Abstract
Gut microbiota play a fundamental role in the nutrition of many vertebrate herbivores through foregut and hindgut fermentation of plant carbohydrates. Some species of marine herbivorous fishes contain moderate to high levels of short-chain fatty acids in the hindgut, indicating the importance of hindgut fermentation. Herbivorous fish hindgut microbiota are diverse and can vary with geographic location, but data on the scale of geographic variation involving a few km of separation are limited. Here, we used the 16S rRNA gene to describe community composition of the gut microbiota of the herbivorous species Kyphosus vaigiensis and K. cinerascens collected in the vicinity of Lizard Island, northern Great Barrier Reef, Australia, in 2011 and 2017. Microbiota community structure differed between posterior hindgut sections, host species, sampling years and two mid-shelf and outer reef locations approximately 20 km apart. Hindgut bacterial community composition varied remarkably between mid-shelf and outer reef locations, and among individual fish on the mid-shelf reef. In both fish species, the most abundant phyla were Pseudomonadota, Bacillota and Bacteroidota, followed by Spirochaetota, Thermodesulfobacteriota and Verrucomicrobiota. There were no clear differences between the host species in terms of the relative abundance and composition of bacterial genera in outer reef samples. In contrast, the dominant genera differed between mid-shelf samples of K. cinerascens and K. vaigiensis, being Endozoicomonas-like (Pseudomonadota) and Brevinema (Spirochaetota), respectively. Endozoicomonas are emerging as important symbionts in many marine hosts worldwide and are thought to be important in the coral sulphur cycle. Differences in microbiota composition were not associated with variation in fish condition, suggesting that the different microbial taxa perform equivalent functional roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gut-associated bacteria play fundamental roles in metazoan physiology (Lee and Hase 2014) and nutrition (Stevens and Hume 1998), such as contributing to the digestion of plants in many herbivores (Choat and Clements 1998; Greene et al. 2020). Algal carbohydrates represent a major source of potential energy for marine herbivorous fishes, which different fish taxa exploit to varying degrees (Krogdahl et al. 2005; Clements et al. 2014, 2017). For example, some taxa including mugilids, scarine parrotfish and some grazing acanthurids employ mechanical trituration in a pharyngeal mill or gizzard-like stomach and depend mainly on rapid gut throughput and digestion of protein, lipid and soluble carbohydrate (Clements et al. 2014). Other marine herbivorous fish taxa, e.g. kyphosids, odacine labrids, aplodactylids and nasine acanthurids, have lengthier gut retention times and rely on gastrointestinal fermentation to salvage energy from refractory carbohydrate (Choat and Clements 1998; Mountfort et al. 2002). Given the algae-rich diet of the latter group of fish taxa, carbohydrate fermentation represents a key process in the marine environment (Choat and Clements 1998). Short-chain fatty acid (SCFA) profiles from the gut of species of the families Acanthuridae, Siganidae, Pomacanthidae and Kyphosidae suggest that the anaerobic microbial populations inhabiting the intestine of these species play a significant role in the nutritional ecology of the host (Clements and Choat 1995; Clements et al. 2009). These fish species contain the highest level of SCFAs in posterior gut sections, also defined as sections IV and V of the intestine (Clements and Choat 1995, 1997), indicating that hindgut sections have the highest levels of microbial fermentation.
Fish gut microbial communities can be highly diverse (Wang et al. 2018) and differ among fish species in taxonomic composition (Sullam et al. 2012). Three bacterial phyla, Pseudomonadota, Fusobacteriota and Bacillota (formerly Proteobacteria, Fusobacteria and Firmicutes, respectively), generally dominate the gut microbiota of fish (Tarnecki et al. 2017), with Vibrionales tending to be the most abundant order in many marine fish taxa (Sullam et al. 2012). Trophic level is also known to play a role in shaping the gut microbiota (Sullam et al. 2012). Although there are remarkable differences among trophic levels in fish gut community composition, common microbial taxa are present in carnivores, omnivores and zooplanktivores (Egerton et al. 2018). For example, the family Vibrionaceae, and the genera Aeromonas and Pseudomonas, are frequently reported in fish gut microbiome studies (Egerton et al. 2018), regardless of fish trophic level.
Dietary composition and geographic location influence microbial community composition in these fish gut communities (Tarnecki et al. 2017; Parata et al. 2020). In general, four processes, namely environmental selection, historical contingency, ecological drift and dispersal, interact with each other in different ways to influence microbial community assembly in the host gut (Costello et al. 2012). These processes can all lead to geographic variation in gut microbial community composition (Sullam et al. 2012; Taranaki et al. 2017; Jones et al. 2018; Senghor et al. 2018; Arroyo et al. 2019). Despite our increasing knowledge of fish gut microbiota, we still lack information on the influence of geographic location on gut community composition, and whether such variation influences community function, such as digestion of foods including seaweed and its constituent carbohydrates.
