Abstract
Sponges (Porifera) are a key component of many coral reef ecosystems. In some biogeographic regions, they are considered the dominant benthic fauna and they have the capacity to fulfil many similar roles to reef-building scleractinians. Certainly, sponges predominate at depth, below the critical thresholds of most coral species. The biological and physical attributes of these biogenic communities contribute essential resources for many reef-associated fishes. However, while fish–sponge interactions have been widely documented, there is no global synthesis of the literature on these interrelationships from the perspective of fish ecology. Here we evaluate coral reef fish–sponge relationships, including the role of sponges in providing food and shelter for fishes, the influence fishes have on sponge distribution and abundance and possible outcomes of climate change on fish–sponge interactions. To date, 16 fish families have been shown to associate with 56 different sponge genera, using them as either a source of shelter (n = 17) or a food source (n = 50), although methodologies for the latter currently lack consistency. We demonstrate that a more comprehensive understanding of fish–sponge interactions has been garnered from tropical Atlantic coral reefs, which has resulted in a strong biogeographic bias. While it is evident that in some areas of the Caribbean fish are key in shaping the distribution and abundance of sponges, it is not yet known whether this conclusion applies to the Indo-Pacific. With increasing stresses such as bleaching events impacting coral reef ecosystems, further work is needed to evaluate whether sponges can fulfil similar functional roles to those previously provided by reef-building scleractinians. Similarly, determining whether sponge expansion will compensate for the negative effects of reef degradation, or contribute to their decline, is vital.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are highly heterogeneous, structurally complex environments that support highly diverse communities of other reef taxa (Syms and Jones 2000; Alvarez-Filip et al. 2009; Stella et al. 2011; Emslie et al. 2014). Complex coral formations support a high diversity of reef-fish assemblages, and the more biotically and physically complex they are, the more fish species they support (Graham and Nash 2013; Darling et al. 2017; Epstein and Kingsford 2019; Torres-Pulliza et al. 2020). Reef-building scleractinians are usually considered the most prominent architects of coral reef seascapes (Kerry and Bellwood 2012; Coker et al. 2014). Their three-dimensional structure creates the reef framework, underpinning an array of ecological processes and services, providing important microhabitats and food for a diverse range of fish species and assemblages (Coker et al. 2014; Richardson et al. 2017; Wilson et al. 2019). For many coral reef fish species, this structural complexity is crucial for survival and reproduction (Hixson and Beets 1989; Jones 1991). It may influence settlement success (Öhman et al. 1998; Jones et al. 2004), ameliorate predation (Jones and Syms 1998; Beukers and Jones 1998) and affect the outcome of competitive interactions (Jones 1988; Kerry and Bellwood 2012). With coral reef ecosystems being subjected to increasingly more regular perturbations (e.g., Wilson et al. 2006; Hughes et al. 2018a, b, c), widespread concerns have arisen regarding the mortality of reef-building scleractinians and the associated loss of three-dimensional reef structure (Hoegh-Guldberg et al. 2007; Veron et al. 2009).
In the aftermath of severe mortality events, new reef configurations are beginning to emerge (Bellwood et al. 2004). As a result, the structure of coral reef benthic communities in many regions has changed markedly in recent decades (Done 1992; Hughes 1994; Connell et al. 1997; McMurray et al. 2018; Russ et al. 2020). Consequently, despite ongoing perturbations it is thought that reefs will persist into the future, albeit with altered compositions (Riegl and Purkis 2009; Pandolfi et al. 2011; Bell et al. 2013). So far, most studies considering fish–habitat interactions have primarily investigated interactions that occur between reef-building scleractinians and fishes (Epstein and Kingsford 2019) and the role of corals per se, as a source of food (Chong-Seng et al. 2014) or shelter (Graham and Nash 2013). Reef-building scleractinians usually represent < 50% of available benthic coral reef structure (Fabricius 1997; Osborne et al. 2011). Soft corals, ascidians, macroalgae and sponges often achieve high abundance, particularly with depth (e.g., Wilkinson and Cheshire 1989; Bridge et al. 2019; Pomponi et al. 2019; Spalding et al. 2019), yet the roles of other benthic structure-forming organisms have seldom been considered (Norström et al. 2009; Tebbett et al. 2019; Oakley-Cogan et al. 2020). Understanding the links that occur between coral reef fishes and their available benthic habitats is critical for determining their functional roles and how these relationships might change over time (Darling et al. 2017; Bellwood et al. 2019).
Coral reef seascapes are comprised of a mosaic of ecologically unique, often highly productive, interlinked coral reef communities that vary with spatial scale and depth gradient, including; coral reef fore-reefs, coral reef flats (Nagelkerken et al. 2000), mangrove forests (Fulton et al. 2020), seagrass meadows (Dorenbosch et al. 2005), and mesophotic coral ecosystems (30-150 m depth) (MCEs) (Lesser and Slattery 2018). Sponges are abundant and conspicuous members of many of these communities (Diaz and Rützler 2001). This is particularly true of Caribbean reef communities, where they are prominent in many of the varied habitats present and can rival both hard and soft coral in terms of distribution, abundance and biomass (McMurray et al. 2015). Where sponge gardens occur, sponges appear to fulfil a similar ecological role to scleractinian corals (Schönberg and Fromont 2012) and, therefore, have the capacity to offer unique refugia for the recruitment, growth, reproduction, feeding, and breeding of many coral reef fish species (Hixon and Beets 1989).
Sponges are tropho-dynamically important within these communities (Reiswig 1981; Karplus 2014). Here, in its simplest form, they facilitate nutrient transfer occur by converting dissolved organic matter (DOM) and particulate organic matter (POM) released by benthic producers (e.g., algae and corals) into particulate detritus available to higher trophic levels, including fishes (de Goeij et al. 2013; Rix et al. 2018) or as sponge biomass consumed by spongivores (McMurray et al. 2018). Recent studies, however, suggest these processes may be far more nuanced, with depth, position and sponge morphotype regulating detrital production (see Lesser and Slattery 2012; Slattery and Lesser 2015; Lesser et al. 2020). Globally, the role of sponge metabolism in contributing to the benthic-pelagic carbon flux is now apparent (Bannister et al. 2011, 2012; Gantt et al. 2019), but see McMurray et al. (2018). Moreover, sponges are fleshy and nutritional organisms (Bergquist 1978; Chanas and Pawlik 1995; Dunlap and Pawlik 1996) that harbor a multitude of symbionts/ cryptobentic organisms that have the capacity to act as a food source for invertebrates, turtles and fishes. They also harbor sometimes dense populations of amphipods and other small crustaceans, that are an additional source of food. Hence, sponges have the potential to be significant sources of food to a diverse range of taxa.
Furthermore, sponges are an important source of three-dimensional structural complexity (Pawlik 2011; Powell et al. 2014; Cabaitan et al.2016), with many species exhibiting morphologies that would be supportive of epibiotic relationships (Schönberg et al. 2016; Cabaitan et al. 2016). Their association with recruiting spiny lobster is already well documented in the Caribbean (Butler et al. 2017). Consequently, sponges likely play a key role in supporting species richness and biomass where other structure is lacking (Stoner and Titgen 2003; Ryer et al. 2004; Seemann et al. 2018) and generally form a component of the mosaic of organisms that make up the reef architecture and constitute a foundation species. Globally, there are now many examples of often species-specific fish–sponge associations that have been documented (Fig. 1; Bell and Carballo 2008; Karplus 2014). However, our understanding of the general significance of fish–sponge interactions remains poorly understood. This is especially true outside of the Caribbean, where most of the foundational work on fish–sponge interactions has been undertaken. An increased interest in the role of sponges within coral reef ecosystems has been documented of late. This research effort has been particularly prevalent in regions where sponges, along with other structure-forming organisms, are becoming dominant members of the benthic fauna (Bell et al. 2013, 2015; Pawlik et al. 2018). A number of recent reviews have highlighted the importance of sponges (e.g., Wulff 2006, 2012, 2021; Webster and Taylor 2012; Karplus 2014; Becerro et al. 2012; Bell et al. 2015; Pawlik and McMurray 2020; Edmunds et al. 2020). These studies have focused both on the roles of sponges in benthic communities (including their functional roles) and their relationships with other organisms. However, there is still comparatively little published in terms of an evaluation of sponge interactions with fishes. Wulff’s (2012) review that focused on the ecological interactions of sponges was extensive, but due to the sheer number of sponge associations that occur, concentrated on their ecological interactions with other invertebrate species (e.g., bryozoans, polychaetes, echinoderms, cnidarians). Taylor et al. (2007), Webster (2007) and Webster and Taylor (2012) considered the importance of sponges’ microbial symbionts and sponge diseases. Pawlik and McMurray (2020) recently reviewed both the ecological importance and biogeochemical importance of sponges on coral reefs. To date, Wulff (2006), Karplus (2014) and Pawlik et al. (2018) have been the only authors to focus their reviews specifically on fish–sponge interactions, despite the number of published studies available for consolidation.
Examples of fish–sponge associations. a Oxycheilinus digramma (SL 30 cm) and Cribrochalina spp.; b Salarias segmentatus (SL 11 cm) and Spheciospongia sp.; c Pleurosicya elongata (SL 4 cm) and Ianthella basta; d Coradion melanopus (SL 15 cm) and Xestospongia testudinaria; e Cephalopholis cyanostigma (SL 40 cm) and X. testudinaria; f Cirrhitichtys falco (SL 7 cm) and X. testudinaria. Photo credits: A. Coppock (a, b, e, f). G. Jones (c, d)
Wulff (2006) considered the feeding strategies adopted by fish to consume sponges. Assessing the true extent of fish predation on sponges, however, is difficult and few studies prior to the late 1980s attempted to quantify it (Hourigan et al. 1989). Sponges are adept in their ability to re-aggregate, regenerate and remodel themselves, frequently rendering such interactions inconspicuous, hindering observational changes over time (Wulff 2006). As such, a number of different methods have been adopted to quantify spongivory over the years: gut content analysis (Randall and Hartman 1968; Mortimer et al. 2021), underwater visual census (UVC) (Wulff 1994, 1997a, b) video census (Dunlap and Pawlik 1996, 1998; Mortimer et al. 2021), feeding assays (Pawlik et al. 1995; Chanas and Pawlik 1995; Lindquist and Hay 1996; Ruzicka and Gleason 2009), but as yet there is no standardized approach. Furthermore, whether fish are ingesting the sponge itself, or one of the many symbiotic or epiphytic organisms (e.g., polychaetes, molluscs, echinoderms or other cryptobenthic fishes) that are known to live on the sponges’ outer surfaces, or within the water filled canals of their interior (Karplus 2014) remains unclear. However, the implications of consuming sponge material may be the same from the perspective of sponges themselves.
As a result, our current understanding of spongivory is fragmentory, and as noted by Bell et al. (2020), has a strong biogeographic bias toward the tropical Atlantic, where there is evidence that fish predation has important impacts on sponge assemblages and sponge dynamics (Loh and Pawlik 2014). This has led to considerable confusion when attempting to clarify the roles of top-down and bottom-up controls on sponge distribution and abundance, both within and between different biogeographic regions, and has resulted in polarized opinions amongst sponge ecologists. Pawlik et al. (2018) provided a comprehensive review of the top-down role of fish predation versus nutrient supply in mediating sponge communities, with a primarily Caribbean focus. However, mechanisms for controlling sponge distribution are still highly debated. Karplus (2014) provided a broad over-view of fish–sponge associations from the perspective of sponge ecology. Since then, numerous additional studies on fish–sponge interactions have been published (SCOPUS accessed 06/01/2022). A comprehensive review considering the use of sponges as a source of shelter, through the ‘lens’ of fish ecology, is currently lacking. Furthermore, with global declines in scleractinian coral cover and diversity, there is an increasing need to understand the significance of fish–sponge interactions more broadly in coral reef ecosystems. In many areas, where sponges make a substantial contribution to the remaining biological and physical reef structure, it has been suggested that they might have the capacity to counteract some of effects of reef degradation and provide an alternative habitat for fishes' dependent on structurally complex substratum (Seemann et al. 2018). However, both the role that sponges might fulfil in these new reef formations, or how they might respond to continued perturbations (and the implications this might have for associated fish species), remains unclear (Bell et al. 2013) and is a key area for future research. The overall aim of this review is to provide an up-to-date and geographically broad synthesis of what is known about interactions between fishes and sponges on coral reefs from the perspective of fish ecology. The specific issues addressed in this review are: (1) biogeographic and taxonomic distribution of research into fish–sponge interactions; (2) importance of sponges as a food source for coral reef fishes and the current methods used to quantify spongivory; (3) fish predation on sponges whether it is intense or important, and the role of top-down and bottom up controls in determining sponge distributions; (4) sponge structure as a potential habitat or refuge for various fish species; and (5) the potential implications of disturbances, habitat degradation and climate change on sponges and their associated species.
