Abstract
The Hawaiian Archipelago has served as a natural laboratory to assess genetic connectivity patterns across a broad spectrum of taxonomic and ecological diversity. Almost all these studies were based on a few targeted loci, but technologies now allow us to assess population structure with genomic coverage and greater resolution. Here, we provide a SNP-based analysis for an endemic surgeonfish, Acanthurus triostegus sandvicensis (manini) across the Hawaiian Archipelago and adjacent Johnston Atoll (N = 461). Based on 3649 SNPs, manini showed population structure in the main Hawaiian Islands, but genetic homogeneity across most of the northwestern extent of the archipelago (overall FST = 0.033, P < 0.001). Net migration occurred from Johnston Atoll into Hawai‘i, providing further support for Johnston Atoll being a pathway for dispersal (or colonization) into Hawai′i. These results highlight the higher efficacy of genomic sequencing to characterize fine-scale patterns of connectivity relative to a targeted loci approach and, moving forward, may invoke a reassessment of past connectivity studies in a genomics framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The linear Hawaiian Archipelago has hosted extensive research into genetic connectivity patterns across a variety of taxonomically and ecologically diverse species (e.g., Eble et al. 2009; Andrews et al. 2010; Gaither et al. 2010; Skillings et al. 2011; Timmers et al. 2011; Coleman et al. 2014; Iacchei et al. 2014; Tenggardjaja et al. 2016). This research has cumulated in several metanalyses that aimed to identify common barriers to dispersal across the archipelago (Toonen et al. 2011), assess how life history traits influence population genetic structure (Selkoe et al. 2014), and reveal that high coral cover harbors the greatest genetic diversity (Selkoe et al. 2016). The methodology for these studies was the use of a targeted loci approach, which in some of the earlier cases relied on a single mtDNA marker.
During the past decade, the field of population genetics has steadily shifted toward high-throughput sequencing due in part to a reduction in cost and the ability to generate thousands of loci (Wetterstrand 2019; Kraft et al. 2020). The higher resolution provided by thousands of loci can reveal genomic trends and patterns that could not be detected using a targeted loci approach. For example, we now have the ability to detect discrete patterns of divergent selection (Jansson et al. 2020), can accurately describe adaptive radiations among shallow genetic divergences (Keller et al. 2013), have improved accuracy for genotyping lineages (Bongaerts et al. 2021), have increase ability to detect fine-scale population structure (Kraft et al. 2020), and greater ability to relate genomic trends to the ecology of the organism (Gaither et al. 2015).
Acanthurus triostegus, locally known as manini (family Acanthuridae), has a broad Indo-Pacific distribution, although the Hawai‘i and Johnston Atoll population is recognized as a sub-species (A. triostegus sandvicensis) based on diagnostic differences in coloration and morphology (Randall 1961, 2007). This species has a relatively long pelagic larval stage of ~ 54–77 days, a possible indicator of high dispersal ability. The single study that investigated connectivity of manini within Hawai‘i used allozymes to reveal population structure between Hawai‘i Island and O‘ahu (Planes and Fauvelot 2002). However, studies in other parts of the range have provided conflicting patterns of dispersal. In Northern Australia, manini had no significant (mtDNA) population structure across the Torres Strait (Mirams et al. 2011), a known biogeographic barrier (Voris 2000). In contrast, allozyme analyses revealed population structure across various spatial scales ranging from adjacent islands to a range-wide assessments (Planes 1993; Planes et al. 1998; Planes and Fauvelot 2002).
The Hawaiian Archipelago is characterized by high endemism in marine fishes (Briggs and Bowen 2012; Toonen et al. 2016), and it has been previous hypothesized that endemic Hawaiian reef fishes are descendants of poor dispersers that could not maintain genetic connectivity with a source population (Hourigan and Reese 1987; Eble et al. 2009). The subspecies distinction of Hawaiian A. triostegus sandvicensis, an otherwise widely distributed and presumanbly highly dispersive species, provides an opportunity to assess patterns of connectivity for a species that exhibits characteristics of both low and high dispersal potential, and gain insight into the utility of species distributions as an indicator of dispersal potential (e.g., Gaither and Rocha 2013). Additionally, this is the first analysis using a genomic approach (restriction site associated DNA sequencing, RADseq), to assess connectivity across the Hawaiian Archipelago for a common coral reef fish. The genomics approach provides a more powerful tool to reveal fine-scale patterns of connectivity thereby allowing us to make comparisons against previously defined genetic breaks across the archipelago.
Although RADseq studies have revealed fine-scale population structure not observed using a targeted locus approach, we caution that this study has limited applications in such a comparative framework. The single previous study demonstrating manini population structure in Hawai‘i was limited to two islands (Planes and Fauvelot 2002), and these locations (Hawai‘i Island and O‘ahu) were subsequently shown to harbor isolated populations among a multitude of diverse marine species (Toonen et al. 2011). Additionally, the numerous connectivity surveys based on a targeted loci approach across the Hawaiian Archipelago are our analytical baseline to compare against our results which are based in a genomics framework. With this in mind, we further ask whether the first genomic survey is concordant with population partitions observed in coral reef-associated species surveyed with a targeted loci approach, and whether additional populations partitions are observed that may indicate increased population resolution.