The family Kyphosidae has a worldwide tropical marine distribution and also occurs in temperate Australasia (Knudsen et al. 2019). Herbivorous Kyphosus spp. display high concentrations of SCFA in the hindgut (Clements and Choat 1995, 1997; Choat and Clements 1998; Clements et al. 2017), indicating that gut microbial communities play an important role in nutrient assimilation from seaweeds. The tropical Kyphosus cinerascens and K. vaigiensis display some of the highest levels of carbohydrate fermentation products among other coral reef associated herbivorous fishes and related omnivorous and planktivorous taxa (Clements et al. 2017). However, levels of SCFA do not differ between the posterior gut segments IV and V in K. vaigiensis, whereas in K. cinerascens section V has the highest levels of SCFA in the gut (Clements and Choat 1995, 1997). Most Kyphosus species have specialized gut anatomy associated with microbial fermentation (Clements and Choat 1997; Johnson and Clements 2021); however, the high level of SCFAs is not restricted to distinct hindgut structures (i.e. hindgut chamber) (Clements and Choat, 1997). In K. vaigiensis, the terminal part of the intestine (section V) forms a hindgut chamber or caecal pouch (Rimmer and Wibe 1987); this feature is absent in K. cinerascens (Clements 1997). The two species also exhibit differences in diet. Previous studies conducted at Lizard Island, Australia, show that Kyphosus vaigiensis has a diet dominated by phaeophytes, while K. cinerascens consumes a high proportion of rhodophytes (Clements and Choat 1997; Choat et al. 2002).
The present study used 16S rRNA gene sequences to characterize hindgut microbial community composition in K. cinerascens and K. vaigiensis collected in 2011 and 2017 from two locations in the vicinity of Lizard Island, Great Barrier Reef, Australia. We sought to determine the differences in community composition between: (1) posterior hindgut sections IV and V, (2) the two Kyphosus species, and (3) mid-shelf reef and outer reef locations separated by approximately 20 kms.
Material and methods
Sample collection
Adult specimens of K. cinerascens (n = 10) and K. vaigiensis (n = 8) were collected by spear gun while snorkelling from the northern Great Barrier Reef, Australia (14 40ʹ S, 145 28ʹ E). Fish were collected from two locations, the Lizard Island complex (mid-shelf reef) and the adjacent outer reefs (Fig. 1 and Table 1) during March 2011 and December 2017 with some crossover between years and locations (Supplementary Table 1). Fish were transported on ice to the laboratory at the Lizard Island Research Station (LIRS). Fish length and weight were recorded for each specimen, and intestinal content was collected from posterior gut sections IV and V following Choat et al. (2002). Gut content (digested and partially digested material in the lumen) samples were fixed in 80% ethanol and shipped to the University of Auckland, New Zealand, for further analysis in PC1 laboratories following standard, clean laboratory procedures.
DNA extraction
Sufficient DNA for sequencing was obtained from 16 samples each of gut sections IV and V (see Table 1). Prior to DNA extraction, the 80% ethanol-fixed samples were pelleted for three minutes at 20,800 × g (Eppendorf 5804 centrifuge, Hamburg, Germany) and supernatant was removed. DNA extractions were carried out according to the DNeasy PowerSoil DNA Isolation Kit (Qiagen, Hilden, Germany) manufacturer’s instructions.
PCR amplification and sequencing
The hypervariable V3-V4 region of the 16S rRNA gene was amplified from the hindgut samples. For the 2011 samples, PCR amplification was performed using the following Illumina tagged and barcoded primers: 341F (5′-CCTACGGGNGGCWGCAG-3′) forward (Herlemann et al. 2011) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) reverse (Caporaso et al. 2011). For the 2017 samples, the forward primer was the same, while the reverse primer was changed to 785R (5′-GACTACHVGGGTATCTAATCC-3′) (Herlemann et al. 2011). The primer set in 2017 was changed as the 341F/785R primer pair was suggested to yield improved coverage of bacterial taxa by Klindworth et al. 2013. PCR reactions (25 μL) for the 2011 samples were carried out using 1 × HotStarTaq Plus Master Mix in triplicate (Qiagen), and for the 2017 samples using 2 × KAPA HiFi HotStart (KAPA Biosystems, Woburn, USA) (single reaction per sample). For the 2011 samples, thermocycling conditions consisted of an initial denaturation step of 5 min at 94 °C, followed by 30 cycles of 94 °C for 30 s, 50 °C for 45 s, 68 °C for 90 s, and a final extension of 10 min at 68 °C. For the 2017 samples, the denaturation step was 3 min at 95 °C, followed by 25 cycles of 95 °C for 30 s 55 °C for 30 s, 72 °C for 30 s, and a final extension of 5 min at 72 °C. For the 2011 samples, the resulting amplicons were cleaned using ZR-96 DNA Clean and Concentrator-5 (Deep Well) kits (Zymo Research), and amplicons from the 2017 samples were cleaned using Agencourt AMPure XP magnetic beads (Beckman Coulter, CA, USA).
In silico PCR testing of both primer sets (2011 and 2017) against the SILVA database 138 (as used for taxonomic assignment) resulted in only a 0.55% difference in the number of organisms (reference sequences) targeted relative to the size of the entire SILVA database, indicating that the 2011 and 2017 primer sets yield highly similar results. Only 5 taxa differed between the two primer sets, all of which were members of phylum Chloroflexota class Anaerolineae (genera OLB13, Thermomarinilinea, Bellilinea, ADurb.Bin120, and RBG-16–58-14). For both sets of samples, library preparation and 2 × 300 bp paired end sequencing was performed using the Illumina MiSeq platform with V3 chemistry at Auckland Genomics (University of Auckland, NZ).