Biogeographic and taxonomic distribution of studies on fish–sponge interactions
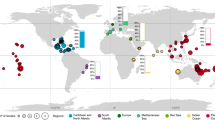
Our findings have been broadly separated into two distinct biogeographic regions (as per Maldonado et al. 2017; Sambrook et al. 2019): the tropical Atlantic (primarily the Caribbean, but also including the Gulf of Mexico, Central America) and the Indo-Pacific, with its much greater geographic range and extent of reef development (Fig. 2a, b). These regions differ in their benthic community structure, associated fish assemblages and nutrient supply (Roff and Mumby 2012; Sambrook et al. 2019). In contrast to the reef area, there have been few studies on fish–sponge interactions in the Indo-Pacific (Fig. 2c). Over half of the fish–sponge interactions described here (58%) were identified in the tropical Atlantic, a region now considered to be a sponge-dominated (McMurray et al. 2018; Pawlik et al. 2018) (Fig. 2d). A particular focus has been apparent in the Caribbean where, in some locations (e.g., the Bahamas, Belize and the Florida Keys) these interactions have been examined on multiple occasions. With this research effort, we are now beginning to gain a thorough understanding of the fish–sponge associations and interactions that occur within this region. However, the Caribbean is frequently considered a much simpler system, in terms of ecosystem modeling, than other coral reef regions worldwide. It has a far more homogeneous environment and comparatively lower levels of diversity (Loh and Pawlik 2017).
World map (a) illustrating the geographic distribution of studies documenting fish–sponge associations on coral reefs, from the literature. Circles are indicative of the locations where these associations were documented, or where studies took place. Insert (b) illustrates the distribution of studies throughout the tropical Atlantic region. Bar graphs showing the number of fish–sponge association studies conducted at each geographic location. c represents locations that fall within the Indo-Pacific region. d represents locations that fall within the tropical Atlantic region
The coral reefs of the Indo-Pacific are remarkably different to those of the tropical Atlantic (Wilkinson and Cheshire 1990). The Indo-Pacific is known for much higher levels of diversity and heterogeneity, making it a far more complex ecosystem to study (Roberts et al. 2002). Certainly, the Indo-Pacific biogeographic region is known for its exceptionally high levels of sponge biodiversity (Bell and Smith 2004; van Soest et al. 2012; de Goeij et al. 2017; Rovellini et al. 2019). Here, although reef-building scleractinians have long been considered the dominant benthic fauna (Norström et al. 2009), sponges still make up a substantial component of the substrata (Diaz and Rützler 2001; Fromont et al. 2006; de Voogd et al. 2009; Fromont et al. 2016). Some sponge genera present here, frequently those considered phototrophic foliose phyllosponges (Wilkinson 1987) (e.g., Ianthella spp., Lamellodysidia spp., Carterospongia spp. and Phyllospongia spp.), are noticeably absent from Caribbean coral reefs (Maldonado et al. 2017). Furthermore, localized physical factors, including light level, water turbulance and depth have been shown to have significant implications for sponge distribution and abundance (Wilkinson and Cheshire 1989; Sadeghi et al. 2008; Bell and Barnes 2000; Slattery and Lesser 2012).
Sponge species diversity and biomass increase with depth (Wilkinson and Cheshire 1989; Lesser and Slattery 2018; Hooper 2019). Their influence is likely to be highest at depths greater than 20 m and on mesophotic reefs (Bridge et al. 2019), beyond the realm of many photosynthetic organisms (including corals) and may even extend to interreefal habitats (Pitcher et al. 2019). Complicating matters further, localized spongivory patterns are likely to be far more varied. Fish (e.g., Siganus puellus) and starfish species (e.g., Protoreaster spp., Oreaster spp.) present here but missing from the tropical Atlantic may be key sponge predators in some habitats (Hoey et al. 2013; Pawlik et al. 2018) These differences in the prominence of fishes, sponges, the degree of habitat complexity and increases in abundance and species diversity with depth may well account for the disparity seen between the number of fish–sponge interactions recorded for each geographic location (and the number of studies conducted). The number of coral reef fish–sponge associations being documented within the Indo-Pacific, however, is steadily rising (e.g., Powell et al. 2014, 2015; Mortimer et al. 2021) but the degree to which generalizations established for the Caribbean apply more broadly needs to be assessed with some focus on comparisons over a similar range of depths.
Where studies have identified fish–sponge interactions, fish species are defined as either spongivorous or sponge-dwelling (Fig. 3). Of the species identified within this study, 51% have been classified as spongivorous fish species and the remaining 49% classed as sponge-dwelling. To date, only three gobiid species, from Caribbean waters (Elacatinus louisae, E. tenox and E. xanthipora), have been identified as potentially being both sponge-dwelling and spongivorous. All three species demonstrate either obligate, morphologically unspecialized associations or facultative associations with sponges. Little is known, however, regarding where, when or what they feed upon (Tyler and Böhlke 1972; Karplus 2014).
Fish species known to interact with sponges. Turquoise is indicative of fish species considered sponge-dwelling species. Orange is indicative of those species considered spongivorous. Blue is indicative of those species that are potentially both spongivorous and sponge-dwelling. Most studies, thus far, have focused on obligate sponge-dwelling gobies. Here, fish species identified as spongivorous have been compiled from studies using a variety of dietary analysis techniques including visual observations, feeding assays, gut content analysis (GCA) and stable isotope analysis (SIA). In this instance the term ‘spongivorous’ encompasses both obligate spongivores and facultative spongivores
Much of what we know in relation to sponge-dwelling fishes is the result of a concerted effort during the 1970’s that aimed to describe fish species that are associated with sponges. Sponges were collected, and their relationships with fish inhabitants recorded (Karplus 2014). Consequently, our knowledge of sponge-dwelling fish species is primarily related to obligate sponge-dwelling fish species, particularly obligate sponge-dwelling gobiid species (≥ 90%). In contrast, comparatively little is known about fish that use sponges as a shelter in either a facultative or fortuitous way. In a similar manner, studies concerning spongivory have typically focussed upon specific fish families (Pomacanthidae, Chaetodontidae, and Tetraodontidae). Although, some fish species commonly considered generalist feeders (Scaridae and Siganidae) have been identified as opportunistic sponge feeders.
Analysis of the literature to date has revealed 56 different genera of sponge that are being used by fishes in some way (Fig. 4), 50 of these sponge genera are used as a source of food and 17 as a source of shelter. The number of species used is unknown. While some genera are represented by multiple species, e.g., Callyspongia spp. incorporates Callyspongia vaginalis, C. procumbens and C. armigera, and more; others are comprised of solely one species or genera e.g., Acarus sp., Aplysina sp. and Velinia sp. Identification of sponges down to the species level has not been consistent. In some instances, the genera are the lowest classification level, whereas other studies have not specified the type of sponge observed. Clearly, sponges are difficult to identify in the field (Wulff 2001), particularly for those outside the field of sponge ecology. This is often attributed to a lack of reliable field guides, resulting in problems identifying sponges down to species level (Diaz and Rützler 2001). While the sponges of tropical Atlantic fore-reefs are comparatively easy to identify, thanks to an array of online resources (e.g., www.spongeguide.org (Zea et al. 2014)). Similar resources are lacking for sponges that form part of the wider tropical Atlantic seascape, or indeed the Indo-Pacific (Diaz and Rützler 2001; Wulff 2001; Bell and Smith 2004). This has frequently led to them being overlooked or mis-identified (Schönberg and Fromont 2012; Bell et al. 2013, 2018). This is particularly true with long-term monitoring where only minor attention is given to sponges, typically reporting them as a single entity. Complicating matters further, sponge morphologies are not static, and sponge morphological diversity can be complex and variable, with many species exhibiting multiple growth forms (Boury-Esnault and Rützler 1997; Bell et al. 2020), often dependent on surrounding environmental variables (Battershill et al. 2010; Bell and Barnes, 2000). As a result, full taxonomic identification is a time-consuming process (Hooper 2003; Fromont et al. 2006, 2016) whereby sponges require identification via the presence of in situ observations, siliceous spicules and molecular or genetic markers (Nielsen et al. 2018). As such, the capacity for precise species identification in dietary studies is limited. Where sponges have been identified for use as shelter, far fewer sponge species have been identified. This is most likely because these studies frequently targeted specific sponge species with the aim of establishing which fish species demonstrated obligate relationships with them (Karplus 2014).
Sponges as a food source
Spongivores are organisms that are both anatomically and physiologically adapted to consume sponges (Wulff, 2021). Sponges were initially considered to be of low nutritional value, and so avoided, a fact often attributed to the minimal levels of predation seen (Burns and Ilan 2003). However, many reef fishes have strong jaws and pharyngeal teeth, capable of grinding the limestone skeletons of hard corals and molluscan shells, so siliceous spicules and spongin should not be problematic (Pawlik 2011). Yet, the morphological adaptations of fishes that allow for successful foraging upon sponges have rarely been considered beyond their initial species description (e.g., Hourigan et al. 1989; Konow and Bellwood 2011). Angelfishes (Pomacanthidae) of the genera Pomacanthus and Holacanthus are considered the archetypal spongivorous fish species of the tropical Atlantic and the Indo-Pacific (Randall and Hartman, 1968; Hobson 1974; Batista et al. 2012; Lorders et al. 2018). They, along with several other prominent reef fish families (e.g., Chaetodontidae Monacanthidae, Scaridaee, Siganidae and Ostraciidae) are considered important consumers of sponges and have been the target of much research concerning sponge predation by fishes (e.g., Randall and Hartman 1968; Ayling 1981; Wulff 1994; Dunlap and Pawlik 1996, 1998). The vast bulk of this work has been conducted within Caribbean, where sponges are now, respectively (relative to coral abundance), either the dominant benthic fauna or rising in abundance (Pawlik 2011; Loh and Pawlik 2014; Pawlik et al. 2018). This has resulted in a strong biogeographic bias (Bell et al. 2020) and to date Wulff (1997b) has been the only researcher to compare and contrast sponge predation between the tropical Atlantic and the eastern Pacific.
Randall and Hartman (1968) established that several Caribbean angelfish species fed predominantly upon sponges. Here, they revealed that fish were consuming small volumes of multiple different sponge species, which led them to conclude that the fish were adopting a ‘smorgasbord’ or rotational feeding strategy, thought to either reduce potential chemical build up (Wulff 1994, 2006) or result from the limited availability of their preferred food source/s (Dunlap and Pawlik 1996). A further two feeding strategies specialist and opportunistic, have since been described. Trunkfishes (genus: Acanthostracion), for example, demonstrate a specialized feeding strategy, concentrating their efforts on consuming only one or two sponge species (e.g., León and Bjorndal 2002; Wulff 1994, 2006). Wulff (1994) observed 85% of trunkfish bites to occur upon Aplysina fulva (Wulff 1994). Parrotfish, however, exhibit an opportunistic feeding strategy, only preying upon cryptic sponges when the opportunity arises (e.g., after a cyclone) (Wulff 1997a, 1997b; Dunlap and Pawlik 1998).
Transplant experiments have revealed that spongivorous fish species (e.g., Scarus guacamaia, Sparisoma chrysipterum) not only actively consumed sponges, but in some cases, exhibit preferences for specific sponge species (e.g., Pawlik 1998; Dunlap and Pawlik 1998; Hill, 1998; Wulff 2021). Various researchers (e.g., Pawlik et al. 1995, 1998, 2011; Chanas and Pawlik 1995; Lindquist and Hay 1996; Lindquist 2002; Burns et al. 2003; Marty et al. 2016) subsequently considered the role of the anti-predatory defence mechanisms (physical and chemical) of both sponges (Pawlik 1993; Pawlik et al. 1995) and sponge larvae (Lindquist and Hay 1996; Lindquist 2002) in dictating these preferences and/or deterring predation. While the presence of physical defences (siliceous spicules) did little to hinder predation (Chanas and Pawlik 1995, 1996; Rüzicka and Gleason 2009), chemical defences (chemical exudates and secondary metabolites) proved far more effective (e.g., Pawlik et al. 1995; Chanas and Pawlik 1995; Lindquist and Hay 1996; Burns et al. 2003; Santonja et al. 2018). As a result, sponges were classed as either ‘palatable’; they are undefended, bear the brunt of predation yet persist but survive via rapid recruitment, faster rates of wound healing and increased growth rates (Uriz et al. 1996; Walters and Pawlik 2005; Loh and Pawlik 2014). Albeit sometimes in cryptic growth forms and restricted to refugia (Pawlik 1998, 2011; Loh and Pawlik 2009, 2013; Marty et al. 2016; Loh et al. 2015). Or ‘unpalatable’; those which produce chemical compounds and secondary metabolites making them distasteful to predators, promoting learned avoidance (Pawlik 1993; Pawlik et al. 1995; Maldonado et al. 2016). Thus, some sponge predators consistently appear to avoid unpalatable (defended) sponges in favor of palatable sponge taxa (Pawlik 2011; Lukowiak et al.2018), while others appear to exhibit a consumer tolerance or an evolved adaptation allowing them to circumvent distastefulness, when exposed to chemical exudates (Hill and Hill 2002).
For those fish species that have been categorized as consumers of ‘unpalatable’ sponge species, we know little regarding their adaptations for consuming chemical exudates. A few marine species (e.g., gastropods and butterflyfishes) are known to feed regularly on allelochemically rich organisms (Vrolijk and Targett 1992; Slattery and Gochfeld 2016; Maldonado et al. 2016). While those that exhibited generalist feeding strategies demonstrated learned avoidance, specialist feeders were capable of bio-transforming allelochemicals via enzymatic detoxification (Slattery and Gochfeld 2016; Maldonado et al. 2016). These alternate responses were thought to reflect varying detoxification capabilities against chemical defences, dependent on degree of dietary specialization (Slattery and Gochfeld 2016). Similar studies are yet to be conducted for spongivorous fishes. However, understanding of the role of allelochemical biotransformation and detoxification, in spongivorous fishes, holds potential significance for understanding patterns of predation (Vrolijk and Targett 1992). Similarly, larval predation is frequently considered a major source of early mortality (Lindquist and Hay 1996). Yet, the role of sponge larvae as a fish food source still needs to be properly considered. It is a field of study that is currently vastly underrepresented. Sponges, particularly those that brood, produce large, conspicuous larvae (Lindquist and Hay 1996), presumably an easy prey target. However, Caribbean sponge larvae appear to be unpalatable to co-occurring fishes, with fish predators recognizing and avoiding feeding assays where chemically defended larvae were present (Lindquist and Hay 1995).