Material and methods
Taxon sampling and DNA extraction
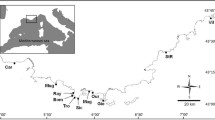
Between 2003 and 2006, 461 tissue samples (primarily fin clips) of manini from 10 locations were collected from across the Hawaiian Archipelago including islands in the Main Hawaiian Islands (Hawai‘i Island, Maui, O‘ahu, and Kaua‘i), the Northwestern Hawaiian Islands (French Frigate Shoals, Maro Reef, Pearl and Hermes Atoll, Midway Atoll, and Kure Atoll), and Johnston Atoll using pole spears with SCUBA or snorkeling (Table 1; Fig. 1). Multiple collections occurred around each island and were grouped together, including across different years, to obtain a genetic signature for each sample location. Tissues were preserved in salt-saturated DMSO buffer (Amos and Hoelzel 1991) and stored at room temperature. Genomic DNA was extracted using Omega Bio-Tek E-Z 96® Tissue DNA Kit (Norcross, GA, USA) following the manufacturers protocol and resuspended in nanopure water. High molecular weight was confirmed by visualizing on a 1.5% agarose gel with GelRed® (Biotium, Inc. Fremont, CA, USA).
Collection locations and sample sizes of Acanthurus triostegus sandvicensis in parentheses. Solid line indicates the regions of the Main Hawaiian Islands and the Northwestern Hawaiian Islands which, in 2006, was designated the Papāhanaumokuākea Marine National Monument. Filled darker areas represent current coastlines while light areas represent the maximum historical above-water island area. Photo credit: Keoki Stender
Library preparation and sequencing
RADseq library preparation and sequencing were conducted by the Genomics Core Laboratory at Texas A&M Corpus Christi, starting with 150 ng of high-molecular weight genomic DNA per sample and following the double-digest RAD (ddRAD) protocol (Peterson et al. 2012). Briefly, this process included digesting each sample with Mspl and EcoRI (New England Biolabs, Ipswich, MA, USA) followed by cleaning with PEG solution using magnetic beads. Samples were then normalized to the same concentration followed by ligation of adapters. After digestion and ligation, a PCR was performed using dual-indexed primers. Fragments of between 325 and 400 bp were selected using BluePippin (Sage Science, Beverley, MA, USA), and a Fragment Analyzer was used to visualize library size range followed by a qPCR to determine molarity of libraries. The resulting libraries were sequenced on an Illumina HiSeq® 4000 (150 paired-end reads, performed by NYU Langone Health Genome Technology Center). Sequence data for the samples were demultiplexed based on the barcodes from the adapters using process_radtags (Catchen et al. 2013). Individual libraries were sequenced across two or three independent runs to increase the number of sequence reads for each sample and to ensure congruence in nucleotide assignments. Fastq sequences were deposited in NCBI’s Sequence Read Archive (accession numbers: SAMN27733595-27734046), and the associated metadata are available at GEOME (Deck et al. 2017).
Genotyping and de novo assembly of RADseq libraries
Raw reads obtained from Illumina runs were assessed for sequence quality using FastQC 0.10.1 (Andrews 2010), to remove low-quality bases (Phred quality score threshold of 30). As a reference genome is not available for A. triostegus, a de novo reference catalog was assembled using Rainbow 2.0.4 (Chong et al. 2012) as performed in the dDocent pipeline (Puritz et al. 2014a, 2014b) using a minimum depth of 15 and maximum of eight mismatches to form reference contigs. The reference contigs were clustered based on a 75% similarity threshold. After generating the reference catalog, reads were mapped using bwa 0.7.17 (Li and Durbin 2009) and SNP detection was performed using FreeBayes 1.10.54 (Garrison and Marth 2012). Variant calls were subjected to several filtering steps to reduce false positives. The data set was filtered to remove all genotypes with < 5 reads per individual. SNPs were retained if they were genotyped in 95% of individuals, had a minor allele count of 3 or higher, an average depth of less than 20, and a minor allele frequency of 0.05. Using the –thin function in vcftools 0.1.12a (Danecek et al. 2011) and setting it to maximum contig size, we retained only the first SNP found along a contig. Using vcftools, we removed SNPs that were not in Hardy–Weinberg Equilibrium. SNPs below the significance threshold value (P = 0.01) were excluded from the dataset.
Population genetic analyses
genodive 2.0b27 (Meirmans and Van Tienderen 2004) was used to generate genetic diversity indices, as well as to test for population structure. Genetic structure among sample locations was evaluated with an analysis of molecular variance (AMOVA) in arlequin 3.5.1.2 (Excoffier et al. 2005). Deviations from null distributions were tested with nonparametric permutation procedures (N = 9999). Pairwise FST statistics were generated to assess genetic structure between locations. False discovery rates were controlled for and maintained at α = 0.05 among all pairwise tests (Benjamini and Yekutieli 2001; Narum 2006). Given the potential for population structure within the larger high islands, specimens from the east and west side of the islands of Hawai‘i Island, Maui, and O‘ahu were initially analyzed as separate sample sites, but no genetic differentiation was identified. The results presented here combined all specimens from both sides as a single island population.
