Abstract
Coral disease is becoming increasingly problematic on reefs worldwide. However, most coral disease research has focused on the abiotic drivers of disease, potentially overlooking the role of species interactions in disease dynamics. Coral predators in particular can influence disease by breaking through protective tissues and exposing corals to infections, vectoring diseases among corals, or serving as reservoirs for pathogens. Numerous studies have demonstrated the relationship between corallivores and disease in certain contexts, but to date there has been no comprehensive synthesis of the relationships between corallivores and disease, which hinders our understanding of coral disease dynamics. To address this void, we identified 65 studies from 26 different ecoregions that examine this predator–prey-disease relationship. Observational studies found over 20 positive correlations between disease prevalence and corallivore abundance, with just four instances documenting a negative correlation between corallivores and disease. Studies found putative pathogens in corallivore guts and experiments demonstrated the ability of corallivores to vector pathogens. Corallivores were also frequently found infesting disease margins or targeting diseased tissues, but the ecological ramifications of this behavior remains unknown. We found that the impact of corallivores was taxon-dependent, with most invertebrates increasing disease incidence, prevalence, or progression; fish showing highly context-dependent effects; and xanthid crabs decreasing disease progression. Simulated wounding caused disease in many cases, but experimental wound debridement slowed disease progression in others, which could explain contrasting findings from different taxa. The negative effects of corallivores are likely to worsen as storms intensify, macroalgal cover increases, more nutrients are added to marine systems, and water temperatures increase. As diseases continue to impact coral reefs globally, a more complete understanding of the ecological dynamics of disease—including those involving coral predators—is of paramount importance to coral reef conservation and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are threatened marine ecosystems, with many reefs experiencing severe decreases in coral cover over the past few decades (Burke et al. 2011). One of the drivers of this loss is disease (Weil 2004; Weil et al. 2006). Coral disease can reduce coral survivorship (Precht et al. 2016), recruitment (Richardson and Voss 2005), and reproductive output (Petes et al. 2003; Weil et al. 2009), affecting entire communities that depend on the structure provided by corals. For instance, white diseases drove the population decline of Acropora corals across the Caribbean at the end of the twentieth century (Aronson and Precht 2001) and currently Stony Coral Tissue Loss Disease is decimating local populations of over twenty species of scleractinian corals across 17 countries and territories, posing a significant threat to conservation and restoration efforts (Walton et al. 2018).
Despite the importance of coral disease to reef health, we have an incomplete understanding of what factors increase disease incidence (i.e., proportion of corals that develop disease signs during a particular time period), disease prevalence (i.e., proportion of corals that have disease signs during a particular time period), and disease progression (i.e., the rate at which disease signs progress on a coral colony). The majority of disease-related studies to date have focused on the effects of abiotic factors such as temperature and nutrients on disease incidence, prevalence, or progression (e.g., Bruno et al. 2003, 2007; Ruiz-Moreno et al. 2012; Howells et al. 2020). Much less attention has been paid to the influence of biotic interactions (e.g., predation, competition, facilitation) and how these interactions shape coral disease dynamics (but see examples such as: Sussman et al. 2003; Nugues et al. 2004; Cole et al. 2009; Pollock et al. 2013; Sweet et al. 2013; Casey et al. 2014; Nicolet et al. 2018b). However, the role of biotic interactions in spreading disease both through vectoring (e.g., promoting primary infections) and wounding (e.g., promoting secondary infections) is well known in a variety of other systems, such as forests (Paine et al. 1997; García-Guzmán and Dirzo 2001), coastal plains (Fraedrich et al. 2008), croplands (Costa 1976), and salt marshes (Silliman and Newell 2003).
Coral predators (i.e., corallivores) are a diverse group of species that consume the mucus, tissues, or skeleton of living soft and hard corals (Cole et al. 2008; Rotjan and Lewis 2008). Through consumptive activities, corallivores can break through corals’ protective tissues and mucus layers, potentially exposing corals to infections (discussed in Nicolet et al. 2013). Corallivores also likely influence disease recurrence, as predators can act as vectors of disease or serve as reservoirs for pathogens (e.g., Sussman et al. 2003). Several studies have examined the links between specific corallivores and diseases (e.g., Dalton and Godwin 2006; Nugues and Bak 2009; Nicolet et al. 2013), but to date there has been no comprehensive synthesis of the relationships among corals, corallivores, diseases, and external stressors. The primary literature has shown that in some cases corallivores can increase disease incidence (Aeby and Santavy 2006) while in others they can decrease disease progression (Cole et al. 2009); that some corallivores are both positively (Grober-Dunsmore et al. 2006) and negatively (Greene et al. 2020) correlated with disease prevalence; that one corallivore can increase disease incidence while a closely related corallivore does not (Gignoux-Wolfsohn et al. 2012); and that a corallivore may transmit one disease while not transmitting another (Nicolet et al. 2018b). This review examines these apparent inconsistencies and synthesizes decades of research to look for general patterns between corallivores and disease.