Palatability, however, is consumer dependent (Wulff 2017, 2021) and responses to anti-predatory mechanisms will be species specific and differ with ontogenetic life stage (Pawlik et al. 1995; Chanas et al. 1997; Lindquist 2002; Wulff 2017, 2021). There are many chemically undefended sponges present in abundance on coral reefs, which do not appear to form a substantive part of the diet of spongivorous fishes (Randall and Hartman, 1968; Hill and Hill 2002). Furthermore, many spongivorous fish species have been identified as consuming ‘unpalatable’ sponges. This indicates that either the defences may have a limited impact on more specialized fish species (Hill and Hill 2002), or in the absence of their preferred food source consumption of ‘unpalatable’ sponge species occurs (Wulff 2021), indicative of a feeding preference hierarchy. Although the use of feeding assays has been key in elucidating the preference and/or avoidance reactions for some spongivorous fishes, such methods should only be used to test specific questions (e.g., nutritional quality) and may not always be representative of in situ behaviors over broad spatial scales (Wulff 2021).
Data on spongivory within the Indo-Pacific is sparse (but see Powell et al. 2014, 2015; Mortimer et al. 2021) (Padilla Verdín et al. 2010). Several fish species, however, have recently been identified, via in situ observations, as potential sponge consumers in the Wakatobi region of Indonesia (e.g., Acanthurus pyroferus, Escenius pictus, Centropyge bicolor, Pygoplites diacathus) (Powell et al. 2015; Mortimer et al. 2021). Mortimer et al. (2021) and Powell et al. (2015) specifically targeted fish species that exhibited some degree of interaction with sponges and were considered sponge-grazers. However, discoveries by Hobson (1974), Sano (1989), Eagle and Jones (2004) and Hoey et al. (2013) were identified incidentally as part of more broad scale dietary analysis studies. In order to successfully and effectively assess the true extent of spongivory (particularly via in situ visual observations) an intricate knowledge of both the fish and sponge species present within the region is required (Wulff 2021). Our current knowledge of Indo-Pacific sponge species is inadequate (Bell et al. 2020). Although research to date has allowed a better understanding of how fish–sponge communities function at small spatial scales and within specific biogeographic regions. A concerted effort is now needed to characterise both fish and sponge assemblages at broader spatial scales throughout the Indo-Pacific (Bell et al. 2020). Only then can we understand the full extent of any fish–sponge interactions.
Our understanding of the implications of fishes feeding on the symbiotic, inquilinist or epizoic animals that inhabit sponges is also in its infancy. Identifying dietary targets such as autotrophic and/or cyanobacteria via behavioral observations alone is an impossible task (Clements et al. 2017). Stable isotope analysis (SIA) is, therefore, a valuable tool for providing insight into the use of algae, detritus and bacteria as food targets by fishes (Clements et al. 2017). A number of studies have classified parrotfishes as opportunistic sponge feeders (e.g., Dunlap and Pawlik 1998). But it is sometimes unclear what prey item is being targeted (Wulff 1997a). Burkepile et al. (2019) indicated that sponges are low preference dietary items for some parrotfishes. Instead, it has been suggested that parrotfish are microphages, preying upon the microscopic bacteria inhabiting sponges and other benthic structure forming taxa (Goldberg 2013; Clements et al. 2017; Nicholson and Clements 2020). Limited research has been conducted to quantify this. As such, disentangling which prey items (microbial or sponge) are being consumed continues to be a research priority. Only then can we fully understand the role of parrotfishes in shaping sponge distribution.
Opportunities for directly observing the feeding behaviors and prey choices of fishes are limited (Amundson and Sanchez-Hernandez 2019), and dietary sources, are often ambiguous. As such, several different methods have been adopted to quantity spongivory, summarized in Table 1. Far too frequently throughout the literature, these individual methods have been used in isolation and not in conjunction with one another, leading to sometimes skewed interpretations of the data. Adopting multi-method approach is beneficial when characterising trophic interactions and should be considered as standard (Nielsen et al. 2018; Mortimer et al. 2021). In situ observations, conducted via underwater visual censuses (UVCs) have allowed for the identification of numerous sponge-grazing fish species and detailed insight into their associated feeding behaviors (e.g., Wulff 1994, 1997a, b; Dunlap and Pawlik 1996, 1998; Mortimer et al. 2021). In addition, when conducted by deploying video cameras this has ensured minimal disturbance and prevented sampling bias due to diver presence (Emslie et al. 2018; Sambrook et al. 2020; Mortimer et al. 2021). However, video cameras when used in isolation, may also produce subjective results. When solely observing bites, as is the case with many in situ observation studies, a couple of concerns are apparent. Firstly, this method involves the indirect measurement of predation, and unless observations are undertaken carefully it may prove difficult to ascertain, whether predation is in fact occurring, and/or which prey source is being targeted (Huang et al. 2008; Pawlik et al. 2018). Secondly, as feeding behaviors are often only observed for short periods of time, whether the results are an accurate reflection of true feeding practices remains unclear. Indeed, for some fish species observing bites on the surface of the sponge does not equate to sponge consumption (Nagelkerken et al. 2009; Mortimer et al. 2021).
Gut content analysis (GCA) is considered by some to be the only legitimate method for quantifying sponge consumption (Pawlik et al. 2018). It provides undisputed evidence of ingestion, reflecting the contents of the most recent meal consumed (Wulff 2006), and is therefore thought to be critical in understanding dietary preferences (Pawlik et al. 2018; Amundson and Sanchez-Hernandez 2019). However, this may not be a complete representation of overall diet. The importance of certain food items (i.e., those that are more difficult to digest) may be over-estimated in comparison to soft bodied, easily digestible prey organisms (e.g., soft corals and gelatinous organisms), confounding our interpretation of dietary preferences (Baker et al. 2014; Amundson and Sanchez-Hernandez, 2019). GCA is also problematic when attempting to identify which specific sponge species are being consumed. Many sponge species exhibit multiple spicule morphotypes, while some lack spicules altogether (e.g., Order: Verongiida) (Bergquist 1978), and with limited data on sponge morphology or precise in situ environmental conditions assigning spicule types to a single sponge species is impossible (Lukowiak et al. 2018). Since Randall and Hartman (1968), the use of GCA to verify sponge predation has rarely been conducted. It is a destructive method with ethical implications as it requires high levels of spearing and fish mortality (often of fish that are considered keystone species) (Huang et al. 2008; Mortimer et al. 2021), which has the capacity to substantially alter local community dynamics (Slattery and Gochfeld 2016). Furthermore, in the absence of additional data, for example fish feeding behaviors and/ or sponge abundance and species composition, it cannot be determined whether sponge consumption is deliberate or incidental. Whether sponge consumption is due to preference or sponge availability also cannot be verified (Wulff 2021).
Using direct methods of diet quantification (i.e., GCA) is still necessary, however, in preventing functional misidentifications (Bellwood et al. 2019). Where in depth analysis via GCA has been conducted in the tropical Atlantic (e.g., Randall and Hartman 1968; Hobson 1974; Hourigan et al. 1989; Batista et al. 2012) it is evident that some angelfish species, e.g., Holacanthus ciliaris and H. tricolor, feed almost exclusively upon sponges (≥ 90% by volume) (Fig. 5A). For Caribbean pomacanthids (Pomacanthus arcuatus and P. paru) feeding habits are more varied. It is apparent sponges are not consumed as frequently (30–70% by volume) in this family, yet still represent a key dietary element (Randall and Hartman 1968; Batista et al. 2012). Differences in sponge consumption were noticeable for P. paru. Here, Randall and Hartman noted that in the Caribbean sponges formed up to 70% of P. paru’s diet. Conversely, Batista et al. (2012) noted, in Brazilian waters, while sponges were consumed (32% by volume), algae formed the more substantive part of their diet. It is thought that differences in the availability of sponges at each location may be accountable for this variation. Other fish families, such as Monacanthidae, Ostraciidae and Tetradontidae, exhibit a far more diverse dietary range. While sponges make up much of the diet of Cantherhines macrocerus (86%) (Randall and Hartman, 1968, Caribbean), the same is not true for other tropical Atlantic species examined. Here, stomach contents identified that sponges comprised 11–30% of all food sources consumed, with algae, polychaetes, tunicates and cnidarians being consumed more frequently.
Dietary breakdown of spongivorous fish species that have undergone gut content analysis (GCA) (% by volume). Gut contents have been classified into major classes where appropriate. Letters above each stacked bar represent the study from which the data was extracted.: (RH) Randall and Hartman 1968; (Hob) Hobson 1974; (H) Hourigan et al. 1989; (EJ) Eagle and Jones 2004; (B) Batista et al. 2012. (HY) Hoey et al. 2013, (M) Mortimer et al. 2021. Italicized letters on the x-axis denote fish species. Cp. v: Centropyge vrooliki, Hc: Holacanthus cilliaris, Ht: H. tricolor, Pa: Pomacanthus arcuatus, Pi: P. imperator, Pj: P. jenkinski, Pp: P. paru, Py. d: Pygoplites diacanthus, Px: P. xanthometapon, Cm: Cantherhines C. macrocerus, Cp: C. pullus, Ca.j: Canthergaster jactator Ca.r: Ca.rostrata, Lt: Lactophrys triqueter, Ap: Acanthostracion polygonius, Aq: A. quadricornis, Ab.s: Abudefduf soridus, Ch.f: Chaetodipterus faber, Cht.u: Chaetodon unimaculatus, Z co: Zanclus cornutus, S pn: Siganus punctatus, S pl: S. puellus. Both obligate and facultative spongivores have been described here using GCA
Gut content analysis of Indo-Pacific fish species reveals similar patterns, although, we can clearly see that the species composition of spongivorous fishes may be markedly different (Fig. 5B). Here, pomacanthids Pygoplites diacanthus and Pomacanthus imperator were prolific sponge predators (≥ 94% by volume) (Hobson 1974; Mortimer et al. 2021). Zanclidae (Zanclus cornutus) and Siganidae (Siganus puellus) also demonstrated notable sponge predation (73–85% and 63–74% by volume, respectively). Similarly, several fish families (Siganidae, Pomacentridae and Chaetodontidae) exhibited a far more varied dietary range (5.7–53% by volume). In addition to, but not included in Fig. 5, Hobson (1974), Sano (1989) and Mortimer et al. (2021) identified a number of fish species where a small volume (< 5%) of sponge was consumed by numerous species (e.g., Chaetodon auriga, C. kleinii, Ctenochaetus binotatus Forcipiger flavissimus), indicating that ingestion was either deliberate but low preference, or incidental. Ultimately, it is likely that the importance of sponges as a food source varies with both fish species and location.—The benefits of adopting a multi-method approach are beginning to be appreciated. A good example of this is demonstrated by Nagelkerken et al. (2009). Here, as part of a broader scale study considering dietary overlap in butterflyfishes potential spongivorous fishes Chaetodon adiergastos and Coradion chrysozonus were observed in situ, and then sampled for gut content and stable isotope analysis. SIA does not allow for the identification of individual species being consumed, but instead provides a measure of dietary sources over time (Post 2002), thus eliminating any potential bias resulting from incidental ingestion and/ or mistaken observations. Despite both species being classed as spongivorous and being observed to feed from the surface of sponges, this was not verified through subsequent analyses. Up to 94% of their gut contents were deemed unidentifiable. The remainder rarely contained sponge spicules, but instead a diverse array of invertebrate species. These findings were supported by stable isotope analysis, suggesting that their diets were in fact a mix of sponge tissue and benthic invertebrates (C. chrysozonus) or composed mainly of macrofauna (Ch adiergastos). Similar outcomes were noted by Mortimer et al. (2021), where despite recording numerous bites onsponge tissue (UVC—video footage) by Chaetodon kleinii and Forcipiger flavissimus (> 7500 and > 1500, respectively) sponge tissue remains identified via GCA only amounted to 2%. Future studies, therefore, should adopt multiple methodologies to corroborate findings (Mortimer et al. 2021), Novel advances in technology and scientific methodologies will allow for this, enabling scientists to assign functional roles and unravel the true extent to which macroscopic fish species rely on sponges as part of their diet.
Recent research has continued to refine our knowledge of the feeding preferences and strategies adopted by spongivorous fishes and elucidated further predation behaviors. We now know that different coral reef fish families will preferentially prey upon different sponge species (Wulff 2021). In addition, from the three main feeding strategies described by Randall and Hartman (1968), and Wulff (1997a, 2006), Wulff (2021) has since defined two spongivory styles; routine spongivory and opportunistic spongivory. However, in this instance opportunistic feeding does not solely apply to herbivorous and omnivorous fish species and may also occur when obligate spongivores encounter cryptic sponge species that are usually inaccessible to them. (Wulff 2021). Concurrently Mortimer et al. (2021), in a comparable manner to corallivore classification, classed specialised spongivorous fishes as obligate spongivores, but noted that sponges were also a major prey item for other sponge-grazers. Here, when a substantial part of the fish’s diet was comprised of sponges (≥ 80%), fish were considered obligate spongivores, but where sponges made up the smaller dietary component (70%) fish were noted as regular sponge consumers, adopting a more facultative approach. Thus, obligate spongivores that demonstrate either a ‘smorgasbord’ or specialist feeding strategy will routinely consume sponges and can be considered sponge focussed predators (Mortimer et al. 2021; Wulff 2021). Conversely, both obligate spongivores and facultative spongivores (more commonly defined as herbivores or omnivores) will exhibit opportunistic feeding behaviors (Mortimer et al. 2021; Wulff 2021). Yet, throughout the literature the overarching definition of ‘spongivore’ encompasses all these variations. It is therefore imperative that the scientific methodologies used, fish feeding strategies observed and fish functional group are considered carefully before findings are used to infer spongivore interactions, or lack thereof at larger biogeographic scales.