We used three approaches to explore population structure indicated by AMOVA. First, genetic partitioning was assessed using structure 2.3.2 (Pritchard et al. 2000), a Bayesian method that estimates ancestry and categorizes individuals into discrete populations. The simulation was run for 1 million generations with the first 100,000 discarded as burn-in. Five replicates of each simulation from K = 1 to 10 genetic clusters were run. We determined the most likely number of genetic clusters (K) using structure harvester 0.6.93 (Earl and von Holdt 2012). structure results were analyzed using the online tool clumpak (http://clumpak.tau.ac.il/index.html) (Kopelman et al. 2015) which integrates the program clumpp 1.1.2 (Jakobsson and Rosenberg 2007) and minimizes the variance across all iterations. clumpak then created the final visualized output. Second, the genetic relationship among populations was resolved with a Principle Component Analysis (PCA) implemented using genodive and following a covariance transformation matrix. Finally, we conducted a Discriminant Analysis of Principal Components (DAPC), a hybrid linear discriminant analysis of principal components, using the R package ‘Adegenet’ 2.2.1 (Jombart 2008). Since PCA analyses require no missing data (and DAPC uses PCA), we replaced missing data with the mean allele frequency for each locus using the function scaleGen, a function of Adegenet. Following the cross-validation algorithm using 500 iterations, we retained the first 20 principal components and three discriminant function, using the population label as the dependent variable. DAPC differs from PCA analyses in that while PCA analyses search for the direction of largest total variance, DAPC maximizes the separation between groups while minimizing within group variation (Jombart et al. 2010).
Testing the direction of gene flow between Johnston Atoll
Johnston Atoll is the closest shallow habitat to Hawai‘i, many endemic species are shared between the two regions (Randall 2007), and so Johnston is considered part of the Hawaiian biogeographic province (Toonen et al. 2016). To examine the direction and magnitude of gene flow between Johnston Atoll and Hawai‘i, migration rates among islands were calculated using the same SNP dataset as above in migrate-n (Beerli and Felsenstein 2001; Beerli 2006). Our initial results found many of the island in the NWHI (French Frigate Shoals, Maro Reef, Pearl and Hermes, Midway) and a single population in the MHI (O’ahu) showed no structure with Johnston Atoll, indicating that these islands are likely nodes for colonization into or out of Hawai’i. With this in mind, we set the migrate-n model assuming all these populations in the analysis had equal exchange of migrants (panmictic). During the migrate-n run, 5,000,000 steps were sampled and recorded every 1000 steps under a constant mutation rate model, with the first 1,000,000 steps discarded as burn-in. Several test runs were conducted following a Bayesian search strategy to determine the appropriate prior values for the parameters θ (four times effective population size multiplied by mutation rate per site per generation, 4Neµ) and M (immigration rate divided by the mutation rate, m/µ). In the final analyses, we set the mean prior values to 0.05 for θ and 500 for M in both directions. During the migrate-n run, 5,000,000 steps were sampled and recorded every 1000 steps under a constant mutation model, with the first 1,000,000 steps discarded as burn-in. After checking for data convergence, the mode and 95% percentiles of θ and M were used to calculate the effective migrants per generation (Nem = (M* θ)/4).
Results
After the initial trimming, filtering, and demultiplexing, we retained 80,955 loci. Following a second filtering step which accounted for coverage, minimum allele frequency, presence among individuals in the dataset, retaining one SNP per contig, and excluding loci out of HWE, we identified 3649 loci that met all the criteria for downstream analyses.
Molecular diversity indices are summarized in Table 1. The number of alleles present in all populations ranged from 1.17 to 1.46 at Kure and Kaua‘i with an average of 1.31 across all populations. The effective number of alleles was similar across locations and ranged from 1.05 to 1.07 with an average of 1.06 across all populations. Total heterozygosity ranged from 0.031 at Maui to 0.045 at Kure with an average of 0.039 across all populations. Inbreeding coefficients revealed that the influence of inbreeding is negligible across all populations.
Population pairwise FST values are summarized in Table 2. In the main Hawaiian Islands (MHI), prior to correction for false discovery rates, population structure was found to be significant between all islands. After correcting for false discovery rates (corrected α = 0.009) the highest differentiation was observed between Hawai‘i Island and O‘ahu (FST = 0.071, P < 0.001); however, Hawai‘i Island no longer was differentiated from Kaua‘i, the only pairwise comparison that lost significance after applying the correction. In the Northwestern Hawaiian Islands (NWHI), all islands except Kure (the furthest NW island) grouped with Johnston Atoll to form one panmictic population. Kure was differentiated from the other NWHI with the greatest differentiation between Kure and Pearl and Hermes (FST = 0.070, P < 0.001). When comparing between the MHI and the NWHI, O‘ahu was not significantly differentiated from Johnston Atoll and the islands in the NWHI with the exception of Kure (FST = 0.073, P < 0.001). Kure showed significant differences from Maui (FST = 0.057, P < 0.001) but was not differentiated from Hawai‘i Island or Kaua‘i. Johnston Atoll also showed significant differentiation from Hawai‘i‘Island, Maui, and Kaua‘i. The AMOVA found significant differences among populations overall (FST = 0.033, P < 0.001; Table 3).
Population clustering
The structure analysis recovered two clusters (Fig. 2, Table S1; K = 2, Mean Ln P(K) = − 32,588.04; second closest). The analysis found one population consisting of Hawai‘i,‘Maui, Kaua‘i, and Kure, and a second population consisting of the remaining islands in the NWHI, O‘ahu, and Johnston Atoll. Various levels of admixture were observed in all locations. The cluster of k = 3 recovered patterns consistent with the two clusters recovered for K = 2 (Fig. S1; K = 3, Mean Ln P(K) = − 32,504.16). The PCA revealed two distinct clusters similar to what was observed in the structure results: one cluster consisting of Hawai‘i, Maui, Kaua‘i, and Kure and a second cluster consisting of the remaining islands in the NWHI, O‘ahu, and Johnston Atoll (Fig. 3). The two distinct clusters are more apparent when the PCA was grouped by individuals (Fig S2) and can also be observed in the DAPC analysis results, which aggregates genetically similar population together (Fig S3).