16S rRNA gene amplicon analysis
For both set of samples, raw sequences were filtered using Cutadapt v3.3 (Marcel 2011; Callahan et al. 2016) to remove primers and adapters. The following analysis was carried out in R v4.0.1 using DADA2 pipeline v1.16.0 (Callahan et al. 2016). Sequencing reads were quality filtered and trimmed using filterAndTrim, dereplicated using derepFastq and merged using mergePairs. The amplicon sequence variant (ASV) table was generated using makeSequenceTable and chimeras removed using RemoveBimeraDenovo command. Taxonomy of ASVs was assigned using DADA2 (Callahan et al. 2016) with SILVA SSU Ref NR99 v138 database (Quast et al. 2013). Finally, sequences were rarefied to 1234 with QIIME v2020.6 (Bokulich et al. 2018), which was the overall sample minimum value, leaving 2875 ASVs across samples. Phyla taxonomy was revaluated and updated using the updated NCBI phylum taxonomy (Oren and Garrity 2021).
Fish and microbial community analysis
The length–weight relationship (LWR) for each fish species was calculated following Ricker (1973). QGIS 3.2 Bonn (QGIS Development Team, 2018) was used to differentiate the fish collection locations. Hindgut microbial community and alpha diversity indices, observed ASVs, Chao1, Shannon (Shannon and Weaver 1949) and Pielou’s evenness (Pielou 1969), were plotted using R v4.0.1 with Rstudio software version 1.2.1335 and R packages ggplot2 v3.3.5 and vegan v2.5–7. Bacterial community beta diversity was plotted with nonmetric multidimensional scaling (NMDS) of weighted UniFrac distances (Lozupone and Knight 2005). Statistical analysis of alpha diversity indices and NMDS was performed in R using analysis of variance (ANOVA) and permutational multivariate analysis of variance (PERMANOVA, through adonis2), respectively.
Endozoicomonas phylogenetic tree
A phylogenetic tree was constructed using the 18 ASV Endozoicomonas-like sequences and three Actinobacter ASV sequences (used as an outgroup to root the tree) recovered from this study and 14 reference sequences of full length 16S rRNA recovered from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Taxonomy of ASVs was annotated based on the SILVA SSU Ref NR99 v138 database (Quast et al. 2013). Sequences were aligned with MUSCLE using Geneious (v2020.05, https://www.geneious.com). 16S full-length amplicons were trimmed to increase the quality of the alignment with the regions V3-V4 of the ASVs. A maximum-likelihood phylogenetic tree was built using FastTree v2.1.10-gimkl-2018b (Price et al. 2009, 2010). Finally, the tree was visualized using iTOL (Letunic and Bork 2021).
Results
Hindgut microbiota composition
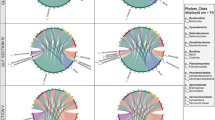
Based on 16S rRNA gene sequence data, the relatively dominant phyla, on average, across most fish were Pseudomonadota, Bacillota and Bacteroidota, followed by less abundant phyla that included Spirochaetes, Thermodesulfobacteriota and Verrucomicrobiota (Fig. 2). The dominant bacterial taxa in Kyphosus species differed between the two geographic areas and gut sections (Fig. 2). In the mid-shelf reef, Pseudomonadota were the most abundant phylum in fish hindgut sections IV (72.8 ± 7.7%, mean ± SE) and V (45.7 ± 13.4%, mean ± SE) of K. cinerascens, and in section V (56.3 ± 7.1%, mean ± SE) of K. vaigiensis. In contrast, in the outer reef, Bacillota were highly abundant in the communities of both sections IV (38.3 ± 5.1%, mean ± SE) and V (51.7 ± 7.7%, mean ± SE) of K. vaigiensis and in section IV (52.8 ± 12.4%, mean ± SE) of K. cinerascens. While Thermodesulfobacteriota were not the most abundant members of the hindgut communities, they comprised up to 5% of some communities. They tended to be more abundant in section IV than V of both fish species, regardless of location fish were collected, whereas Spirochaetes exhibited the greatest difference between fish species, as they tended to be more abundant in K. vaigiensis from the mid-shelf reef, particularly in section IV (40.7 ± 16.7%, mean ± SE).
From left to right: photographs of Kyphosus study species; illustrations of their main dietary components: Laurencia majuscula, Sphacelaria tribuloides and Cladophora rugulosa for Kyphosus cinerascens and Udotea argentea and Turbinaria ornata for K. vaigiensis (following Choat et al. 2002); gastrointestinal tract morphology and sampling regions for the two study species; and mean ASVs relative abundances for sections IV and V from mid-shelf and outer reef samples of each fish species
In K. cinerascens from mid-shelf reef Endozoicomonas-like sequences (Pseudomonadota) were numerically dominant in section IV of all fish studied, where they comprised over 50% of each community (all 2017 samples), and up to 85.2% in sample G184IV (Fig. 3). They were also very abundant in the corresponding section V communities (also 2017 samples), but strikingly less abundant overall in the outer reef samples of the same fish species, regardless of year sampled (2011 or 2017). Gut communities of K. cinerascens from the outer reef exhibited no clear trends in microbial community composition and varied considerably between individual fish. For example, the most abundant genera were Macellibacteroides in section IV sample M96IV (53.6%), and Enterovibrio in section V sample M235V (78.9%), yet their relative abundance was very low in the other samples from the same gut sections (Fig. 3).