Impact of fish predation on sponge abundance
Predation can have a major influence on the abundance and composition of benthic marine organisms (Hixon 1983; Batista et al. 2012; Boaden and Kingsford 2015) particularly where competition for space is high (Chadwick and Morrow 2011). It may be ‘intense’, but not necessarily ‘important’ in terms of influencing individuals and population sizes. For example, fish predation on soft corals can be intense, but does not appear to be important by influencing population size or mortality (Garra et al. 2020). The relative importance of top-down (predation) versus bottom-up (food availability) processes that influence the distribution and abundance of populations and assemblages is an active area of research for coral reef ecologists (Graham et al. 2015, 2017; 2020; Houk et al. 2018; Russ et al. 2020). The continued degradation (and associated loss of reef-building scleractinians) arising from repeated climatic and anthropogenic disturbances has prompted rapid changes to local benthic communities. The influence of spongivory in shaping the distribution, abundance, species composition and morphology of sponges has, therefore, become another highly contested area of research in sponge ecology (Pawlik et al. 2018; Wulff 2017, 2021; Lesser and Slattery 2020).
Historically, spongivory by hawksbill turtles (Eretmochelys imbricata) was thought to restrict the distribution, abundance and morphology of sponges within the tropical Atlantic (Meylan 1988; León and Bjorndal 2002; Pawlik et al. 2018). Until relatively recently, following substanital declines in hawksbill numbers, the role of fish in shaping these patterns had not been widely reported. It is now thought that spongiviorous fishes might play a more prominent predatory role in this region and that fish controlling interactions are more common than initially anticipated (Bjorndal et al. 2016; Pawlik et al. 2018). Additionally, sponge fragmentation, occurring as a by-product of fish predation, is an important method of both asexual (Wulff 1991) and sexual (Maldonado and Uriz 1999) reproduction in sponges, allowing them to maximize their dispersal capacity. As such, this reproductive strategy relies upon predation to inform distribution.
Several studies have demonstrated sponge predation, both routine and opportunistic, to be important in determining sponge habitat restriction (e.g., Dunlap and Pawlik 1996, 1998; Wulff 2005, 2017; Pawlik et al. 2018). Sponge species that were usually prominent in mangroves or seagrass meadows, were readily consumed by angelfishes and parrotfishes when transplanted to the coral reef fore-reefs. Similarly, caging experiments have revealed cryptic sponges thrive where predation is minimized, expanding beyond their cryptic refugia (Wulff 1997a). This suggests that, particularly within certain areas of the Caribbean, fish predation upon sponges is the primary selective agent in structuring sponge communities (Pawlik 1998; Pawlik et al. 2008; Lukowiak et al. 2018). Subsequent studies (Pawlik et al. 2013, 2015; Loh and Pawlik 2014) established that localized increases in sponge growth and abundance resulted from increased fishing pressure on key spongivorous species (Pomacentridae). This grazing pressure may, therefore, have a strong influence on abundance, distribution and morphology of sponges (Pawlik 1998; Loh and Pawlik 2009; Batista et al. 2012). However, the potential feedback loop arising from predator induced sponge fragmentation, increased asexual reproduction and subsequent population expansion were not considered in these studies. More recently Wulff’s (2017) study noted the presence of top-down controls between reef sites, but not within, indicating that such controls will likely be more nuanced than initially anticipated.
Predation may also affect the growth of sponges. Predation of Cliona tenuis, a bio-eroding sponge found in the Caribbean, by the parrotfish Sparisoma viride resulted in reduced growth rates (Márquez and Zea 2012). Similarly, Hill (1998) reported that angelfish (family: Pomacanthidae) were critical in regulating sponge-coral competition by reducing growth in Chrondrilla nucula. Although such interactions are likely to be species specific, spongivorous fish could delay or prevent the sponge’s advance over the coral (Hill 1998; Wulff 2006; Márquez and Zea 2012). As such, this is now regarded as a key interaction in mediating sponge-coral encounters (Hill 1998; Loh et al. 2015).
In contrast to the top-down theory, Trussell et al. (2006) and Lesser (2006) argued that bottom-up factors, such as the availability of pico-plankton in the form of particulate organic matter (POM) and dissolved organic matter (DOM) to be paramount in regulating the trophic ecology of sponges. Both POM and DOM are important requirements in sponge maintenance and growth. Similarly, Lesser and Slattery (2013) and Slattery and Lesser (2015) indicated that sponge abundance is controlled via resource availability. Here, substantial increases in sponge size and growth rate, with depth (> 30 m), occurred for both defended and undefended sponge species. Spongivory levels were considered consistent (Lesser and Slattery 2013, 2018; Lesser et al. 2018). POM increases are also known to occur with increasing depth (Lesser and Slattery 2020). Contrary to a number of recent opinions (e.g., Bell et al. 2013), it has been also suggested that the prospect of a regime shift to sponge domination is unlikely to happen. Even where sponge predation is occurring, sponges will be limited by the resources available to them (Lesser and Slattery 2020).
The role of top-down or bottom-up processes in the wider coral reef seascape remains unclear. Within MCEs, for example, trophodynamic pathways may differ markedly from the shallow regions where sponge ecologists have routinely focussed (Brokovich et al. 2008). Fish biodiversity and abundance in MCEs typically peaks at ≤ 30 m and declines with increasing depth thereafter (Pyle et al. 2019). Furthermore, changes to the composition of reef fish assemblages also occur with increased depth (Malcom et al. 2011; McDonald et al. 2016; Kane and Tissot 2017), with a disproportionate likelihood of finding novel or endemic species (Kane et al. 2014). Sponges, conversely, often dominate at depth, where percent cover and abundance of sponges rise (Slattery and Lesser 2012, 2015; Lesser and Slattery 2018). In contrast to the abundance of literature available describing the trophic pathways and relationships that exist between fish assemblages and habitat structures on shallow (≤ 30 m) coral reefs, our knowledge of these relationships in deeper waters (30–150 m) is limited (Brokovich et al. 2008). Current data is inherently biased toward certain areas of tropical Atlantic waters and Hawaii, with other biogeographic regions largely undocumented. Until further data collection has occurred our understanding of site specific differences may well be apparent but unaccounted for (Lesser and Slattery 2018). Thus, it is currently inappropriate to speculate on the nature of these relationships for other MCE regions (Pomponi et al. 2019) and coral reef seascapes.
While it appears for some biogeographic regions (e.g., Pawlik 1998; Pawlik et al. 2008; Lukowiak et al. 2018) that predation by fishes on sponges is intense, and can be important for determining patterns of abundance, this may not be the case elsewhere. Where ecosystems are more heterogeneous, the possibility of top-down versus bottom-up control within the ecosystem may not hold the same degree of importance in shaping sponge distributions. There is likely to be a complex interplay between controls (Wulff 2017) and both routine and opportunistic spongivory may have the capacity to control distributions (Wulff 2017) while pulse events of planktonic productivity drive bottom-up inputs. However, we currently have no knowledge of how such controls might work in the Indo-Pacific (Bell et al. 2020). In addition to fishes and turtles, starfish (Protoreaster spp.) may play a prominent role in localized spongivory (Pawlik et al. 2018) and as such may also contribute to top-down controls. To truly assess localised (and more widespread) spongivory, and the potential influence of top-down versus bottom-up control, we need a thorough understanding of fish and sponge species compositions, local fishing pressures, nutrient flows and localised biophysical factors, but the likelihood that bottom-up and top-down alternate at many locations and for other ecosystems is high. Research on sponge communities and the processes driving them would benefit from long-term studies. Few data sets exist that consider sponge abundance over time. Only through temporal observations can control mechanisms truly be identified (Easson et al. 2013; McMurray et al. 2015; Wulff 2017; Edmunds et al. 2020; Gochfeld et al. 2020).
Sponges as a fish habitat
Increased structural complexity is agreed to be advantageous for fish assemblages (Hixon and Beets 1989). Sponges are often key structures contributing to the three-dimensional architecture of a coral reef, providing an important source of vertical relief (Pawlik 2011; Powell et al. 2014; Cabaitan et al. 2016), this is likely to be especially true with increasing depth (Wilkinson and Cheshire 1989; Hooper 2019; Pomponi et al. 2019). The use of sponges as a refuge from predation (usually fish predation) has been well documented for numerous species (e.g., Abdo 2007; Huang et al. 2008). Here, the sponges’ structure, physical defences and chemical defences are used to the tenants’ advantage. It is possible that many fish species use sponges in this manner, sheltering from physical stressors, or as protection from foraging predators and interfering competitors (Safriel and Ben-Eliahu 1991). To illustrate this point, White et al. (2007) showed that immature sponge-dwelling sharknose cleaner gobies, Elacatinus evelyane, native to the Caribbean, demonstrated significantly faster growth rates when residing on sponges than members of the same species residing in corals, suggesting that these associations must be beneficial. However, despite this progress, little is known regarding most of these partnerships (Karplus 2014).
Early reports (e.g., Radcliffe 1917) described fish–sponge living arrangements for a limited number of species. fish–sponge habitat associations have since been categorized into three overarching groups (Tyler and Böhlke 1972): (1) obligatory sponge dwellers (either morphologically specialized or morphologically unspecialized); (2) facultative sponge dwellers; and (3) fortuitous sponge dwellers. We now have a substantial body of work elucidating the relationships between sponges and several obligate, cryptobenthic fish species (Tyler and Böhlke 1972; Larson 1990; Randall and Lobel 2009) predominantly species from the family Gobiidae. Most sponge associated fishes, however, are only facultatively or fortuitously associated with their hosts (Tyler and Böhlke 1972). Consolidating the available literature will highlight important future research directions and provoke new research questions.
Obligate sponge dwellers, as defined by Tyler and Böhlke (1972), are those fish species that are either morphologically highly specialized or morphologically unspecialized and are only known to live on or within sponges. Tubular type sponge morphologies, prevalent throughout the tropical Atlantic, were the most common sponge association described as exhibiting these associations. It was noted that a key trait displayed by all obligate sponge-dwelling fish species is their small size, with many species rarely being larger than 5 cm (Total Length). This size constraint often arises as a consequence of sponge osculum diameter (Henkel and Pawlik 2005), which mediates access to the internal cavity of the sponge. As a result, the majority (90%) of all obligate sponge-dwelling fishes belong to the family Gobiidae (Karplus 2014). As a result of a concerted effort throughout the 1970s, obligate sponge-dwelling gobies are now loosely categorized into three distinct groups.
The first group of gobies is comprised of those of the genera Evermannichtys, Risor and Pariah, commonly found throughout the tropical Atlantic. They frequently exhibit localized morphological and phenotypical adaptations for living inside sponges and are usually located either inside the water canals or within the large cavity of the sponge. The second group, commonly found throughout the Western Atlantic, includes gobies belonging to the genus Elacatinus. These gobies usually occupy the lumens and outer surfaces of either tubular or finger-like sponges. Finally, the third group consists of the genera Luposicya, Phyllogobius, Pleurosicya and Bryaninops. These gobies are known to inhabit the upper and lower surfaces of leaf-shaped/ foliose sponges within the Indo-Pacific (Karplus 2014).
A substantial volume of work has attempted to elucidate these relationships, and as a result a number of adaptations designed to assist with survival and fitness in obligate sponge-dwelling fishes have been identified. These include elongated body shapes and modified ctenoid scales (Böhlke and Robins 1969; Tyler and Böhkle 1972), thought to facilitate movement and assist grip within the sponge’s internal passages, and adapted dentition (e.g., Risor ruber and Luposicya lupus) (Tyler and Böhkle 1972; Larson 1990) to aid with species specific feeding strategies. Despite this adapted dentition, many obligate sponge-dwelling fishes are not thought to feed on the sponge itself, their preferences are thought to be mucus, resident invertebrate, or resident parasitic polychaetes (Randall and Lobel 2009; Karplus 2014).
Most fishes are, however, only facultatively associated with their sponge hosts. Tyler and Böhkle (1972) defined these facultative fish species as morphologically generalized fishes that spend at least a portion of their lives either within or on sponges, but that are also known to occur in other habitats. Since these initial studies, limited research has been conducted to elucidate these facultative relationships. Where progress on the subject has been made, this is predominantly with regard to temperate, polar and deep-water fish species (e.g., flatfish and halibut), where interrelationships with sponges may be far less complex and they are primarily used either as a temporary shelter or a source of prey organisms (Freese and Wing 2003; Stoner and Titgen 2003; Ryer et al. 2004; Karplus 2014).