Scatter diagram based on Principal Components Analysis between Hawaiian populations of Acanthurus triostegus sandvicensis using 3649 SNPs. Abbreviations: Hawai‘i, HAW; Maui, MAU; O‘ahu, OAH; Kaua‘i, KAU; French Frigate Shoals, FFS; Maro Reef, MAR; Pearl and Hermes Atoll, PH; Midway Atoll, MID; Johnston Atoll, JOH; Kure Atoll, KUR
Estimation of migration rates
Migration estimates determined by the migrate-n analysis ranged from 23.6 (French Frigate Shoals to Johnston Atoll) to 47.0 (Pearl and Hermes to French Frigate Shoals) effective number of migrants per generation (Table S2). The average movement among islands was 30.3 migrants per generation. The strongest rate of migration from Johnston Atoll to the Hawaiian Archipelago was observed between Johnston Atoll and French Frigate Shoals (Nem = 32.8). O’ahu was found to have the highest rates of migration to Johnston Atoll among the MHI (Nem = 34.2).
Discussion
Patterns of dispersal across the archipelago
Studies of connectivity along the Hawaiian Archipelago have added to our understanding on how dispersal patterns are shaped and how biodiversity is exchanged in the marine realm. The results of this study corroborate previously identified patterns of connectivity. However, this analysis also revealed novel patterns, presumably based on increased sampling and genomic coverage, which can change our understanding of dispersal dynamics in the Hawaiian biogeographical province.
Among the previous patterns corroborated with SNP data, allozyme analysis of manini had revealed structure between Hawai‘i Island and O‘ahu (Planes and Fauvelot 2002). The multi-species genetic breaks identified by Toonen et al. (2011) found that most islands in the MHI are distinct at a population level, a pattern consistent with our findings for manini (Table 2). Along the rest of the range through the NWHI, our observation of higher connectivity is generally concordant with trends observed with targeted loci in other species. We recovered a highly connected population extending up the NWHI from French Frigate Shoals to Midway Atoll that also includes Johnston Atoll. However, in most species surveyed to date, the furthest northwestern genetic break was found between Midway Atoll and Pearl & Hermes (Toonen et al. 2011). The results of our study also found a break further northwest between Midway Atoll and Kure. Nonetheless, previous studies revealed a recurring pattern of highly connectivity in the middle of the archipelago (e,g., from Nihoa to Pearl and Hermes) (e.g., Eble et al. 2009, 2011b; Andrews et al. 2014; Tenggardjaja et al. 2016, 2018).
The high connectivity of manini between Johnston Atoll and the archipelago is a pattern documented in other reef fish species (Ramon et al. 2008; Craig et al. 2010; DiBattista et al. 2011; Fernandez-Silva et al. 2015). Johnston Atoll is the nearest land mass to the Hawaiian Archipelago, 885 km southwest of French Frigate Shoals, and is included in the Hawaiian biogeographic province based on high faunal similarity. Many endemic Hawaiian reef fishes are found there (Randall 2007; Briggs and Bowen 2012), and it is likely a stepping stone for Indo-Pacific biodiversity to colonize into Hawai‘i (Bowen 2016). Johnson Atoll has been implicated as the source of propagules (larvae) found in the middle of the archipelago (Rivera et al. 2004; Gaither et al. 2011; Andrews et al. 2014) and this theme is supported by our findings of net migration from Johnston Atoll into the NWHI (Table S2). Dispersal from Johnston Atoll into the archipelago is further supported by biophysical models. Kobayashi (2006) identified two potential corridors into the archipelago from Johnston Atoll for species with PLDs greater than 40 days—one being French Frigate Shoals. Dispersal out of Hawai‘i generally occurs in a westward trajectory toward Japan rather than a southernly trajectory toward Johnston Atoll (Eble et al. 2011b). Here, we find dispersal of manini out of Hawai’i follows a southern trajectory from O’ahu to Johnston Atoll.
We observed no significant population partitions between O’ahu and the NWHI, and no significant population partitioning between the broader MHI (excluding O‘ahu) and Kure (Table 2, Fig. 2). This pattern of genetic connectivity between the ends of the archipelago, to the exclusion of intermediate habitats, makes little geographic sense and has bedeviled genetic assessment of other species (e.g., Gaither et al. 2011). However, this pathway begins to make sense in light of a potential pathways of larval transport from O’ahu to Johnston Atoll, which is known to export propagules in the NWHI. In this scenario, Johnston Atoll acts as a conduit for connectivity between O’ahu and the middle of archipelago. It is not clear what biological or physical drivers could facilitate this pattern, although it is consistent with a previous population assessment of the sea cucumber, Holothuria atra, which indicated net export from the Hawaiian Archipelago to Johnston Atoll (Skillings et al. 2011). The other notable difference from previous studies is the shift in the most northwestern genetic break to between Midway and Kure. Other than these anomalous patterns, the results of our study are mostly consistent with patterns shared across multiple species (Toonen et al. 2011), including the large expanse of genetic homogeneity found among the atolls and low islands in the middle of the archipelago.