Relative abundance of the 25 most abundant bacterial genera in gut sections IV and V of K. cinerascens and K. vaigiensis from mid-shelf and outer reef samples. Data is based on 16S rRNA gene ASVs, and relative abundances are summed to genus level or the nearest higher rank. Labels with M = samples collected in 2011; those with G = 2017
Remarkably, the Endozoicomonas-like genus was also very abundant (61.5%) in one individual of K. vaigiensis (section V, mid-shelf reef). The gut microbial community was highly distinct between K. vaigiensis collected at mid-shelf reef (section V) and outer reef (sections IV and V samples) locations, and the prevalent gut taxa were Oscillospiraceae and Enterovibrio (Fig. 3). Other prevalent taxa associated with K. vaigiensis were Acinetobacter and Pseudomonas. While not possible to compare differences in sampling location in K. vaigiensis without considering the potential for temporal effects (all but one fish from the mid-shelf reef were from 2017 and both fish from the outer reef were from 2011), we found the genus Brevinema (Spirochaetes) dominated or were prominent in certain groups of samples and not others. In particular, Brevinema dominated section IV gut communities of K. vaigiensis from the mid-shelf reef, but were largely absent from section V samples from the same fish species and year (2017). Brevinema were also notable community members in six out of seven 2011 samples in sections IV and V (all out reef and one of two mid-shelf reef samples), indicating considerable variation across gut sections and time or space.
One possible reason for the difference in microbial composition between fish collected from mid-shelf reef and outer reef sites across two different sampling years could be variation in fish condition, dysbiosis or an aberrant gut microbiota in some of the fish in one of the two locations. However, our data show that mid-shelf reef and outer reef fish all fell on the same length–weight line (R2 = 0.9479 for K. cinerascens and R2 = 0.9423 for K. vaigiensis, Fig. 4), indicating that all fish were in similar condition.
Relationship between length and weight of Kyphosus specimens. K. vaigiensis shown in orange and K. cinerascens in black. Circles represent mid-shelf specimens, and triangles represent outer reef specimens. The length–weight relationship is based on the equation log(W) = log a + b log (L) (a is the intercept and b is the slope of the equation)
Alpha and beta diversity analysis
Weighted UniFrac beta diversity was used to analyse the differences in microbial community structure between hindgut sections, fish species, sampling year and geographic location. Findings indicate that no one factor alone explained all variation, and factors contributing to variation differed between posterior gut sections in the study fish. Again, geographic location (PERMANOVA, p-value < 0.05), sampling years (PERMANOVA, p-value < 0.01) and fish (PERMANOVA, p-value < 0.001) appeared to influence the gut microbiota of section IV in the two fish species, wherein the outer reef samples (which are a mix of 2011 and 2017 and fish species) clustered together, while mid-shelf samples from both fish species formed a discrete cluster (all 2017, Fig. 5). In contrast, community structure in hindgut section V (Fig. 5) was significantly influenced by fish species (PERMANOVA, p-value < 0.01).
To evaluate alpha diversity, we assessed a range of metrics based on 16S rRNA gene ASVs. Diversity indices (Shannon and Pielou) and richness estimates (Chao1, ASVs richness) of combined sections IV and V showed no significant difference between location, time or fish species (Fig. 6a), as also confirmed using ANOVA (p-value > 0.1). Despite sampling different locations and years, microbial community alpha diversity of combined gut sections appeared to be broadly similar (based on ASVs, Fig. 6a). However, when considering genus-level data, where sections IV and V were treated separately (Fig. 3), there is a discernible difference in alpha diversity among samples, particularly in section IV. We therefore also calculated alpha diversity metrics (richness and Shannon index) for each gut section separately based on ASVs (Fig. 6b). These confirmed statistically significant differences in alpha diversity between fish species at each location in section IV across sampling years, but not section V (Fig. 6b, Shannon index). Overall, alpha diversity results revealed similar richness between communities when sections were combined (Fig. 6a), although this was largely due to equivalent community diversity in section V among fish from the two locations. Microbial communities in hindgut section IV were highly distinct due to low alpha diversity in fish from the mid-shelf reef (Fig. 3).
a Boxplots of alpha diversity indices (Pielou’s evenness, Chao1, Richness (ASVs) and Shannon) of combined hindgut sections IV and V; b alpha diversity (ASVs richness and Shannon diversity) of sections IV and V separately. Colours represent K. cinerascens (black) and K. vaigiensis (orange) in mid-shelf and outer shelf reef locations
Endozoicomonas phylogenetic tree
To examine the relationship of fish-associated Endozoicomonas-like sequences to previously described Endozoicomonas, we built a phylogenetic tree with reference sequences of Endozoicomonas from GenBank. The Endozoicomonas-like ASV sequences displayed high similarity and formed a distinct clade to that containing reference sequences of Endozoicomonas (Fig. 7). Accordingly, we designated these Kyphosus-associated ASVs as Endozoicomonas-like throughout our study.
Phylogenetic tree of 18 ASVs of Endozoicomonas-like 16S rRNA sequences recovered in the present study and 14 reference sequences from GenBank of 16S rRNA sequences of Endozoicomonas associated with different marine organisms. The scale bar corresponds to 0.1 substitutions per nucleotide position. 1000 bootstraps (values represented on the tree)
Discussion
Microbiota community composition in this study differed considerably between posterior hindgut sections, fish species, two mid-shelf and outer reef locations approximately 20 km apart, and sampling years. The dominant phyla in both fish species were Pseudomonadota, Bacillota and Bacteroidota, followed by Spirochaetota, Thermodesulfobacteriota and Verrucomicrobiota. No single microbial genus was dominant across individual fish of the same species in the microbial composition of outer reef samples. Conversely, the dominant genera differed between mid-shelf samples of K. cinerascens and K. vaigiensis, being Endozoicomonas (Pseudomonadota) and Brevinema (Spirochaetota), respectively.