Tyler and Böhlke’s (1972) final category, fortuitous sponge dwelling fishes, describes a wide variety of fish families, some of which have only been collected from sponges on a limited number of occasions. This definition of a fortuitous sponge-dwelling fish also covers those species that demonstrate known breeding associations with sponges but are not obligate sponge-dwellers. Here, the process of egg-brooding prompts fish that are usually documented as occupying different habitats to deposit their eggs either the in sponge or within its internal passages. In addition to the eggs being protected from predation via the sponge’s physical and chemical defences, it is thought that deposition ensures a continuous oxygen supply as well as potential antibacterial, antiviral and anti-fungal benefits (Munehara 1991; Karplus 2014). Our knowledge about the reproductive strategies of sponge-dwelling fishes, however, is restricted to a study by Colin (1975) conducted over 40 years ago. The distinction between facultative sponge-dwellers and fortuitous sponge-dwellers is somewhat blurred. Tyler and Böhlke (1972) observed that non-obligatory fish–sponge associations were poorly understood, a fact reiterated by Karplus (2014). This observation remains true today, particularly with regard to coral reef fish–sponge associations. Moreover, whether commercially important coral reef fish species use sponges as a source of shelter at key ontogenetic stages has not been examined. However, fishes are using sponges for shelter as part of the biotic architecture of reefs.
The behavioral preferences and post-settlement processes of sponge-dwelling fish, obligate or otherwise, have rarely been determined (Majoris et al. 2018). Pawlik et al. (2002) concluded that olfactory cues emitted by sponges were of little importance in deterring fish (predation). However, the reverse may well be true for fish that are attempting to locate suitable areas of shelter. Sponge-dwelling brittlestars (Ophiothrix suensonii, O. lineata) (Henkel and Pawlik 2005), and crustaceans (Hemimysis margalesi and Palaemon serratus) (Santonja et al. 2018) have been shown to actively detect and select live sponge habitats over a similar, alternative refuges. Similarly, Majoris et al. (2018) recently began to refine our understanding of settlement distributions and habitat preferences for the neon goby Elacatinus lori, by demonstrating the importance of visual cues at settlement in locating specific sponge habitats, predominantly Agelas fistularis. This suggests that some fish species not only prefer sponge habitats, but also have the mechanisms for recognizing and locating live sponges (Henkel and Pawlik 2005; Santonja et al. 2018). Given that fishes are known for their ability to identify specific chemosensory cues at low concentrations (Sweatman, 1983, 1985; Belanger et al. 2006), it is entirely possible that similar processes (both visual and chemosensory) are responsible for directing both the juvenile and adults of other fish species toward suitable sponge sites.
The future of fish–sponge interactions
Climate change is rapidly emerging as a universal threat to the integrity and function of coral reef ecosystems (Hughes et al. 2017, 2018c). Many scientists now agree that changes in climatic conditions are happening at a much faster rate than previously anticipated, potentially reducing the capacity for hard corals and reef communities to adapt successfully (Hoegh-Guldberg et al. 2007; Przeslawski et al. 2008). The most substantial of these alterations to benthic communities has been a decline in hard coral cover, due to increases in both natural and anthropogenic stressors that have induced recruitment failures, bleaching and mortality (Bellwood et al. 2004). This has resulted in reduced topographic complexity and habitat homogenization (Komyakova et al. 2013; Seemann et al. 2018). The effects of this coral loss on associated fish species and assemblages are now well established and have frequently been linked to declines in species richness, abundance and biodiversity (Jones et al. 2004; Silveira et al. 2015; Richardson et al. 2017; Komyakova et al. 2013, 2018).
Consequently, space has become available to be rapidly colonized by other benthic organisms, and as a result a variety of different reef configurations have begun to emerge (Norström et al. 2009; Tebbet et al. 2019). If reef-building scleractinians are unable to recover, a phase shift may occur, whereby reefs reach an alternative stable state, characterized by changes in ecosystem processes, function and community structure (Norström et al. 2009). To date, phase shifts on coral reefs have almost always been associated with shifts from hard coral dominated environments to those dominated by macroalgae (e.g., Done 1992; Norström et al. 2009). As a result, the past two decades have seen much research effort devoted to understanding the implications of habitat shifts on coral reefs toward algal dominated environments (e.g., Done 1992). Until recently, the prospect of a shift toward a non-algal alternate state (Norström et al. 2009), including the possibility of a sponge dominated scenario has largely been overlooked (Bell et al. 2013, 2018). The likelihood of a sponge dominated assemblage becomes increasingly plausible where coral bleaching and other perturbations affect hard and soft corals in deep water environments (Frade et al. 2018).
In the absence of high levels of hard coral cover, it has been suggested that sponges may be pivotal for sustaining both the biomass and species richness of coral reef fish communities (Seemann et al. 2018). Sponges generally grow faster and reproduce more quickly (relatively speaking) than many reef-building coral species (Santavy et al. 2013), and therefore, have the capacity to provide considerable physical and biological structure, where vertical relief is otherwise lacking (Kuffner et al. 2007; Santavy et al. 2013). The possibility of a sponge dominated reef, therefore, is not unfounded. Whether they can fulfil similar roles to reef-building scleractinians, in terms of providing shelter or acting as an important source of food, or whether they can counteract the effects of coral reef degradation (Seemann et al. 2018), is not yet known. For obligate corallivorous fishes this will not be possible. However, for more generalist fish species it is possible that the vertical relief and three-dimensional structure provided by the sponge could act as a suitable substitute (Bell et al. 2013; Powell et al. 2015). It has been demonstrated that structurally complex sponge species, such as the Spaghetti Sponge (Callyspongia samerensis), might serve as a nursery site for some reef fish species that usually seek shelter offered by branching coral species (Cabaitan et al. 2016). Similarly, large, robust sponge species (for example the barrel sponges Xestospongia muta, and X. testudinaria) could provide important structural habitat, similar to that of massive mound corals (Acosta et al. 2015). These massive sponges may play a vital role in supporting species richness and biomass and expanding trophic levels in otherwise depauperate fish communities (Seeman et al. 2018).
A growing volume of research has begun to consider the response of sponges to climate change and other environmental stressors (e.g., ocean acidification, increases in sea surface temperature and tropical cyclones) or future environmental change (Gochfeld et al. 2020). Although the available evidence suggests that sponges might not be particularly threatened (Bell et al. 2013), little information is available regarding the responses of most sponge species to environmental pressures, with few species being studied extensively (Bell et al. 2015). The skeletons of sponges, specifically Demospongiae, are frequently made of siliceous glass or proteins, in theory, rendering them less susceptible to erosion due to ocean acidification, thus giving them a competitive advantage (Pawlik 2011). Additionally, in contrast to many reef-building scleractinians, which exhibit a sensitivity to increases in sea surface temperatures—often resulting in the expulsion of zooxanthellae (and bleaching)—sponges are thought to be comparatively more tolerant to increases in sea surface temperatures (Przeslawski et al. 2008; Bell et al. 2013; Ramsby et al. 2018) suggesting that sponges could be well adapted to act as an alternative habitat structure. In the Caribbean, where sponges are now considered the dominant benthic structure (Pawlik 2011; Pawlik et al. 2018; Loh and Pawlik 2014), and in Indonesia where high levels of sedimentation have resulted in a reduced diversity but increased abundance of particular sponge species (Powell et al. 2014), concerns have been raised. The environmental implications arising from both increases and decreases in sponge abundance are not fully understood (Wulff 2012). Sponge abundance is known to fluctuate (Edmunds et al. 2020). Increases in sponge cover might come at the expense of other sessile organisms, such as reef-building scleractinians, but could improve substrate stability and/or water quality. Conversely, decreases in the abundance of sponges could lead to reduced water quality due to a lack of filtration, loss of reef architecture and an increase in predator-sponge ratios which might result in a more unstable substrate (Wulff 2012). Either outcome will have significant implications for local fish populations and assemblages.
Sponges may be impacted by environmental stressors in other capacities. For example, they are prone to both fungal and bacterial infections, which can decimate entire populations (e.g., Cebrian et al. 2011; Stabili et al. 2012; Easson et al. 2013; Ereskovsky et al. 2019). Sponges are not usually considered susceptible to bleaching. They typically occur below critical thermocline thresholds where they are less vulnerable to disturbances (e.g., Wilkinson and Cheshire 1989; Graham et al. 2015). However, a growing volume of evidence suggests that continued rates of sea surface temperature increase could be problematic in the future (Hooper 2019). Unlike corals, sponges, have not commonly been shown to die after bleaching (McMurray et al. 2011). However, a few recent studies have begun to look at the consequences for sponges after the expulsion of their photosynthetic endosymbionts. The giant barrel sponge, Xestospongia muta, along with the encrusting several clionid sponge species (Hill et al. 2016), are examples of sponge species that are known to be vulnerable to a form of bleaching (Vicente 1990; Williams and Bunkley-Williams 1990). McMurray et al. (2011) argued that unlike, hard corals, the bleaching of X. muta had no significant effects. Sponges were able to regain their pigmentation, implying that cyanobacterial symbionts provide little or no benefit to their host species. Contradicting this, Ramsby et al. (2018) demonstrated that although temperature increases up to 30 degrees Celsius had a negligible effect on encrusting sponge species Cliona orientalis (GBR, Australia), a rise of a further two degrees, to 32 degrees Celsius, increased respiratory rates, reduced energy reserves and induced bleaching. In this instance pigmentation could not be regained. Thus, responses to increased sea surface temperatures may vary with both species and location.
Increases in sea surface temperature are presumed to indirectly accelerate the spread of encrusting (bio-eroding) sponge species, through inducing stress on corals (Márquez and Zea 2012). Not only could this contribute to reduced levels of structural complexity as the sponge envelops the coral but accelerated rates of bio-erosion dissolving the substratum could result in reduced levels of structural stability (Ramsby et al. 2018). As the structural shelter provided hard corals is crucial to increased biodiversity, this overgrowth may prompt subsequent implications regarding fish settlement and early survival, as well as post-recruitment interactions (Hixon and Beets 1989). At the very least, a change from a coral-dominated to sponge-dominated ecosystem will substantially alter the chemosensory seascape, having important implications for larval fish recruitment. Until it is verified how sponges might respond to both localized and global environmental pressures, whether they might help or hinder the survival of coral reef fish assemblages cannot be fully understood. However, any contribution to reef architecture, in the absence of other habitat formers such as reef-building scleractinians, is likely to benefit at least some fishes.
Conclusions
We are gradually gaining a better understanding of fishes’ relationships with sponges. Sponges contribute to a mosaic of biotic architecture, especially with depth, which influences assemblages of fishes by providing food and shelter. Studies need to continue to reach beyond Caribbean waters and encompass a far wider range of biogeographic regions. Only through identifying the sponge species present in other biogeographic regions, specifically across large areas of the Indo-Pacific, can we understand the factors influencing sponge distribution in these more complex systems. Fish clearly have the ability to affect assemblages of sponges, be that through mortality or enhancing asexual reproduction through the production of fragments. The contribution of predation to a top-down affect combined with potential bottom-up processes on population abundance is yet to be determined. The relative importance of these processes is likely to vary by ocean basin and locations within oceans. For example, there is strong evidence that reefs in the tropical Atlantic are heavily structured via top-down predation by fishes, but it is unclear whether the same is true of the Indo-Pacific. Similarly, the extent to which sponges are used as a food source by Indo-Pacific fishes needs to be clarified. Multiple methods include in situ behavioral observations, gut content and stable isotope analysis techniques will provide more clarity on the nature of spongivory. Sponges undoubtedly have the capacity to act as an alternative source of shelter for a number of cryptobenthic fishes, particularly those considered obligate sponge-dwellers. We now need to focus on narrowing our current knowledge gap in relation to facultative and fortuitous sponge-dwelling fishes. Given the current rate of climate related hard coral degradation within many coral reef ecosystems worldwide, it is imperative that the roles played by major benthic groups such as sponges is understood.
References
Abdo DA (2007) Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from Southwest Australia. Mar Biol 152:845–854
Acosta C, Barnes R, McClatchey R (2015) Spatial discordance in fish, coral, and sponge assemblages across a Caribbean atoll reef gradient. Mar Ecol 36:167–177
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbean Coral Reefs: Region-Wide Declines in Architectural Complexity. Proc Royal Soc B 276:3019–3025
Amundson P, Sanchez-Hernandez J (2019) Feeding studies take guts – critical review and recommendations of methods for stomach contents analysis in fish. J Fish Biol 95:1364–1373
Ayling AM (1981) The role of biological disturbance in temperate subtidal encrusting communities. Ecology 62:830–847
Baker R, Buckland A, Sheaves M (2014) Fish gut content analysis: robust measures of diet composition. Fish. Fish. 15:s 170–177.