Here, we conduct a review of the corallivore-disease literature and summarize these findings to better understand the role of corallivores in the disease dynamics of corals. We divide the literature into six sections that span the range of corallivore-disease relationships studied to date. The first section, “correlations between corallivores and disease,” looks at observational studies that correlate corallivore presence with disease prevalence, but do not test these relationships experimentally. The second section, “corallivores as drivers of disease”, analyzes experimental studies that tested whether corallivores increase, decrease, or have no effect on disease incidence, prevalence, or progression. The third section, “wounding and disease,” examines the potential role of wounding in coral disease and examines results from studies that mimicked wounding by a corallivore. “Corallivore feeding on disease fronts” discusses the body of literature showing corallivores feeding on disease lesions or preferentially infesting disease margins. Finally, sections five and six explore how non-corallivore species and anthropogenic stressors may interact to alter the corallivore-coral-disease relationship. We synthesize key results within each of these sections, examine the extent to which different corallivore families increase the likelihood of corals contracting disease, examine current knowledge gaps in the field, and explore avenues for future research.
Methods
To collect relevant peer-reviewed research, we searched the Web of Science database (ISI Thomson Reuters) for English articles using the following parameters: Topic = (diseas* OR pathogen OR fung* OR bacteria* OR infect*) AND Topic = (coral OR “coral reef”) AND Topic = (corallivore OR predat*) for any paper published through 2020, which yielded 210 papers. Additional papers were located by following citations from selected studies from this search. We then examined studies and selected those that: (1) directly manipulated corallivores to examine their effect on disease incidence, prevalence, or progression, (2) examined relationships between corallivores and disease in the lab or field, (3) removed diseased tissue to test for debridement effects, or (4) mechanically simulated wounding by a corallivore (Table 1, Electronic Supplementary Material). We included studies that examined disease explicitly or examined corallivore impacts on the microbiome more generally, given how few putative coral disease agents have been identified. Additionally, although organisms like damselfish and surgeonfish are generally herbivorous, we included them as corallivores as they have been observed biting corals and feeding along diseased margins (Chong-Seng et al. 2011; Kellogg et al. 2017). This selection process resulted in a total of 65 studies.
We then classified studies based on whether authors used observational (e.g., surveys, behavioral observations), experimental (i.e., manipulative), or both methods to examine the relationship between a corallivore and disease. From each article we extracted: the corallivore(s) involved, the disease(s), the key findings, and the location(s) of the study. Key findings included: (1) whether corallivores increased, decreased, or had no effect on disease incidence, prevalence, or progression in a manipulative study; (2) whether corallivore abundance was negatively or positively correlated with disease prevalence in an observational study; (3) whether researchers found potential pathogens inside corallivores (e.g., guts, mouthparts, feces); and (4) whether corallivores were found to feed on disease margins. We recorded a distinct observation for each unique species-disease-study combination, so a study could have multiple “observations” if it examined multiple corallivore species or diseases. Study sites were classified based on the 232 ecoregions and 12 realms defined in the Marine Ecoregions of the World system (Spalding et al. 2007), which group regions biogeographically. Ecoregions are areas composed of similar species distinct from the regions around them (e.g., “Floridian”). Realms are large, continent-scale areas with shared evolutionary history (e.g., “Tropical Atlantic”). If studies were conducted in multiple regions, we counted them once for each region.
Although we discuss specific white diseases (e.g., white band disease, wite plague II) in the text if they are defined in a given study, we group all white and white-like diseases together for analysis because not all white diseases have clear definitions or etiological agents (Bourne et al. 2015). Additionally, many of the studies determined the type of white disease from macroscopic appearance (e.g., lesion progression rate, lesion morphology), which is often insufficient to distinguish among white diseases (Bythell et al. 2004; Bourne et al. 2015). Indeed, researchers using visual signs to identify diseases have found different putative pathogens for the same “disease” in different studies (e.g., Table 1, Electronic Supplementary Material). For instance, although white plague II in the Caribbean appears to be caused by Aurantimonas coralicida, studies of white plague from outside the Caribbean appear to have different microbial communities that lack A. coralicida (reviewed in Bourne et al. 2015), which suggests the diseases are different despite appearing visually similar. Researchers have also created names for particular forms of white disease without morphological, histological, or etiological details that distinguish that form from other white diseases (Bourne et al. 2015). Further, some white diseases are in part distinguished based on lesion progression rate, which is not measured in all surveys and experiments. Thus, we use the term “white diseases” to describe coral tissue loss that is unrelated to bleaching and without a colored band or other clear diagnostic feature. Studies that surveyed multiple diseases and quantified disease prevalence as a sum of all observed diseases were termed “General” disease.