Bacillota and/or Pseudomonadota (formerly Firmicutes and Proteobacteria) taxa are often the dominant groups in herbivorous fishes such as surgeonfish (Miyake et al. 2015; Ngugi et al 2017; Nielsen et al. 2017; Parata et al. 2020) and butterflyfish (Clever et al. 2020). Bacillota are associated with high-fibre diets and SCFA production in humans (Nobel et al. 2018), and were associated with carbohydrase production in surgeonfish (Ngugi et al. 2017). Bacillota were the most abundant taxon in the temperate fish species Kyphosus sydneyanus, Odax pullus and Aplodactylus arctidens (Clements et al. 2007), all of which have diets dominated by macroalgae and moderate to high levels of SCFA in the hindgut (Mountfort et al. 2002). Although K. vaigiensis and K. cinerascens both contain high levels of SCFA in the hindgut (Clements et al. 2017), in our present study Bacillota were only dominant in fish collected from outer reef sites.
Spirochaetes were also a notable phylum distinguishing the gut communities in K. vaigiensis from those in K. cinerascens, particularly in section IV from mid-shelf reef. Sparagon et al. (2022) examined the gut microbiota of five Kyphosus individuals collected in Hawaii, and based on their supplementary photo of their Kyphosus specimens we were able to identify three individuals (fish 4, 5 and 8) as K. vaigiensis and one individual (fish 6) as K. cinerascens. The gut microbiota of some of the samples from these fish species reported by Sparagon and colleagues were in some cases similar to the gut microbiome of K. vaigiensis and K. cinerascens in our study (i.e. the presence of the Spirochaete genus Brevinema and the Bacteroidota genus Alistipes). Spirochaetes are often reported as pathogenic in humans (Lee and Hampson. 1994); however, they can play a beneficial role in termites and in other terrestrial herbivores through acetogenesis and dinitrogen fixation (Ohkuma et al. 2015; Tokuda et al. 2018; Blankenchip et al. 2018). Spirochaetes have also been suggested to play an important ecological role in herbivorous fish (Clements et al. 2009; Parata et al. 2020).
A number of studies collectively show that the gut microbial community structure is distinct between species of tropical herbivorous fish (Smriga et al. 2010; Miyake et al. 2015; Jones et al. 2018; Scott et al. 2020). For example, according to Scott et al. 2020, the most abundant phyla in the gut microbiota of the herbivorous fish Acanthurus coeruleus, Acanthurus tractus, Scarus taeniopterus. Sparisoma aurofrenatum and Sparisoma viride were Proteobacteria, Firmicutes and Planctomycetes (now known as Pseudomonadota, Bacillota and Planctomycetota, respectively; Oren and Garrity 2021). While species composition was relatively consistent between these fish species, the relative abundance of each microbial taxonomic group differed strongly (e.g. Sp. aurofrenatum harboured a less diverse intestinal microbiome than Sc. Taeniopterus or A. tractus) (Scott et al. 2020). This indicates considerable functional diversity within coral reef fish, as also suggested by our results (i.e. we found different bacterial taxa within individual fish of the same species, suggesting that different microbial taxa can perform equivalent functional roles). Microbial communities in faecal samples of Acanthurus nigricans were dominated by Pseudomonadota (non-Vibrionaceae), Bacteroidota (formerly Bacteroidetes), and Bacillota, while those in Chlorurus sordidus (now C. spilurus) and Lutjanus bohar were dominated by Pseudomonadota-Vibrionaceae (Smriga et al. 2010). These observations are consistent with findings here, with an overall high abundance of Pseudomonadota, Bacillota and Bacteroidetes in K. cinerascens and K. vaigiensis gut microbial communities. Additionally, we found that the relative abundances of microbial taxa in K. cinerascens and K. vaigiensis were influenced by different factors, including gut sections, host fish species, geographic locations and sampling years. Similarly, Jones et al. (2018) reported that latitudinal gradient influences the gut microbiota of the rabbitfish Siganus fuscescens collected from over 2000 km on the coast of Western Australia.
Variation in community composition of gut microbiota could drive host niche differentiation (Greene et al. 2020). For example, the gut microbiomes in some Madagascar lemurs can vary depending on host diet, gut morphology and geographic location (Greene et al. 2020). Similarly, in our study the dominant genera, Endozoicomonas-like and Brevinema, varied depending on host fish species, hindgut section and location or time (Fig. 3). Both Endozoicomonas and Brevinema were the most abundant groups in butterflyfish on Caribbean coral reefs (Clever et al. 2020), and Brevinema was one of the most prevalent genera in surgeonfish from the Great Barrier Reef (Parata et al. 2020).