Factors influencing dispersal
Hawaiian manini are a subspecies (A. triostegus sandvicensis) endemic to Hawaii and Johnston Atoll (Randall 1961, 2007) which may provide insight into their dispersal capabilities. Previous researchers have hypothesized that endemic Hawaiian reef fishes are descendants of poor dispersers (Hourigan and Reese 1987; Eble et al. 2009); after a rare colonization event into Hawai‘i they were unable to maintain connectivity with the parent population in the Central Pacific. However, research on manini in Australia indicated that seascapes rather than dispersal potential had a larger influence on genetic patterns (Liggins et al. 2016). Nonetheless, interspecific patterns vary and studies that have investigated population structure of endemic Hawaiian reef fishes, including groupers, damselfish, and surgeonfishes, have shown that endemic species have higher levels of population structure across the archipelago, relative to widespread species (Rivera et al. 2004; Ramon et al. 2008; Eble et al. 2009; Tenggardjaja et al. 2018). An exception to this pattern are the three endemic butterflyfishes which were all found to be genetically homogenous across their distribution (Craig et al. 2010). For widespread Indo-Pacific species, very little to no population structure is observed across the archipelago (Craig et al. 2007; Eble et al. 2009, 2011a; Reece et al. 2010; DiBattista et al. 2011; Andrews et al. 2014).
The population structure of manini has traits that are intermediate between the expectations for endemic versus widespread species. Population structure is observed across the MHI and Kure, but with genetic homogeneity across the remainder of the NWHI and Johnston Atoll. Research based on allozymes, which characterized genetic structure of manini across the entire Indo-Pacific range, found that the Hawaiian population was genetically distinct from the rest of the range, albeit with FST values that indicate an isolated population rather than a deep evolutionary (and taxonomic) designation (Planes and Fauvelot 2002). A genomic SNP-based survey across the entire Indo-Pacific range of manini would be necessary to properly characterize the evolutionary distinction of the Hawaiian color morph.
These contrasting patterns of genetic homogeneity across large expanses and population structure across relatively short distances have been demonstrated elsewhere in the range of manini. Population structure has been documented within the Polynesian Archipelago, on either side of the Torres Strait (Liggins et al. 2016), and even within the lagoon at New Caledonia, based on allozyme analyses (Planes et al. 1996, 1998). However, manini have also maintained genetic connectivity across vast expanses of the Indo-Pacific (Planes and Fauvelot 2002; Mirams et al. 2011). Grulois et al. (2020) reported a break only on the scale of Indian and Pacific populations based on microsatellites. To further confound our attempts at generalization, recent evidence from a parentage analysis conducted on O‘ahu found that the majority of manini larvae settle less than 30 km from their spawning grounds, even in the face of strong currents (Coleman et al., submitted). Based on these contrasting patterns, the dispersal ability of manini is clearly not static and is likely influenced by a variety of abiotic and biotic factors.
Habitat preference and larval behavior are known to play a key role in dispersal and settlement queues (Jones 2015). The ecosystem of the MHI differs greatly from the relatively pristine ecosystem of the NWHI, which was designated as the Papahānaumokuākea Marine National Monument in 2006, thereby limiting anthropogenic influences. The MHI are made of up of high islands with steady freshwater run off that transport nutrients into surrounding water, whereas the older NWHI consists of low islands, atolls, and oligotrophic waters. The human impact in the MHI has also led to degraded reefs, overfishing, and pollution, among other pressures that have distorted natural processes in this region (Bahr et al. 2015; Wedding et al. 2018). Many species that are common in the NWHI are rare or unknown in the MHI such as Acropora corals (Grigg et al. 1981) as well as many endemic fishes (Kosaki et al. 2017). Intriguingly, the area of genetic homogeneity in the NWHI has high concordance with a community cluster based on the numerical abundance of endemic and non-endemic fish species (Friedlander et al. 2020). The area of ecological homogeneity corresponds approximately to the area of genetic homogeneity.
Since juvenile and adult stages are relatively sedentary, the key to understanding higher connectivity in the NWHI must focus on larvae. Considering the geological, oceanographic, and trophic conditions, we posit three non-exclusive hypotheses:
-
(1)
Larvae might disperse more in oligotrophic waters of the NWHI, because of low food supply and/or longer time to become competent for settlement. This might be resolvable by examining otolith growth rings in newly settled recruits in the MHI versus NWHI.
-
(2)
High islands may be an easier target for larvae to find. Freshwater runoff, louder ambient noise, and higher nutrient load could provide cues for larvae to navigate, recruit, and settle. This might be resolvable with behavioral studies.
-
(3)
High islands may provide protection from regional currents, a bit of 'shade' with meandering eddies that promote local recruitment. This might be resolvable with fine-scale biophysical models.
While these hypotheses merit further consideration, the geological and ecological difference between the NWHI and MHI does not seem to explain why O‘ahu manini shows high connectivity with the NWHI or why Kure shows connectivity with the MHI. While a pathway through Johnston Atoll is an intriguing new possibility, the underlying factors promoting or inhibiting dispersal across the archipelago remain elusive.
Conclusions
As the field of population genetics continues to evolve, a suite of tools are becoming available to evaluate patterns of connectivity on a genomic scale (Germer et al. 2000; Andrews and Luikart 2014; Puritz et al. 2014b). The exponential increase in data will continue to revolutionize our ability to identify factors that influence a range of processes from population connectivity to species divergence, including ecological important traits (Hohenlohe 2014), historic role of hybridization in shaping biodiversity (Meier et al. 2017), genetic basis for species interactions and adaptation (Allendorf et al. 2010; Hohenlohe et al. 2010), among others.
The utility of targeted marker analysis is not diminished and can effectively be used in concert with genomic data to describe evolutionary and contemporary patterns of connectivity, along with the associated mechanisms facilitating these patterns (Gaither et al. 2015). As we move forward in assessing connectivity across the Hawaiian Archipelago, it may be worthwhile to revisit some of the studies that used targeted loci and integrate them with a genomics perspective to uncover contemporary patterns of dispersal and identify the mechanisms that shaped the evolution of Hawai‘i's unique biodiversity.
