–833
van der Ent E, Hoeksema BW, de Voogd NJ (2016) Abundance and genetic variation of the coral-killing cyanobacteriosponge Terpios hoshinota in the Spermonde Archipelago, SW Sulawesi, Indonesia. J Mar Biol Assoc UK 96:453–463
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Wiegand S, Jogler M, Jogler C (2018) On the maverick Planctomycetes. FEMS Microbiol Rev 42:739–760
Wong HL, Smith DL, Visscher PT, Burns BP (2015) Niche differentiation of bacterial communities at a millimeter scale in Shark Bay microbial mats. Sci Rep 5:15607
Wongthong P, Harvey N (2014) Integrated coastal management and sustainable tourism: a case study of the reef-based SCUBA dive industry from Thailand. Ocean Coast Manag 95:138–146
Wood SA, Kelly LT, Bouma-Gregson K, Humbert JF, Laughinghouse HD, Lazorchak J, McAllister TG, McQueen A, Pokrzywinski K, Puddick J, Quiblier C (2020) Toxic benthic freshwater cyanobacterial proliferations: challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw Biol 65:1824–1842
Yang SY, Chen HJ, Ho MJ, Chen YJ, Huang YY, Chow WS, Tang SL, Jeng MS, Chen CA (2018) Outbreak of coral-killing cyanobacteriasponge, Terpios hoshinota, in Taiping Island (Itu Aba), Spratlys, South China Sea. Bull Mar Sci 94:1543–1544
Yeemin T, Sutthacheep M, Pettongma R (2006) Coral reef restoration projects in Thailand. Ocean Coast Manag 49:562–575
Acknowledgements
We are grateful to the Conservation Diver team at Koh Tao, J. Gijsbers Alejandre and S. Oosterbroek for their assistance and material supply during the field campaign. The project was funded by Naturalis Biodiversity Center, Leiden, with additional funding from the Jan-Joost ter Pelkwijkfonds and the Alida M Buitendijkfonds. While writing the article, the first author was supported through 4D-REEF, a European Union's Horizon 2020 research and innovation programme, under the Marie Skłodowska-Curie grant agreement No 813360. We want to thank two anonymous reviewers for their constructive comments which helped us to improve our manuscript.
Author information
Authors and Affiliations
Contributions
BWH, PMV, RM and TMS designed the study; TMS performed fieldwork and labwork; BWH, RM and AGCLS provided infrastructure/material/technical support; DFRC and TMS analyzed the data; and TMS, DFRC, NCMG, RM, PMV, AGCLS and BWH contributed to the writing, reviewing, and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there is no conflict of interest in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stuij, T.M., Cleary, D.F.R., Gomes, N.C.M. et al. High diversity of benthic cyanobacterial mats on coral reefs of Koh Tao, Gulf of Thailand. Coral Reefs 42, 77–91 (2023). https://doi.org/10.1007/s00338-022-02304-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02304-0