Endozoicomonas are emerging as diverse and important symbionts in many marine hosts worldwide (Neave et al. 2016) and were one of the most abundant genera in planktivorous damselfish and cardinalfish collected from Lizard Island (Parris et al. 2016), where the present study was conducted. Endozoicomonas species have been suggested to play a role in the coral sulphur cycle via dimethyl sulfoniopropionate (DMSP) metabolism to produce dimethyl sulphide (DMS) (Tandon et al. 2020). DMSP is also produced in significant quantities by red algae (Dacey et al. 1994), a major dietary component of K. cinerascens (Choat et al. 2002). We examined the phylogenetic relationship of the Endozoicomonas-like sequences in our data set to reference Endozoicomonas sequences from other marine taxa to explore patterns of host-symbiont association. The Endozoicomonas-like ASVs formed a distinct clade to that containing the reference Endozoicomonas sequences, indicating that our ASV sequences comprise a novel K. cinerascens-associated clade that is closely related to Endozoicomonas found in other marine animals.
Differences in populations of gut symbionts of marine herbivorous fish have been detected previously between mid-shelf and outer shelf reef locations on the northern Great Barrier Reef (Arroyo et al. 2019), showing that it is possible that geographic comparisons could be confounded by temporal factors. The Lizard Island Complex and nearby outer reefs were affected by two major cyclones in 2014 and 2015, and a mass bleaching event in 2016 (Madin et al. 2018; Zawada et al. 2019). Our 2011 samples were collected prior to these disturbances, while the 2017 sampling was collected one year after the bleaching event. It is possible that these disturbances influenced community assembly in our gut communities, either through historical contingency, ecological drift or dispersal (Costello et al. 2012), and so we explored this in more detail.
We observed a strong difference in community composition at genus level between sections IV and V in K. vaigiensis from mid-shelf reef (Fig. 3). This could be due to the presence in this species of a hindgut chamber (Clements and Choat 1997). Specimens of Kyphosus cinerascens lack a hindgut chamber, and share broadly similar bacterial taxa between sections IV and V, particularly on the mid-shelf reefs, such as the genus Endozoicomonas-like. This may indicate that the presence of a hindgut chamber that is separated from the proximal intestine by a sphincter increases total microbiota diversity. Community composition also differed markedly between the two host species. This could be due to a diet dominated by green (Udotea argentea) and brown (Turbinaria ornata) algae in K. vaigiensis (Choat et al. 2002) (Fig. 2), and by a mixture of red (Laurencia majuscola), brown (Sphacelaria tribuloides) and green (Cladophora rugulosa) algae in K. cinerascens (Choat et al. 2002) (Fig. 2). Gut morphology and diet have a strong influence on microbial community composition in animals (Ley et al. 2008), especially herbivores (Greene et al. 2020; Escalas et al. 2021). In addition, we observed variation among individual fish in their gut microbiota for both fish species and gut sections, especially in K. cinerascens from the outer reefs and in K. vaigiensis from mid-shelf reefs (Fig. 3). Although individual conspecific fish displayed variation in gut microbial communities, these gut communities should be consistent in overall metabolic function, as both fish species have consistently high levels of SCFA in the hindgut (Clements and Choat 1995, 1997).
The importance of gut microbiota to nutrition in kyphosid fishes (Mountfort et al. 2002; Clements et al. 2014) raised the possibility that highly variant gut communities may influence fish condition and health. We tested this by examining the relationship between length and weight for all of our sampled fish (Fig. 4). Fish that are nutritionally compromised would be expected to weigh less for a given length than fish with a healthy gut microbiota. However, we detected no difference in length/weight between either sampling locations or sampling years. This suggests that the considerable variation in gut microbiome community composition did not influence community function, and that community assembly is labile and driven by factors other than microbiota phylogeny.
This study suggests that spatial variation, even over scales of only tens of kilometres, can influence gut community composition in herbivorous fishes. There is a significant difference in the hindgut microbial community composition in K. cinerascens between mid-shelf and outer-shelf reefs, as seen in Naso tonganus over the same spatial scales (Arroyo et al. 2019), while marked variation in K. vaigiensis may be attributed to either spatial or temporal factors, similarly to the variation in microbial composition observed in S. fuscescens with latitudinal changes in Jones et al. (2018). We also observed considerable interspecific variation in the hindgut microbiota of fish living in the same geographic area at the same time, i.e. outer-shelf reef for K. cinerascens and mid-shelf reef for K. vaigiensis. Our data also suggest that the Endozoicomonas-like genus, which dominated communities in mid-shelf samples of K. cinerascens, most likely makes an important contribution to metabolic function and immune defence, as Endozoicomonas does in corals and other invertebrates (Neave et al. 2016; Tandon et al. 2020; Jensen et al. 2021).
Future work should examine the level of spatial and temporal variation in gut community composition in other herbivorous fishes over larger spatial scales, while taking into account the influence of potential spatial and/or temporal variation in diet. Our study suggests that while there must be strong environmental selection for functionality in these gut communities, this is not strictly tied to particular bacterial taxa, and the effects of variation in many factors including diet and location, and the interaction of different processes including historical contingency, random sampling and dispersal may also influence community assembly (Costello et al. 2012).
Data availability
All raw reads associated with this work have been deposited at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/bioproject/) under BioProject ID: PRJNA783643.