Change history
15 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00338-022-02280-5
References
Allendorf FW, Hohenlohe PA, Luikart G (2010) Genomics and the future of conservation genetics. Nat Rev Genet 11:697–709
Amos B, Hoelzel AR (1991) Long term preservation of whale skin for DNA analysis. Rept Intl Whal Comm Spec Issue 13:99–103
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc
Andrews KR, Karczmarski L, Au WW, Rickards SH, Vanderlip CA, Bowen BW, Gordon GE, Toonen RJ (2010) Rolling stones and stable homes: social structure, habitat diversity and population genetics of the Hawaiian spinner dolphin (Stenella longirostris). Mol Ecol 19:732–748
Andrews KR, Luikart G (2014) Recent novel approaches for population genomics data analysis. Mol Ecol 23:1661–1667
Andrews KR, Moriwake VN, Wilcox C, Grau EG, Kelley C, Pyle RL, Bowen BW (2014) Phylogeographic analyses of submesophotic snappers Etelis coruscans and Etelis “marshi” (Family Lutjanidae) reveal concordant genetic structure across the Hawaiian Archipelago. PLoS ONE 9:e91665
Bahr KD, Jokiel PL, Toonen RJ (2015) The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3:e950
Beerli P (2006) Comparison of Bayesian and maximum-likelihood inference of population genetic parameters. Bioinformatics 22:341-345
Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc Natl Acad Sci 98:4563–4568
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Bongaerts P, Cooke IR, Ying H, Wels D, den Haan S, Hernandez-Agreda A, Brunner CA, Dove S, Englebert N, Eyal G (2021) Morphological stasis masks ecologically divergent coral species on tropical reefs. Curr Biol 31:2286–2298
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr 39:12–30
Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA (2013) Stacks: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Chong Z, Ruan J, Wu C-I (2012) Rainbow: an integrated tool for efficient clustering and assembling RAD-seq reads. Bioinformatics 28:2732–2737
Coleman RR, Gaither MR, Kimokeo B, Stanton FG, Bowen BW, Toonen RJ (2014) Large-scale introduction of the Indo-Pacific damselfish Abudefduf viagiensis into Hawai’i promotes genetic swamping of the endemic congener A. abdominalis. Mol Ecol 23:5552–5565
Craig MT, Eble JA, Bowen BW, Robertson DR (2007) High genetic connectivity across the Indian and Pacific Oceans in the reef fish Myripristis berndti (Holocentridae). Mar Ecol Prog Ser 334:245–254
Craig MT, Eble JA, Bowen BW (2010) Origins, ages and population histories: comparative phylogeography of endemic Hawaiian butterflyfishes (genus Chaetodon). J Biogeogr 37:2125–2136
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Deck J, Gaither MR, Ewing R, Bird CE, Davies N, Meyer C, Riginos C, Toonen RJ, Crandall ED (2017) The Genomic Observatories Metadatabase (GeOMe): A new repository for field and sampling event metadata associated with genetic samples. PLOS Biol 15:e2002925
DiBattista JD, Wilcox C, Craig MT, Rocha LA, Bowen BW (2011) Phylogeography of the Pacific blueline surgeonfish, Acanthurus nigroris, reveals high genetic connectivity and a cryptic endemic species in the Hawaiian Archipelago. J Mar Biol 2011:839134. https://doi.org/10.1155/2011/839134:17
Earl DA, von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Eble J, Toonen R, Bowen B (2009) Endemism and dispersal: comparative phylogeography of three surgeonfishes across the Hawaiian Archipelago. Mar Biol 156:689–698
Eble JA, Rocha LA, Craig MT, Bowen BW (2011a) Not all larvae stay close to home: insights into marine population connectivity with a focus on the brown surgeonfish (Acanthurus nigrofuscus). J Mar Biol 2011:12
Eble JA, Toonen RJ, Sorenson L, Basch LV, Papastamatiou Y, Bowen BW (2011b) Escaping paradise: larval export from Hawaii in an Indo-Pacific reef fish, the yellow tang Zebrasoma flavescens. Mar Ecol Prog Ser 428:245–258
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Fernandez-Silva I, Randall JE, Coleman RR, DiBattista JD, Rocha LA, Reimer JD, Meyer CG, Bowen BW (2015) Yellow tails in the Red Sea: phylogeography of the Indo-Pacific goatfish Mulloidichthys flavolineatus reveals isolation in peripheral provinces and cryptic evolutionary lineages. J Biogeogr 42:2402–2413
Friedlander AM, Donovan MK, DeMartini EE, Bowen BW (2020) Dominance of endemics in the reef fish assemblages of the Hawaiian Archipelago. J Biogeogr 47:2584–2596
Gaither MR, Rocha LA (2013) Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: evidence for the centre of overlap hypothesis. J Biogeogr 40:1638–1648
Gaither MR, Bowen BW, Toonen RJ, Planes S, Messmer V, Earle J, Robertson DR (2010) Genetic consequences of introducing allopatric lineages of Bluestriped Snapper (Lutjanus kasmira) to Hawaii. Mol Ecol 19:1107–1121
Gaither MR, Jones SA, Kelley C, Newman SJ, Sorenson L, Bowen BW (2011) High connectivity in the deepwater snapper Pristipomoides filamentosus (Lutjanidae) across the Indo-Pacific with isolation of the Hawaiian Archipelago. PLoS ONE 6:e28913
Gaither MR, Bernal MA, Coleman RR, Bowen BW, Jones SA, Simison WB, Rocha LA (2015) Genomic signatures of geographic isolation and natural selection in coral reef fishes. Mol Ecol 24:1543–1557