Bannister RJ, Hoogenboom MO, Anthony KRN, Battershill CN, Whalan S, Webster NS, de Nys R (2011) Incongruence between the distribution of a common coral reef sponge and photosynthesis. Mar Ecol Prog Ser 423:95–100
Bannister RJ, Battershill CN, de Nys R (2012) Suspended sediment grain size and mineralogy across the continental shelf of the Great Barrier Reef: Impacts on the physiology of a coral reef sponge. Cont Shelf Res 32:86–95
Batista D, da Silva Muricy GR, Rustum Andréa B, Campos Villaça R (2012) High intraspecific variation in the diet of the french angelfish Pomacanthus paru in the south-western Atlantic. Braz J Oceanogr 60:449–454
Battershill. C.N., Bergquist, P.R., Cook, S.d C. (2010) Phylum Porifera, Pp. 58–135. In: de Cook SC (Ed.) New Zealand Coastal Marine Invertebrates 1. Pp 640. Canterbury University Press, Christchurch, New Zealand
Becerro MA, Uriz MJ, Maldonado M, Turon X (2012) Advances in sponge science: phylogeny, systematics, ecology. Adv Mar Biol 62:1–355
Belanger RM, Corkum LD, Li W, Zielinski BS (2006) Olfactory sensory input increases gill ventilation in male round gobies (Neogobius melanostomus) during exposure to steroids. Comp Biochem Physiol Part A Mol Integr Physiol 144:196–202
Bell JJ, Barnes DKA (2000) The distribution and prevalence of sponges in relation to environmental gradients within a temperate sea lough: Vertical cliff surfaces. Divers Distrib 6:283–303
Bell JJ, Carballo JL (2008) Patterns of sponge biodiversity and bundance across different biogeographic regions. Mar Biol 155:563–570
Bell JJ, Smith D (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Ass U.K. 84:581–591
Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS (2013) Could some coral reefs become sponge reefs as our climate changes? Glob Change Biol 19:2613–2624
Bell JJ, Mcgrath E, Biggerstaff A, Bates T, Cárdenas CA, Bennett H (2015) Global conservation status of sponges. Conserv Biol 29:42–53
Bell JJ, Rovellini A, Davy SK, Taylor MW, Fulton EA, Dunn MR, Bennett HM, Kandler NM, Luter HM, Webster NS (2018) Climate change alterations to ecosystem dominance: How might sponge-dominated reefs function? Ecology 99:1920–1931
Bell JJ, McGrath E, Kandler NM, Marlow J, Beepat SS, Bachtiar R, Shaffer MR, Mortimer C, Micaroni V, Mobilia V, Rovellini A, Harris B, Farnham E, Strano F, Carballo JL (2020) Interocean patterns in shallow water sponge assemblage structure and function. Biol Rev 95:1720–1758
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bellwood DR, Streit RP, Brandl SJ, Tebbett SB (2019) The meaning of the term ‘function’in ecology: a coral reef perspective. Funct Ecol 33:948–961
Bergquist PR (1978) Sponges. Hutchinson, London
Beukers JS, Jones GP (1998) Habitat complexity modifies the impact of piscivores on a coral reef fish population”. Oecologia 114:50–59
Bjorndal KA, Chaloupka M, Saba VS, Diez CE, Van Dam RP, Krueger BH, Horrocks JA (2016) Somatic growth dynamics of west Atlantic Hawksbill sea turtles: A spatio-temporal perspective. Ecosphere 7:1–14
Boaden AE, Kingsford MJ (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6:46. https://doi.org/10.1890/ES14-00292.1
Böhlke JE, Robins CR (1969) Western Atlantic sponge-dwelling gobies of the genus Evermannichthys: Their taxonomy, habits and relationships Proc Acad Natl Sci 121: 1–24
Boury-Esnault N, Rützler (1997) Thesaurus of sponge morphology. Smithsonian Contributions to Zoology 596:1–55
Bridge TCL, Webster JM, Sih TL, Bongaerts P (2019) The Great Barrier Reef outer shelf. In: Hutchings P, Kingsford MJ, Hoegh-Guldberg O (eds) The Great Barrier Reef: Biology, environment and management. CSIRO Publishing and CRC Press, Australia, pp 229–246
Brokovich E, Einbinder S, Shasar N, Kiflawi M, Kark S (2008) Descending to the twilight-zone: changes in coral reef fish assemblages along a depth gradient down to 65m. Mar Ecol Prog Ser 371:253–262
Burkepile DE, Adam TC, Roycroft M, Ladd MC, Munsterman KS, Ruttenberg BI (2019) Species-specific patterns in corallvory and spongivory among Caribbean parrotfishes. Coral Reefs 38:417–423
Burns E, Ifrach I, Carmeli S, Pawlik JR, Ilan M (2003) Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical Defense”. Mar Ecol Prog Ser 252:105–114. https://doi.org/10.3354/meps252115
Burns E, Ilan M (2003) Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. II. Physical defense. Mar Ecol Prog Ser 252: 115–123 doi
Butler MJ, Dolan TW, Hunt JH, Rose KA, Herrnkind WF (2017) Recruitment in degraded marine habitats: A spatially explicit, individual-based model for spiny lobster. Ecol Appl 15:902–918
Cabaitan PC, Gomez ED, Yap HT (2016) The spaghetti sponge Callyspongia samarensis (Wilson, 1925) provides temporary habitat for reef fish recruits”. Mar Biodivers 46:541–542
Cebrian E, Uriz MJ, Garrabou J, Ballesteros E (2011) Sponge mass mortalities in a warming Mediterranean Sea: are cyanobacteria-harboring species worse off? - PLoS One [https://doi.org/10.1371/journal.pone.0020211]
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. In: Dubinsky Z, Stambler N (Eds) Coral reefs an ecosystem in transition. Springer Dordrecht. [https://doi.org/10.1007/978-94-007-0114-4]
Chanas B, Pawlik JR (1995) Defenses of Caribbean sponges against predatory Reef Fish. II. Spicules, Tissue Toughness, and Nutritional Quality. Mar Ecol Prog Ser 127:195–231
Chanas B, Pawlik JR (1996) Does the skeleton of a sponge provide a defense against predatory reef ish? Oecologia 107:225–231
Chanas B, Pawlik JR, Lindel T, Fenical W (1997) Chemical defense of the Caribbean sponge Agelas clathrodes (Schmidt). J Exp Mar Biol Ecol 208:185–196
Chong-Seng KM, Graham NAJ, Pratchett MS (2014) Bottlenecks to coral recovery in the Seychelles. Coral Reefs 33:449–461
Clements KD, German DP, Piché J, Tribollet A, Choat JH (2017) Intergrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc Lond 120:729–751
Coker DJ, Wilson SK, Pratchett MS (2014) Importance of live coral habitat for reef fishes. Rev Fish Biol Fisher 24(1):89–126
Colin PL (1975) The neon gobies: The comparative biology of the gobies of the genus Gobiosoma subgenus Elacatinus (Pisces: Gobiidae) in the tropical western north Atlantic Ocean. Tropical Fish Hobbyist publications. Neptune. New Jersey. 304 pp. ISBN 0-87-666-450-8
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time”. Ecol Monogr 67:461–488
Darling ES, Graham NAJ, Januchowski- Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits and reef fish assemblages. Coral Reefs 36:561–575
de Goeij JM, Lesser MP, Pawlik JR (2017) Nutrient fluxes and ecological functions of coral reef sponges in a changing ocean. In: Carballo JL, Bell JJ (eds) Climate Change, Ocean Acidification and Sponges. Springer International Publishing, Switzerland pp 373–410
de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W (2013) Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 342:108–110
de Voogd NJ, Becking LE, Cleary DFR (2009) Sponge community composition in the Derawan Islands NE Kalimantan, Indonesia. Mar Ecol Prog Ser 396:169–180
Diaz CM, Rützler K (2001) Sponges: An essential component of Caribbean coral reefs”. Bull Mar Sci 69:535–546
Done TJ, (1992) Phase shifts in coral reef communities and their ecological significance.Hydrobiologia 247: 121–32
Dorenbosch M, Grol MGG, Christianen MJA, Nagelkerken I, van der Velde G (2005) Indo-Pacific seagrass beds and mangroves contribute to fish density and diversity on adjacent coral reefs. Mar Ecol Prog Ser 302:63–76
Dunlap M, Pawlik JR (1996) Video-monitored predation by caribbean reef fishes on an array of mangrove and reef sponges. Mar Biol 126:117–123
Dunlap M, Pawlik JR (1998) Spongivory by parrotfish in Florida mangrove and reef habitats. Mar Ecol 19:325–337
Eagle JV, Jones GP (2004) Mimicry in coral reef fishes: ecological and behavioural responses of a mimic to its model. J Zool Lond 264:33–43
Easson CG, Slattery M, Momm HG, Olson JB, Thacker RW, Gochfeld DJ (2013) Exploring individual- to population-level impacts of disease on coral reef sponges: Using spatial analysis to assess the fate, dynamics, and transmission of Aplysina red band syndrome (ARBS). PLoS ONE 8(11):e79976. https://doi.org/10.1371/journal.pone.0079976
Edmunds PJ, Coblentz M, Wulff JL (2020) A quarter-century of variation in sponge abundance and community structure on shallow reefs in St. John, US Virgin Islands. Mar. Biol. 167: 135 [doi: https://doi.org/10.1007/s00227-020-03740-8]
Emslie MJ, Cheal AJ, Johns KA (2014) Retention of habitat complexity minimizes disassembly of reef fish communities following disturbance: A large-scale natural experiment. PLoS ONE. https://doi.org/10.1371/journal.pone.0105384
Emslie MJ, Cheal AJ, MacNeil MA, Miller IR Sweatman HPA (2018) Reef fish communities are spooked by SCUBA surveys and may take hours to recover. Peer J e4886 [doi:https://doi.org/10.7717/peerj.4886]
Epstein HE, Kingsford MJ (2019) Are soft coral habitats unfavourable? A closer look at the association between reef fishes and their habitat. Environ Biol Fish 102:479–497
Ereskovsky A, Ozerov DA, Pantyulin AN, Tzetlin AB (2019) Mass mortality event of White Sea sponges as the result of high temperature in summer 2018. Polar Biol 42:2313–2318
Fabricius KE (1997) Soft coral abundanceon the central Great Barrier Reef: Effects of Acanthaster planci, space availability and aspects of the physical environment. Coral Reefs 16:159–167
Frade PR, Bongaerts P, Englebert N, Rogers A, Gonzalez-Riviero M, Hoegh-Guldberg O (2018) Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nat Commun. https://doi.org/10.1038/s41467-018-05741-0
Freese LJ, Wing BL (2003) Juvenile red rockfish, Sebastes sp., associations with sponges in the Gulf of Alaska. Mar Fish Rev 38–42
Fromont J, Vanderklift MA, Kendrick GA (2006) Marine sponges of the Dampier Archipelago, Western Australia: patterns of species distributions, abundance and diversity. Biodivers Conserv 15:3731–3750
Fromont J, Wahab MAA, Gomez O, Ekins M, Grol M, Hooper JNA (2016) Patterns of Sponge Biodiversity in the Pilbara. Northwestern Australia Diversity 8(4):14–16
Fulton CJ, Berkström C, Wilson SK, Abesamis RA, Bradley M, Åkerlund C, Barrett LT, Bucol AA, Chacin DH, Chong-Seng KM, Coker DJ, Depczynski M, Eggertsen L, Eggertsen M, Ellis D, Evans RD, Graham NAJ, Hoey AS, Holmes TH, Kulbicki M, Leung PTY, Lam PKS, van Lier J, Matis PA, Noble MM, Pérez-Matus A, Piggott C, Radford BT, Tano S, Tinkler P (2020) Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish. https://doi.org/10.1111/faf.12455
Gantt SE, McMurray SE, Stubler AD, Finelli CM, Pawlik JR, Erwin PM (2019) Testing the relationship between microbiome composition and flux of carbon and nutrients in Caribbean coral reef sponges”. Microbiome 7:1–13
Garra S, Hall A, Kingsford MJ (2020) The effects of predation on the condition of soft corals. Coral Reefs 39:1329–1343
Gochfeld DJ, Olson JB, Chaves-Fonnegra A, Smith TB, Ennis RS, Brandt ME (2020) Impacts of Hurricanes Irma and Maria on coral reef sponge communities in St Thomas, U.S. Virgin Islands Estuaries Coast 43:1235–1247
de Goeij JM, Lesser MP, Pawlik JR (2017) Nutrient fluxes and ecological functions of coral reef sponges in a changing ocean. In: Carballo JL, Bell JJ (eds). Climate change, ocean acidification and sponges: Impacts across multiple levels of organization Cham: Springer International Publishing
Goldberg WM (2013) The biology of reefs and reef organisms. University of Chicago Press, Chicago
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97
Graham NAJ, McClanahan TR, MacNeil MA, Wilson SK, Cinner JE, Huchery C, Holmes TH (2017) Human disruption of coral reef trophic structure. Curr Biol 27:231–236
Graham NAJ, Robinson JPW, Smith SE, Govinden R, Gendron G, Wilson SK (2020) Changing role of coral reef marine reserves in a warming climate”. Nat Commun 11:1–8
Henkel TP, Pawlik JR (2005) Habitat use by sponge-dwelling brittlestars. Mar Biol 146:301–313
Hill MS (1998) Spongivory on Caribbean reefs releases corals from competition with sponges. Oecologia 117:143–150
Hill MS, Hill AL (2002) Morphological plasticity in the tropical sponge Anthosigmella varians: Responses to predators and wave energy. Biol Bull 202:86–95
Hill MS, Walter C, Bartels E (2016) A mass bleaching event involving Clionaid sponges”. Coral Reefs 35:153
Hixon MA (1983) Fish grazing and community structure of coral reefs and algae: A synthesis of recent studies. In: Reaka MS (ed). The Ecology of Deep and Shallow Coral Reefs Symposia Series for Undersea Research. NOAA/ NURP, Washington, D.C. pp 79–87
Hixon MA, Beets JP (1989) Shelter characteristics and Caribbean fish assemblages: Experiments with artificial reefs. Bull Mar Sci 44:666–680