References
Arroyo FA, Pawlowska TE, Choat JH, Clements KD, Angert ER (2019) Recombination contributes to population diversification in the polyploid intestinal symbiont Epulopiscium sp type B. ISME J 13:1084–1097
Blankenchip CL, Michels DE, Braker HE, Goffredi SK (2018) Diet breadth and exploitation of exotic plants shift the core microbiome of Cephaloleia a group of tropical herbivorous beetles. PeerJ 6:e4793
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Caporaso JG (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:1–17
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci 108(Supplement 1):4516–4522
Choat JH, Clements KD (1998) Vertebrate herbivores in marine and terrestrial environments: a nutritional ecology perspective. Annu Rev Ecol Syst 29:375–403
Choat JH, Clements KD, Robbins W (2002) The trophic status of herbivorous fishes on coral reefs. Mar Biol 140:613–623
Clements KD, Choat JH (1995) Fermentation in tropical marine herbivorous fishes. Physiol Zool 68:355–378
Clements KD, Choat JH (1997) Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Mar Biol 127:579–586
Clements KD, Pasch IB, Moran D, Turner SJ (2007) Clostridia dominate 16S rRNA gene libraries prepared from the hindgut of temperate marine herbivorous fishes. Mar Biol 150:1431–1440
Clements KD, Raubenheimer D, Choat JH (2009) Nutritional ecology of marine herbivorous fishes: ten years on. Funct Ecol 23:79–92
Clements KD, Angert ER, Montgomery WL, Choat H (2014) Intestinal microbiota in fishes: what’s known and what’s not. Mol Ecol 23:1891–1898
Clements KD, German DP, Piché J, Tribollet A, Choat JH (2017) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc Lond 120:729–751
Clements KD (1997) Fermentation and Gastrointestinal microorganisms in fishes. In: Mackie R.I., White B.A. (eds) Gastrointestinal Microbiology. Chapman & Hall Microbiology Series. Springer, Boston pp 156–198
Clever F, Sourisse JM, Preziosi RF, Eisen JA, Guerra ECR, Scott JJ, Wilkins LG, Altieri AH, McMillan WO, Leray M (2020) The gut microbiome stability of a butterflyfish is disrupted on severely degraded Caribbean coral reefs. bioRxiv
Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA (2012) The application of ecological theory toward an understanding of the human microbiome. Science 336:1255–1262
Dacey JWH, King GM, Lobel PS (1994) Herbivory by reef fishes and the production of dimethylsulfide and acrylic acid. Mar Ecol Prog Ser 112:67–74
Egerton S, Culloty S, Whooley J, Stanton C, Ross RP (2018) The gut microbiota of marine fish. Front Microbiol 9:873
Escalas A, Auguet JC, Avouac A, Seguin R, Gradel A, Borrossi L, Villéger S (2021) Ecological specialization within a carnivorous fish family is supported by a herbivorous microbiome shaped by a combination of gut traits and specific diet. Front Mar Sci 8:91
Greene LK, Williams CV, Junge RE, Mahefarisoa KL, Rajaonarivelo T, Rakotondrainibe H, O’Connell TM, Drea CM (2020) A role for gut microbiota in host niche differentiation. ISME J 14:1675–1687
Herlemann DRP, Labrenz M, Juergens K, Bertilsson S, Waniek JJ, Anderrson AF (2011) Transition in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J 5:1571–1579
Jensen S, Frank JA, Arntzen MØ, Duperron S, Vaaje-Kolstad G, Hovland M (2021) Endozoicomonadaceae symbiont in gills of Acesta clam encodes genes for essential nutrients and polysaccharide degradation. FEMS Microbiol Ecol 97:fiab070
Johnson KS, Clements KD (2021) Histology and ultrastructure of the gastrointestinal tract in four temperate marine herbivorous fishes. J Morphol 283:16–34
Jones J, DiBattista JD, Stat M, Bunce M, Boyce MC, Fairclough DV, Travers MJ, Huggett MJ (2018) The microbiome of the gastrointestinal tract of a range-shifting marine herbivorous fish. Front Microbiol 9:2000
Klindworth A, Pruesse E, Schweer T, Peplies JJ, Quast C, Horn M, Glockner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1–e1
Knudsen SW, Choat JH, Clements KD (2019) The herbivorous fish family Kyphosidae (Teleostei: Perciformes) represents a recent radiation from higher latitudes. J Biogeogr 46:2067–2080
Krogdahl Å, Hemre GI, Mommsen TP (2005) Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquac Nutr 11:103–122
Lee JI, Hampson DJ (1994) Genetic characterisation of intestinal spirochaetes and their association with disease. J Med Microbiol 40:365–371
Lee WJ, Hase K (2014) Gut microbiota–generated metabolites in animal health and disease. Nat Chem Biol 10:416–424
Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296
Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Madin JS, Baird AH, Bridge TCL, Connolly SR, Zawada KJA, Dornelas M (2018) Cumulative effects of cyclones and bleaching on coral cover and species richness at Lizard Island. Mar Ecol Prog Ser 604:263–268
Marcel M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10–12
Miyake S, Ngugi DK, Stingl U (2015) Diet strongly influences the gut microbiota of surgeonfishes. Mol Ecol 24:656–672
Mountfort DO, Campbell J, Clements KD (2002) Hindgut fermentation in three species of marine herbivorous fish. Appl Environ Microbiol 68:1374–1380
Neave MJ, Apprill A, Ferrier-Pagès C, Voolstra CR (2016) Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl Microbiol Biotechnol 100:8315–8324
Ngugi DK, Miyake S, Cahill M, Vinu M, Hackmann TJ, Blom J, Tietbohl MD, Berumen ML, Stingl U (2017) Genomic diversification of giant enteric symbionts reflects host dietary lifestyles. Proc Natl Acad Sci USA 114:E7592–E7601
Nielsen S, Walburn JW, Verges A, Thomas T, Egan S (2017) Microbiome patterns across the gastrointestinal tract of the rabbitfish Siganus fuscescens. PeerJ 5:e3317
Nobel YR, Snider EJ, Compres G, Freedberg DE, Khiabanian H, Lightdale CJ, Toussaint NC, Abrams JA (2018) Increasing dietary fiber intake is associated with a distinct esophageal microbiome. Clin Transl Gastroenterol 9:199
Ohkuma M, Noda S, Hattori S, Iida T, Yuki M, Starns D, Inoue JI, Darby AC, Hongoh Y (2015) Acetogenesis from H2 plus CO2 and nitrogen fixation by an endosymbiotic spirochete of a termite-gut cellulolytic protist. Proc Natl Acad Sci USA 112:10224–10230
Oren A, Garrity GM (2021) Valid publication of the names of forty-two phyla of prokaryotes. Int J Syst Evol Mirobiol 71:005056
Parata L, Nielsen S, Xing X, Thomas T, Egan S, Vergés A (2020) Age, gut location and diet impact the gut microbiome of a tropical herbivorous surgeonfish. FEMS Microbiol Ecol 96:fiz179.