Garrison E, Marth G (2012) Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:12073907
Germer S, Holland MJ, Higuchi R (2000) High-throughput SNP allele-frequency determination in pooled DNA samples by kinetic PCR. Genome Res 10:258–266
Grigg RW, Wells JW, Wallace C (1981) Acropora in Hawaii. Part 1. History of the scientific record, systematics, and ecology—part I. Pac Sci 35:1–13
Grulois D, Hogan RI, Paygambar S, Planes S, Fauvelot C (2020) New microsatellite DNA markers to resolve population structure of the convict surgeonfish, Acanthurus triostegus, and cross-species amplifications on thirteen other Acanthuridae. Mol Biol Rep 47:8243–8250
Hohenlohe PA (2014) Ecological genomics in full colour. Mol Ecol 23:5129–5131
Hohenlohe PA, Bassham S, Etter PD, Stiffler, Johnson EA, Cresko WA (2010) Population genomics of parallel adaptation in Threespine stickleback using sequenced RAD tags. PLoS Genet 6:e1000862
Hourigan TF, Reese ES (1987) Mid-ocean isolation and the evolution of Hawaiian reef fishes. Trends Ecol Evol 2:187–191
Iacchei M, O’Malley JM, Toonen RJ (2014) After the gold rush: population structure of spiny lobsters in Hawaii following a fishery closure and the implications for contemporary spatial management. Bull Mar Sci 90:331–357
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Jansson E, Besnier F, Malde K, André C, Dahle G, Glover KA (2020) Genome wide analysis reveals genetic divergence between Goldsinny wrasse populations. BMC Genet 21:1–15
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:1–15
Jones GP (2015) Mission impossible: unlocking the secrets of coral reef fish dispersal. In: Mora C (ed) Ecology of fishes on coral reefs. Cambridge University Press, Cambridge, pp 16–28
Keller I, Wagner C, Greuter L, Mwaiko S, Selz O, Sivasundar A, Wittwer S, Seehausen O (2013) Population genomic signatures of divergent adaptation, gene flow and hybrid speciation in the rapid radiation of Lake Victoria cichlid fishes. Mol Ecol 22:2848–2863
Kobayashi DR (2006) Colonization of the Hawaiian Archipelago via Johnston Atoll: a characterization of oceanographic transport corridors for pelagic larvae using computer simulation. Coral Reefs 25:407–417
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) CLUMPAK: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15:1179–1191
Kosaki RK, Pyle RL, Leonard JC, Hauk BB, Whitton RK, Wagner D (2017) 100% endemism in mesophotic reef fish assemblages at Kure Atoll, Hawaiian Islands. Mar Biodivers 47:783–784. https://doi.org/10.1007/s12526-12016-10510-12525
Kraft DW, Conklin EE, Barba EW, Hutchinson M, Toonen RJ, Forsman ZH, Bowen BW (2020) Genomics versus mtDNA for resolving stock structure in the silky shark (Carcharhinus falciformis). PeerJ 8:e10186
Li H, Durbin R (2009) Fast and accurate short read alignment with burrows–wheeler transform. Bioinformatics 25:1754–1760
Liggins L, Treml EA, Possingham HP, Riginos C (2016) Seascape features, rather than dispersal traits, predict spatial genetic patterns in co-distributed reef fishes. J Biogeogr 43:256–267
Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O (2017) Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat Commun 8:14363
Meirmans PG, Van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Mirams A, Treml E, Shields J, Liggins L, Riginos C (2011) Vicariance and dispersal across an intermittent barrier: population genetic structure of marine animals across the Torres Strait land bridge. Coral Reefs 30:937–949
Narum SR (2006) Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv Genet 7:783–787
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double Digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7:e37135
Planes S (1993) Genetic differentiation in relation to restricted larval dispersal of the convict surgeonfish, Acanthurus triostegus, in French Polynesia. Mar Ecol Prog Ser 98:237–246
Planes S, Fauvelot C (2002) Isolation by distance and vicariance drive genetic structure of a coral reef fish in the Pacific Ocean. Evolution 56:378–399
Planes S, Galzin R, Bonhomme F (1996) A genetic metapopulation model for reef fishes in oceanic islands: the case of the surgeonfish, Acanthurus triostegus. J Evol Biol 9:103–117
Planes S, Parroni M, Chauvet C (1998) Evidence of limited gene flow in three species of coral reef fishes in the lagoon of New Caledonia. Mar Biol 130:361–368
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Puritz JB, Hollenbeck CM, Gold JR (2014a) dDocent: a RADseq, variant-calling pipeline designed for population genomics of non-model organisms. PeerJ 2:e431
Puritz JB, Matz MV, Toonen RJ, Weber JN, Bolnick DI, Bird CE (2014b) Demystifying the RAD fad. Mol Ecol 23:5937–5942
Ramon M, Nelson P, Martini E, Walsh W, Bernardi G (2008) Phylogeography, historical demography, and the role of post-settlement ecology in two Hawaiian damselfish species. Mar Biol 153:1207–1217
Randall JE (1961) Contribution to the biology of the convict surgeonfish of the Hawaiian Islands, Acanthurus triostegus sandvicensis. Pac Sci 15:215–272