Hobson E (1974) Feeding relationships of teleostean fish fauna on coral reefs in Kona, Hawaii. Fishery Bulletin 72:915–1031
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoey AS, Brandl SJ, Bellwood DR (2013) Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: Implications for ecosystem function. Coral Reefs 32:973–984
Hooper JNA (2019) Sponges. In: Hutchings P, Kingsford MJ, Hoegh-Guldberg O (eds) The Great Barrier Reef: Biology, environment and management. CSIRO Publishing and CRC Press, Australia, pp 229–246
Hooper JNA (2003) Sponguide: Guide to sponge collection and identification. Queensland Museum, Brisbane, Australia [http://catalog.hathitrust.org/api/volumes/oclc/55992850.html]
Houk P, Cuetos-Bueno J, Kerr AM, McCann K (2018) Linking fishing pressure with ecosystem thresholds and food web stability on coral reefs. Ecol Monogr 88:109–119
Hourigan TF, Stanton FG, Motta PJ, Kelley CD, Carlson B (1989) The feeding ecology of three species of Caribbean angelfishes (Family Pomacanthidae). Environ Biol Fish 24:105–116
Huang JP, McClintock JB, Amsler CD, Huang YM (2008) Mesofauna associated with the marine sponge Amphimedon viridis. Do its physical or chemical attributes provide a prospective refuge from fish predation?” J Exp Mar Biol Ecol 362: 95–100
Hughes TP, T, (1994) Castrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough J, Morrison TH, Palumbi SR, van Nes EH, Scheffer M (2017) Coral Reefs in the Anthropocene. Nature 546:82–90
Hughes TP, Kerry JT, Simpson T (2018a) Large-scale bleaching of corals on the Great Barrier Reef. Ecology. https://doi.org/10.1002/ecy.2092
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett MS, Schoepf V, Torda G, Wilson SK (2018b) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018c) Global warming transforms coral reef assemblages. Nature 556:492–496
Jones GP (1988) Experimental Evaluation of the Effects of Habitat Structure and Competitive Interactions on the Juveniles of Two Coral Reef Fishes. J Exp Mar Biol Ecol 123:115–126
Jones GP, Syms CG (1998) Disturbance, habitat structure and the ecology of fishes on coral eefs. Austral Ecol 23:287–297
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves”. Proc Natil Acad Sci B 101:8251–8253
Jones GP (1991) Post recruitment processes in the ecology of coral reef fish populations: a multifactorial perspective. pp 294–328. In: Sale PF (Ed) The ecology of fishes on coral reefs. Academic Press, San Diego, 754pp ISBN: -0–12–615–180–6
Kane CN, Tissot BN (2017) Trophic designation and live coral cover predict changes in reef-fish community structure along a shallow to mesophotic gradient in Hawaii. Coral Reefs 36:891–901
Kane C, Kosaki RK, Wagner D (2014) High levels of mesophotic reef fish endemism in the Northwestern Hawaiian Islands. Bull Mar Sci 90:693–703
Karplus I, . (2014). The associations between fishes and sponges. In: Symbiosis in fishes:The biology of interspecific partnerships371–430. John Wiley & Sons Ltd
Kerry JT, Bellwood DR (2012) The effect of coral morphology on shelter selection by coral reef fishes. Coral Reefs 31:415–424
Komyakova V, Munday PL, Jones GP (2013) Relative importance of coral cover, habitat complexity and diversity in determining the structure of reef fish communities”. PLoS ONE 8:1–13
Komyakova V, Jones GP, Munday PL (2018) Strong effects of coral species on the diversity and structure of reef fish communities: A multi-ccale analysis. PLoS ONE 13:1–21
Konow N, Bellwood DR (2011) Evolution of high trophic diversity based on limited functional disparity in the feeding apparatus of marine angelfishes (f. Pomacanthidae).” PLoS ONE [doi.org/https://doi.org/10.1371/journal.pone.0024113]
Kuffner IB, Brock JC, Grober-Dunsmore R, Bonito VE, Hickey TD, Wright CW (2007) Relationships between reef fish communities and remotely sensed rugosity measurements in Biscayne National Park, Florida, USA. Environ Biol Fish 78:71–82
Larson HK (1990) A revision of the commensal gobiid fish genera Pleurosicya and Luposicya (Gobiidae), with descriptions of eight new species of Pleurosicya and discussion of related genera”. The Beagle 7:1
León YM, Bjorndal KA (2002) Selective feeding in the hawksbill turtle, an important predator in oral reef ecosystems. Mar Ecol Prog Ser 245:249–258
Lesser MP (2006) Benthic-pelagic coupling on coral reefs: Feeding and growth of Caribbean sponges. J Exp Mar Biol Ecol 328:277–288
Lesser MP, Slattery M (2013) Ecology of Caribbean sponges: Are top-down or bottom up processes more important? PLoS ONE 8(11):e79799. https://doi.org/10.1371/journal.pone.0079799
Lesser MP, Slattery M (2018) Sponge density increases with depth throughout the Caribbean. Ecosphere 9(12):e02525. https://doi.org/10.1002/ecs2.2525
Lesser MP, Slattery M (2020) Will coral reef sponges be winners in the Anthropocene? Glob. Change Biol 26:3202–3211
Lesser MP, Slattery M, Mobley CD (2018) Biodiversity and functional ecology of mesophotic coral reefs. Annu Rev Ecol Evol Syst 49:49–71
Lesser MP, Mueller B, Pankey MS, Macartney KJ, Slattery M, de Goeij JM (2020) Depth-dependent detritus production in the sponge Halisarca caerulea. Liminol Oceanogr 65:1200–1216
Lindquist N (2002) Chemical defense of early life stages of benthic marine invertebrates. J Chem Ecol 28:1987–2000
Lindquist N, Hay ME (1996) Palatability and chemical defense of marine invertebrate larvae. Ecol Monogr 66:431–450
Lindqusit N, Hay ME (1995) Can small rare prey be chemically defended? The Case for Marine Larvae. Ecology 76(4):1347–1358
Loh TL, Pawlik JR (2009) Bitten down to size: Fish predation determines growth form of the Caribbean coral reef sponge Mycale laevis. J Exp Mar Biol Ecol 374:45–50
Loh TL, Pawlik JR (2014) Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs”. Proc Natl Acad Sci B 111:4151–4156
Loh TL, McMurray SE, Henkel TP, Vicente J, Pawlik JR (2015) Indirect effects of overfishing on Caribbean reefs: Sponges overgrow reef-building corals. PeerJ. https://doi.org/10.7717/peerj.901
Lorders FL, Miranda RJ, Nunes JAC, Barros F (2018) Spongivory by fishes on southwestern Atlantic coral reefs: No evidence of top-down control on sponge assemblages. Front Mar Sci 5:1–9
Lukowiak M, Cramer KL, Madzia D, Hynes MG, Norris RD, O’Dea A (2018) Historical change in a Caribbean reef sponge community and long-term loss of sponge predators. Mar Ecol Prog Ser 601:127–137
Majoris JE, D’Aloia CC, Francis RK, Buston PM (2018) Differential persistence favors habitat preferences that determine the distribution of a reef fish. Behav Ecol 29:429–439
Malcom HA, Jordan A, Smith SDA (2011) Testing a depth-based Habitat Classification System against reef fish assemlage patterns in a subtropical marine park. Aquat Conserv: Mar Freshw Ecosyst 21:173–185
Maldonado M, Uriz M (1999) Sexual propogation by sponge fragments. Nature 398:476
Maldonado A, Lavado R, Kniuston S, Slattery M, Ankisetty S, Goldstone JV, Watanabe K, Hoh E, Gadepalli RS, Rimoldi JM, Schlenk OGK, D, (2016) Biochemical mechanisms for geographical adaptations to novel toxin exposures in Butterflyfish. PLoS ONE 11(5):e1054208. https://doi.org/10.1371/journal.pone.0154208
Maldonado M, Aguilar R, Bannister RJ, Bell, JJ, Conway KW, Dayton PK, Diaz C, Gutt J, Kelly M, Kenchington ELR, Leys SP, Pomponi SA, Rapp HT, Rützler K, Tendal OS, Vacelet J, Young CM (2017) Sponge grounds as key marine habitats: A synthetic review of types, structure, functional roles, and conservation concerns In: Bratmanti L, Gori A, Rossi S, Orejas C (Eds) Marine animal forests: The ecology of benthic biodiversity hotspots. Springer [doi:https://doi.org/10.1007/978-3-319-21012-4]
Márquez JC, Zea S (2012) Parrotfish mediation in coral mortality and bioerosion by the encrusting, excavating sponge Cliona tenuis. Mar Ecol 33:417–426
Marty MJ, Blum JE, Pawlik JR (2016) No accounting for taste: Palatability of variably defended Caribbean sponge species is unrelated to predator abundance. J Exp MarBiol Ecol 485:57–64
McDonald C, Bridge TCL, Jones GP (2016) Depth, bay position and habitat structure as determinants of coral reef fish distributions: Are deep reefs a potential refuge? Mar Ecol Prog Ser 561:217–231
McMurray SE, Blum JE, Leichter JJ, Pawlik JR (2011) Bleaching of the giant barrel sponge Xestospongia muta in the Florida Keys. Limnol Oceanogr 56:2243–2250
McMurray SE, Finelli CM, Pawlik JR (2015) Population Dynamics of Giant Barrel Sponges on Florida Coral Reefs. J Exp Mar Biol Ecol 473:73–80
McMurray SE, Stubler AD, Erwin PM, Finelli CM, Pawlik JR (2018) A test of the sponge-loop hypothesis for emergent Caribbean reef sponges. Mar Ecol Prog Ser 588:1–14
Meylan A (1988) Spongivory in hawksbill turtles: A diet of glass. Science 239:393–395
Mortimer C, Dunn M, Haris A, Jompa J, Bell J (2021) Estimates of sponge consumtion rates on an Indo-Pacific reef. Mar Ecol Prog Ser 672:123–140
Munehara H (1991) Utilization and ecological benefits of a sponge as a spawning bed by the little dragon sculpin Blepsias cirrhosus. JPN J Ichthyol 38:179–184
Nagelkerken I, Van Der Velde G, Gorissen MW, Meijer GJ, van't Hof T, den Hartog C (2000) Importance of mangroves, seagrass beds and shallow coral reefs as a nursery for important coral reef fishes, using a visual census technique. Estuar Coast Shelf Sci 51: 31–44
Nagelkerken I, Van Der Velde G, Wartenbergh SLJ, Nugues MM, Pratchett MS (2009) Cryptic dietary components reduce dietary overlap among sympatric butterflyfishes (Chaetodontidae). J Fish Biol 75:1123–1143
Nicholson GM, Clements KD (2020) Resoling resource partitioning in parrotfishes (Scarini) using microhistory of feeding substrata. Coral Reefs 39:1212–1327
Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P (2018) Diet tracing in ecology: Method comparison and selection. Methods Ecol Evol 9:278–291
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:293–306
Oakley-Cogan A, Tebbett SB, Bellwood DR (2020) Habitat zonation on coral reefs: Structural complexity, nutritional resources and herbivorous fish distributions. PLoS ONE 15:1–23
Öhman MC, Munday PL, Jones GP, Caley MJ (1998) Settlement strategies and distribution patterns of coral-reef fishes. (2013) 66th Annual Conference for Protective Relay Engineers. CPRE 2013(225):219–238
Osbourne K, Dolman AM, Burgess SC, Johns KA (2011) Disturbance and the dynamics of coral cover on the Great Barrier Reef. PLoS ONE 6:e17516. https://doi.org/10.1371/journal.pone.0017516
Padilla Verdín C, Carballo JL, Camacho ML (2010) A qualitative assessment of sponge-feeding organisms from the Mexican Pacific coast. Open Mar Biol J 4:39–46
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422
Pawlik JR (1993) Marine invertebrate chemical defenses. Chem Rev 93:1911–1922
Pawlik JR, McFall G, Zea S (2002) Does the odor from sponges of the genus Ircinia protect them from fish predators? J Chem Ecol 28(6):1103–1115
Pawlik JR (1998) Coral reef sponges: Do predatory fishes affect their distribution? Limnol Oceanogr 43:1396–1399
Pawlik JR (2011) The chemical ecology of sponges on Caribbean reefs: Natural products shape natural systems. BioScience 61(11):888–898
Pawlik JR, Loh TL (2017) Biogeographical homogeneity of Caribbean coral reef benthos. J Biogeogr 44:960–962
Pawlik JR, McMurray SE (2020) The emerging ecological and biogeochemical importance of sponges on coral reefs. Annu Rev Mar Sci 12:315–337
Pawlik JR, Chanas B, Toonen RJ, Fenical W (1995) Defenses of Caribbean sponges against predatory reef fish. I. Chemical Deterrency. Mar Ecol Prog Ser 127:183–194
Pawlik JR, Henkel TP, Mcmurray SE, López-Legentil S, Loh TL, Rohde S (2008) Patterns of sponge recruitment and growth on a shipwreck corroborate chemical defense resource trade-off. Mar Ecol Prog Ser 368:137–143
Pawlik JR, Loh TL, McMurray SE, Finelli CM (2013) Sponge communities on Caribbean coral reefs are structured by factors that are top-down, not bottom-up. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0062573
Pawlik JR, McMurray SE, Erwin P, Zea S (2015) A review of evidence for food limitation of sponges on Caribbean reefs. Mar Ecol Prog Ser 519:265–283
Pawlik JR, Loh TL, McMurray SE (2018) A review of bottom-up vs. top-down control of sponges on Caribbean fore-reefs: What’s old, what’s new, and future directions. PeerJ 2018:1–28
Pitcher CR, Doherty PJ, Anderson TJ (2019) Seabed environment, habitats and biological assemblages. In: Hutchings P, Kingsford MJ, Hoegh-Guldberg O (eds) The Great Barrier Reef: Biology. Environment and Management. CSIRO Publishing, Clayton South, Victoria, pp 63–72
Pomponi SA, Diaz MC, Van Soest RWM, Bell LJ, Busutil L, Gochfeld DJ, Kelly M, Slattery M (2019) Sponges. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic Coral Ecosystems, Coral Reefs of the World 12. Springer, New York, pp 563–588