Parris DJ, Brooker RM, Morgan MA, Dixson DL, Stewart FJ (2016) Whole gut microbiome composition of damselfish and cardinalfish before and after reef settlement. PeerJ 4:e2412
Pielou EC (1969) An Introduction to mathematical ecology. John Wiely and Sons, New York
Price MN, Dehal PS, Arkin AP (2009) FastTree: computing large minimum-evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650
Price MN, Dehal PS, Arkin AP (2010) FastTree 2: approximately maximum-likelihood trees for large alignments. PLoS ONE 5:e9490
QGIS Development Team (2018) QGIS. QGIS geographical information system. Open Source Foundation Project. http://qgis.osgeo.org
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ricker WE (1973) Linear regressions in fishery research. J Fish Res Board Can 30:409–434
Rimmer DW, Wiebe WJ (1987) Fermentative microbial digestion in herbivorous fishes. J Fish Biol 31:229–236
Scott JJ, Adam TC, Duran A, Burkepile DE, Rasher DB (2020) Intestinal microbes: an axis of functional diversity among large marine consumers. Proc R Soc Lond B Biol Sci 287:20192367
Senghor B, Sokhna C, Ruimy R, Lagier JC (2018) Gut microbiota diversity according to dietary habits and geographical provenance. Hum Microb J 7:1–9
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, USA
Smriga S, Sandin SA, Azam F (2010) Abundance, diversity, and activity of microbial assemblages associated with coral reef fish guts and feces. FEMS Microbiol Ecol 73:31–42
Sparagon WJ, Gentry EC, Minich JJ, Vollbrecht L, Laurens LML, Allen EE, Sims NA, Dorrestein PC, Wegley Kelly L, Nelson CE (2022) Fine scale transitions of the microbiota and metabolome along the gastrointestinal tract of herbivorous fishes. Anim Microbiome 4:33
Stevens CE, Hume ID (1998) Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev 78:393–427
Sullam KE, Essinger SD, Lozupone CA, O’Connor MP, Rosen GL, Knight ROB, Kilham SS, Russell JA (2012) Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol 21:3363–3378
Tandon K, Lu CY, Chiang PW, Wada N, Yang SH, Chan YF, Chen PY, Chang HY, Chiou YJ, Chou MS, Chen WM (2020) Comparative genomics: dominant coral-bacterium Endozoicomonas acroporae metabolizes dimethylsulfoniopropionate (DMSP). ISME J 14:1290–1303
Tarnecki AM, Burgos FA, Ray CL, Arias CR (2017) Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J Appl Microbiol 123:2–17
Tokuda G, Mikaelyan A, Fukui C, Matsuura Y, Watanabe H, Fujishima M, Brune A (2018) Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc Natl Acad Sci USA 115:E11996–E12004
Wang AR, Ran C, Ringø E, Zhou ZG (2018) Progress in fish gastrointestinal microbiota research. Rev Aquac 10:626–640
Zawada KJA, Madin JS, Baird AH, Bridge TCL, Dornelas M (2019) Morphological traits can track coral reef responses to the anthropocene. Funct Ecol 33:962–975
Acknowledgements
Alessandro Pisaniello was supported by a Ministry of Business, Innovation and Employment (MBIE) Endeavour Grant (project UOAX1808-CR-2). Fieldwork and sequencing for the 2011 samples was supported by a Marsden Fund grant to KDC, ERA and WLW. Fieldwork for the 2017 samples was supported by an internal AUT grant to WLW, and sequencing for these samples to an MBIE Smart Ideas grant to KDC and WLW. The authors thank the New Zealand eScience Infrastructure for the computing resources and Auckland Genomics facility for the NGS sequencing service. The authors also thank Jian Sheng Boey, Emilie Gios and Kevin C. Lee for the advice with the bioinformatic analysis and Vivian Ward for graphic design. We thank Lyle Vail and Anne Hoggett of the Lizard Island Research Station, Will Robbins and Howard Choat for assistance with field sampling.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Fish collection was covered by James Cook University Animal Ethics Committee approvals A1641 and A2237.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic editor Andrew Hoey
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pisaniello, A., Bojarski, L.D., Handley, K.M. et al. Sources of variation in community composition of the hindgut microbiota in two tropical Kyphosus species. Coral Reefs 41, 1523–1535 (2022). https://doi.org/10.1007/s00338-022-02299-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02299-8