Randall JE (2007) Reef and shore fishes of the Hawaiian Islands. University of Hawai‘i Sea Grant College Program, Honolulu
Reece JS, Bowen BW, Joshi K, Goz V, Larson A (2010) Phylogeography of two moray eels indicates high dispersal throughout the Indo-Pacific. J Hered 101:391–402
Rivera MAJ, Kelley CD, Roderick GK (2004) Subtle population genetic structure in the Hawaiian grouper, Epinephelus quernus (Serranidae) as revealed by mitochondrial DNA analyses. Biol J Lin Soc 81:449–468
Selkoe KA, Gaggiotti OE, Andrews KR, Bernal MA, Bird CE, Bolick H, Baums I, Coleman RR, Concepcion GT, Craig MT, DiBattista JD, Eble J, Fernandez-Silva I, Gaither MR, Iacchei M, Polato NR, Rivera MA, Rocha LA, Skillings D, Timmers M, Szabo Z, Bowen BW, Toonen RJ (2014) Emergent patterns of population genetic structure for a coral reef community. Mol Ecol 23:3064–3079
Selkoe KA, Gaggiotti OE, Treml EA, Wren JLK, Donovan MK, Cosortium HRC, Toonen RJ (2016) The DNA of coral reef biodiversity: predicting and protecting genetic diversity of reef assemblages. Proc R Soc Lond B Biol Sci 283:20160354
Skillings DJ, Bird CE, Toonen RJ (2011) Gateways to Hawai ‘i: genetic population structure of the tropical sea cucumber Holothuria atra. J Mar Biol 2011:783030
Tenggardjaja KA, Bowen BW, Bernardi G (2016) Reef fish dispersal in the Hawaiian Archipelago: comparative phylogeography of three endemic damselfishes. J Mar Biol 2016:3251814:17
Tenggardjaja KA, Bowen BW, Bernardi G (2018) Comparative phylogeography of widespread and endemic damselfishes in the Hawaiian Archipelago. Mar Biol 165:1–21
Toonen RJ, Bowen BW, Iacchei M, Briggs JC (2016) Biogeography, marine. In: Kliman RM (ed) Encyclopedia of evolutionary biology, vol 1. Academic Press, Oxford
Toonen RJ, Andrews KR, Baums IB, Bird CE, Concepcion CT, Daly-Engel TS, Eble JA, Faucci A, Gaither MR, Iacchei M, Puritz JB, Schultz JK, Skillings DJ, Timmers M, Bowen BW (2011) Defining boundaries for applying ecosystem-based management: a multispecies case study of marine connectivity across the Hawaiian Archipelago. J Mar Biol 2011:460173
Timmers MA, Andrews KR, Bird CE, deMaintenton MJ, Brainard RE, Toonen RJ (2011) Widespread dispersal of the crown-of-thorns sea star, Acanthaster planci, across the Hawaiian Archipelago and Johnston Atoll. J Mar Biol 2011:934269
Voris HK (2000) Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr 27:1153–1167
Wedding LM, Lecky J, Gove JM, Walecka HR, Donovan MK, Williams GJ, Jouffray J-B, Crowder LB, Erickson A, Falinski K, Friedlander AM, Kappel CV, Kittinger JN, McCoy K, Norström A, Nyström M, Oleson KLL, Stamoulis KA, White C, Selkoe KA (2018) Advancing the integration of spatial data to map human and natural drivers on coral reefs. PLoS ONE 13:e0189792
Wetterstrand KA (2019) DNA Sequencing costs: data from the NHGRI Genome Sequencing Program (GSP). www.genome.gov/sequencingcostsdata
Acknowledgements
This project was funded by the National Oceanic and Atmospheric Administration (NOAA), Project# R/SB-17, sponsored by the University of Hawai‘i Sea Grant College Program, SOEST, under Institutional Grant No. NA14OAR4170071 from the NOAA Office of Sea Grant, Department of Commerce (B.W.B. and Stephen A. Karl). The NOAA Office of National Marine Sanctuaries supported R.R.C. through the Dr. Nancy Foster Scholarship program under Award no. NA15NOS4290067. Additional support was provided by grants from the Howard K.L. Castle Foundation (to M.A. Hixon, R.J. Toonen, and B.W.B) and the National Science Foundation (OCE-1558852 to B.W.B.). We thank Matt Craig, Daniel Coffey, Joshua Copus, Joseph DiBattista, Toby Daly-Engel, Jeff Eble, Michelle Gaither, Stephen Karl, Derek Kraft, Jo-Ann Leong, Cassie Lyons, Carl Meyers, Yannis Papastamatiou, David Pence, Joshua Reece, Mark Royer, Zoltan Szabo, Kim Tenggardjaja, Robert Toonen, ‘Aulani Wilhelm, Jill Zamzow, ToBo Lab members, and the crew of the R/V Hi‘ialakai for their logistical support and/or assistance in the field. Field sampling in the Northwest Hawaiian Islands was made possible by the Papahānaumokuākea Marine National Monument and the logistic prowess of Randall Kosaki. Thanks to Jeff Leis for discussions that produced the three hypotheses about larval connectivity. Thanks to editor Morgan Pratchett and two anonymous reviewers for comments that improved the manuscript. The views expressed herein are those of the author(s) and do not necessarily reflect the views of NOAA or any of its subagencies. This is contribution #JC-06-24 from Hawai’i’s Sea Grant College Program, #1887 from the Hawai‘i Institute of Marine Biology, and #11509 from the School of Ocean and Earth Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Peter Francis Cowman
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Coleman, R.R., Bowen, B.W. Genomic assessment of an endemic Hawaiian surgeonfish, Acanthurus triostegus sandvicensis, reveals high levels of connectivity and fine-scale population structure. Coral Reefs 41, 687–697 (2022). https://doi.org/10.1007/s00338-022-02257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02257-4