Post DM (2002) Using stable isotopes to estimate trophic position: Models, methods and assumptions. Ecology 83(3):703–718
Powell A, Smith DJ, Hepburn LJ, Jones T, Berman J, Jompa J, Bell JJ (2014) Reduced diversity and high sponge abundance on a sedimented Indo-Pacific reef system: Implications for future changes in environmental quality. PLoS ONE 9. https://doi.org/10.1371/journal.pone.0085253
Powell A, Jones T, Smith DJ, Jompa J, Bell JJ (2015) Spongivory in the Wakatobi Marine National Park, southeast Sulawesi, Indonesia”. Pac Sci 69:487–508
Przeslawski R, AhYong S, Byrne M, Wörheides G, Hutchings P (2008) Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob Change Biol 14:2773–2795
Pyle RL, Kosaki R, Pinheiro HT, Rocha LA, Whitton RK, Copus JM (2019) Fishes: Biodiversity. In: Loya Y, Puglise KA, Bridge TCL (Eds) Mesophotic Coral Ecosystems, Coral Reefs of the World 12. pp749–777. Springer New York
Radcliffe L (1917) Description of a new goby, Garmannia spongicola from North Carolina. Proc U.S. Natn Mus 52:423–425
Ramsby BD, Hoogenboom MO, Smith HA, Whalan S, Webster NS (2018) The bioeroding sponge Cliona orientalis will not tolerate future projected ocean warming. Sci Rep 8:1–14
Randall JE, Hartman WD (1968) Sponge-feeding fishes of the West Indies. Mar Biol 1:216–225
Randall JE, Lobel PS (2009) “A literature review of the sponge-dwelling gobiid fishes of the genus Elacatinus from the Western Atlantic, with description of two new Caribbean species. Zootaxa 19:1–19
Richardson LE, Graham NAJ, Pratchett MS, Hoey AS (2017) Structural complexity mediates functional structure of reef fish assemblages among coral habitats. Environ Biol Fish 100:193–207
Riegl BM, Purkis SJ (2009) Model of coral population response to accelerated bleaching and mass mortality in a changing climate”. Ecol Model 220:192–208
Reiswig HM (1981) Partial carbon and energy budgets of the bacteriosponge Verongia fistularis (Porifera: Demospongiae) in Barbados. Mar Ecol 2:273–293
Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS (2018) Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar Ecol Prog Ser 589:85–96
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservatiohn priorities for tropical reefs. Science 295:1280–1284
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27:404–413
Rovellini A, Dunn MR, Fulton EA, Webster NS, Smith DJ, Jompa J, Haris A, Berman J, Bell JJ (2019) Decadal variability in sponge abundance and biodiversity on an Indo-Pacific coral reef. Mar Ecol Prog Ser 620:63–76
Russ GR, Rizzari JR, Abesamis RA, Alcala AC (2020) Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecol Appl. https://doi.org/10.1002/eap.2224
Ruzicka R, Gleason DF (2009) Sponge community structure and anti-predator defenses on temperate reefs of the south Atlantic Bight”. J Exp Mar Biol Ecol 380:36–46
Ryer CH, Stoner AW, Titgen RH (2004) Behavioral mechanisms underlying the refuge value of benthic habitat structure for two flatfishes with differing anti-predator strategies. Mar Ecol Prog Ser 268:231–243
Sadeghi SPA, Yavari V, Loghmani Devin M (2008) First record of sponge distribution in the Persian Gulf, (Hengam Island, Iran). Pak J Biol Sci 11:2521–2524
Safriel UN, Ben-Eliahu MN (1991) The influence of habitat structure and environmental stability on the species diversity of polychaetes in vermetid reefs. In: Bell, SS. McCoy, ED. Mushinsky, HR. (Eds) Habitat structure population and community biology series, Vol 8. Springer. Dordrecht. [doi: https://doi.org/10.1007/978-94—011-3076-9_17s]
Sambrook K, Hoey AS, Andréfouët S, Cumming GS, Duce S, Bonin MC (2019) Beyond the reef: The widespread use of non-reef habitats by coral reef fishes”. Fish Fish 20:903–920
Sambrook K, Bonin MC, Bradley M, Cumming GS, Duce S, Andréfouёt S, Hoey AS (2020) Broadening our horizons: seascape use by coral reef-associated fishes in Kavieng, Papua New Guinea, is common and diverse. Coral Reefs 39:1187–1197
Sano M (1989) Feeding habits of Japanese butterflyfishes (Chaetodontidae) Environ Biol Fishes 1–3: 195–203
Santavy DL, Courtney LA, Fisher WS, Quarles RL, Jordan SJ (2013) Estimating surface area of sponges and gorgonians as indicators of habitat availability on Caribbean coral reefs. Hydrobiologia 707:1–16
Santonja M, Greff S, Le Croller M, Thomas OP, Pérez T (2018) Distance interaction between marine cave-dwelling sponges and crustaceans. Mar Biol 165. https://doi.org/10.1007/s00227-018-3377-0
Schönberg CHL, Fromont J (2012) Sponge gardens of Ningaloo Reef (Carnarvon Shelf, Western Australia) are biodiversity hotspots. Hydrobiologia 687(1):143–161
Schönberg CHL, Hosie AM, Fromont J, Marsh L, O’Hara T (2016) Apartment-Style Living on a Kebab Sponge. Mar Biodivers 46:331–332
Seemann J, Yingst A, Stuart-Smith RD, Edgar GJ, Altieri AH (2018) The importance of sponges and mangroves in supporting fish communities on degraded coral reefs in Caribbean Panama”. PeerJ 2018. https://doi.org/10.7717/peerj.4455
Silveira CB, Silva-Lima AW, Francini-Filho RB, Marques JSM, Almeida MG, Thompson CC, Rezende CE, Paranhos R, Moura RL, Salomon PS, Thompson FL (2015) Microbial and sponge loops modify fish production in phase-shifting coral reefs. Environ Microbiol 17:3832–3846
Slattery M, Gochfeld DJ (2016) Butterflyfishes exhibit species-specific responses to change in Pacific coral reef benthic communities. Mar Biol 163:246
Slattery M, Gochfeld DJ, Diaz MC, Thacker RW, Lesser MP (2016) Variability in chemical defense across shallow to mesophotic depth gradient in the Caribbean sponge Plakortis angulospiculatus. Coral Reefs 35:11–22
Slattery M, Lesser MP (2012) Mesophotic coral reefs a global model of community structure and function. Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia
Slattery M, Lesser MP (2015) Trophic ecology of sponges from shallow to mesophotic depth (3 to 150m): Comment on Pawlik et al. (2015). Mar Ecol Prog Ser 527:275–279
Spalding HL, Amando-Filho GM, Bahia RG, Ballatine DL, Fredericq S, Leichter JJ, Nelson WA, Slattery M, Tsuda RT (2019) Macroalgae. In: Loya Y, Puglise KA, Bridge TCL (Eds) Mesophotic Coral Ecosystems, Coral Reefs of the World 12. pp507- 536. Springer New York
Stabili L, Cardone F, Alifano P, Tredici SM, Piraino S (2012) Epidemic mortality of the sponge Ircinia variabilis (Schmidt, 1862) associated to proliferation of a Vibrio bacterium. Microb Ecol 64:802–814
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol 49:43–104
Stoner AW, Titgen RH (2003) Biological structures and bottom type influence habitat choices made by Alaska flatfishes. J Exp Mar Biol Ecol 292:43–59
Sweatman HPA (1983) Influence of conspecifics on choice of settlement sites by larvae of twp pomacentrid fishes (Dascyllua aruanus and D. reticulatus) on coral reefs. Mar Biol 75:225–229
Sweatman HPA (1985) The influence of adults of some coral reef fishes on larval recruitment. Ecol Monogr 55:469–485
Syms C, Jones GP (2000) Disturbance, habitat structure , and the dynamics of a coral-reef fish community Ecology 81 (10): 2714–29
Taylor MW, Radax R, Steger D, Wagner M (2007) Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347
Tebbett SB, Streit RP, Bellwood DR, (2019) Expansion of a colonial ascidian following consecutive mass coral bleaching at Lizard Island, Australia. Mar Environ Res 144:125–129
Torres-Pulliza D, Dornelas MA, Pizarro O, Bewley M, Blowes SA, Boutros N, Brambilla V, Chase TJ, Frank G, Friedman A, Hoogenboom MO, Williams S, Zawada KJA, Madin JS (2020) A Geometric Basis for Surface Habitat Complexity and Biodiversity. Nature Ecol Evol. https://doi.org/10.1038/s41559-020-1281-8
Trussell GC, Lesser MP, Patterson MR, Genovese SJ (2006) Depth-specific differences in growth of the reef sponge Callyspongia vaginalis: Role of bottom-up effects. Mar Ecol Prog Ser 323:149–158
Tyler JC, Böhlke JE (1972) Records of sponge-dwelling fishes, primarily of the Caribbean. Bull Mar Sci 22:601–642
Uriz MJ, Turon X, Becerro MA, Galera J (1996) Feeding deterrence in sponges. The role of toxicity, physical defenses, energetic contents, and life-history tage. J Exp Mar Biol Ecol 205:187–204
Van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ, Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA (2012) Global diversity of sponges (Porifera). PLoS ONE 7(4):e35105. https://doi.org/10.1371/journal.pone.0035105
Veron JEN, Hoegh-Guldberg O, Lenton TM, Lough JM, Obura DO, Pearce-Kelly P, Sheppard CRC, Spalding M, Stafford-Smith MG, Rogers AD (2009) The coral reef crisis: The critical importance of <350 ppm CO2. Mar Poll Bull 58:1428–1436
Vicente VP (1990) Response of sponges with autotrophic endosymbionts during the coral-bleaching episode in Puerto Rico. Coral Reefs 8:199–202
Vrolijk NH, Targett NM (1992) Biotransformation enzymes in Cyphoma gibbosum (Gastropoda:Ovulidae): implications for detoxification of gorgonian allelochemicals. Mar Ecol Prog Ser 88:237–246
Walters KD, Pawlik JR (2005) Is there a trade-off between wound-healing and chemical defenses among Caribbean reef sponges? Integr Comp Biol 45:352–358
Webster NS (2007) Sponge disease: A global threat?”. Environ Microbiol 9:1363–1375
Webster NS, Taylor MW (2012) Marine sponges and their microbial symbionts: Love and other relationships. Environ Microbiol 14:335–346
White JW, Grigsby CJ, Warner RR (2007) Cleaning behavior is riskier and less profitable than an alternative strategy for a facultative cleaner fish. Coral Reefs 26:87–94
Wilkinson CR (1987) Interocean differences in size and nutrition of coral reef sponge populations. Science 236(4809):1654–1657
Wilkinson CR, Cheshire AC (1989) Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs 8:127–134
Wilkinson CR, Cheshire AC (1990) Comparisons of sponge populations across the barrier reefs of Australia and Belize: Evidence for higher productivity in the Caribbean. Mar Ecol Prog Ser 67:285–294
Williams EH, Bunkley-Williams L (1990) The World-wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res Bull 330–338
Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: Are Reef Fishes at Risk or Resilient?”. Glob Change Biol 12:2220–2234
Wilson SK, Robinson JPW, Chong-Seng K, Robinson J, Graham NAJ (2019) Boom and bust of keystone structure on coral reefs. Coral Reefs 38:625–635
Wulff JL (1991) Asexual fragmentation, genotype success and population dynamics of erect branching sponges. J Mar Biol Ecol 149:227–247
Wulff JL (1997a) Parrotfish predation on cryptic sponges of Caribbean coral reefs. Mar Biol 129:41–52
Wulff JL (1997b) Causes and consequences of differencein sponge diversity and abundance between the Caribbean and the Eastern Pacific of Panama. Proc. 8th Int. Coral Reef Sym 2:1377–1382
Wulff JL (2001) Assessing and monitoring coral reef sponges: Why and how?”. Bull Mar Sci 69:831–846
Wulff JL (2005) Trade-offs in resistance to competitors and predators, and their effects on the diversity of tropical marine sponges. J Anim Ecol 74:313–321
Wulff JL (2006) Ecological interactions of marine sponges. Can J Zool 84:146–166
Wulff JL (2017) Bottom-up and top-down controls on coral reef sponges: disentangleing within-habitat and between-habitat processes. Ecology 98(4):1130–1139
Wulff JL (1994) Sponge feeding by Caribbean angelfishes, trunkfishes and filefishes. In: (van Soest RWM, Van Kempen, Braekman (Eds) Sponges in time and space, 265–271. Rotterdam.
Wulff JL (2012) Ecological interactions and the distribution, abundance, and diversity of sponges.In: Adv Mar Biol. 61:273–344.
Wulff JL (2021) Targeted predator defenses of sponges shape community organization and tropical marine eosystem function. Ecological Monographs 91(2) [doi:https://doi.org/10.1002/ecm.1438]
Zea S, Henkel TP, Pawlik JR (2014) The Sponge Guide: a picture guide to Caribbean sponges. 3rd Edition. Available online at www.spongeguide.org
Acknowledgements
We would like to thank Kim Pritchard for her feedback and editing on this manuscript, and Steve Whalan for some thought-provoking conversations and ideas. Thanks also go to three anonymous reviewers for their constructive feedback which improved this manuscript. Financial support was provided by a James Cook University Postgraduate Research Scholarship (AC) and an Australian Research Council Centre of Excellence of Coral Reef Studies research grant awarded to GJ.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Editor Alastair Harborne
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coppock, A.G., Kingsford, M.J., Battershill, C.N. et al. Significance of fish–sponge interactions in coral reef ecosystems. Coral Reefs 41, 1285–1308 (2022). https://doi.org/10.1007/s00338-022-02253-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02253-8