To examine the magnitude of corallivore effects, we isolated a subset of studies that looked at disease incidence in corals exposed to corallivory or simulated corallivory versus control corals. To be included, studies had to provide the total number of corals in each treatment, monitor fragments for disease signs, and provide the number of infected corals in each treatment. We calculated the risk ratio for each study as the risk of developing disease signs in the corallivore treatment (number of treatment corals infected/number of corals in treatment) over the risk of developing disease signs in the control treatment, where a risk ratio of 1.5 indicates a coral exposed to a corallivore is 1.5 times as likely to develop disease signs and a risk ratio of 1 indicates no effect. We used a Haldane-Anscombe correction (Haldane 1940; Anscombe 1956) to account for the large number of zeros in our dataset. All analyses were conducted in R version 4.0.3 (R Core Team 2020) using the tidyverse (Wickham et al. 2019), sf (Pebesma 2018), RColorBrewer (Neuwirth 2014), here (Müller 2020), patchwork (Pedersen 2020), ggsn (Baquero 2019), and rnaturalearth (South 2017) packages.
Results and Discussion
The number of studies on corallivores and disease has generally increased over time, with no year having more than six publications (Fig. 1a). Although initial studies were experiments, starting in 1997 there has been a mix of experimental and observation-based research (Fig. 1a). There was at least one relevant study in 26 different ecoregions (Fig. 1b), but the results were concentrated in just a few regions, mainly in the US and Australia. Over 46% of studies came from three ecoregions (Floridian, Torres Strait Northern Great Barrier Reef, and Hawaii). At a larger scale, nearly half of the studies were conducted in the tropical Atlantic (41.1%, n = 30), while 34.2% were from the Central Indo-Pacific (n = 25), 17.8% (n = 13) from the Eastern Indo-Pacific, 4.1% from the Western Indo-Pacific (n = 3), 1.4% (n = 1) from temperate Australasia, and 1.4% (n = 1) from the temperate Northern Atlantic.
-
1.
Correlations between corallivores and disease
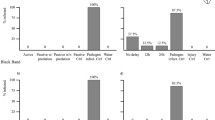
a Number of relevant studies through time that looked at the effects of corallivores on coral disease, divided up by whether the study was observational (e.g., a survey), experimental (e.g., a manipulative experiment), or both. b Map of where relevant studies in Panel A were conducted, grouped by marine ecoregions (Spalding et al. 2007). If a study was conducted in multiple ecoregions, it was counted once for each ecoregion. c Coralliophila snails on Acropora palmata in the Florida Keys. Photo by L.S. d Corallivorous fish feeding scars in French Polynesia. Photo by J.R
Observational studies suggest a strong relationship between corallivore presence and disease prevalence (although see Ross 2014; Scott et al. 2017). In one of the first studies to document such a relationship, researchers found a positive correlation between disease prevalence in the Red Sea and densities of the corallivorous snail, Drupella cornus (Antonius and Riegl 1997). In the following decades, other studies found similar positive correlations between corallivorous snails and disease, including increased tissue loss with fish and snail predation (Rodríguez-Villalobos et al. 2014); Drupella sp. in Vietnam and a more pathogenic coral microbiome (Bettarel et al. 2018); Drupella predation in Japan and growth anomalies (Muko and Nadaoka 2020); predation scars in the Arabian Sea and black band disease (Ranith et al. 2017); Coralliophila galea (formerly abbreviata, Dillwyn, 1823) in the USVI and white diseases (Bright et al. 2016); corallivorous snails and white diseases in Guam (Greene et al. 2020); and Drupella scars in Australia and disease (Onton et al. 2011). Corallivorous snails (family Muricide) were most frequently positively correlated with disease prevalence and were associated with six different disease categories (Fig. 2a). Other invertebrates, such as the bearded fireworm, Hermodice carunculata (family Amphinomidae) (Vargas-Ángel et al. 2003; Miller and Williams 2007; Moreira et al. 2014); the flamingo tongue snail, Cypoma gibbosum (family Ovulidae) (Nagelkerken et al. 1997; Slattery 1999); the furry coral crab, Cymo melanodactylus (family Xanthidae) (Pratchett et al. 2010; Pollock et al. 2013); and cryptochirid crabs (family Cryptochiridae) (Pratte and Richardson 2016) were also positively correlated with disease in at least one study. Surveys may have found these positive correlations because diseased corals attract corallivorous invertebrates, because corallivorous invertebrates cause coral disease, or a mixture of both.
Corallivores whose presence has been shown to be correlated with disease prevalence (a and b), corallivores that have been shown to alter disease incidence or progression through manipulative experiments (c and d), and corallivores that had no effect on disease incidence or progression in an experimental setting (e). BBD = black band disease, BrB = brown band disease, GA = growth anomalies, and SEB = skeletal eroding band
Surveys also revealed a relationship between corallivorous fishes and disease prevalence, although the relationship was more variable than the relatively consistent positive correlation between invertebrates and disease prevalence. Unlike with invertebrates, whether the relationship between disease prevalence and corallivorous fish was positive (i.e., more disease in areas with more fish) or negative (i.e., more disease in areas with fewer fish) varied by study and disease (Fig. 2). All 16 invertebrate studies found a positive correlation between corallivore abundance and disease, while 7 fish studies found positive correlations and 4 found negative correlations. For instance, although butterflyfishes (family Chaetodontidae) have been positively correlated with a suite of coral diseases (Raymundo et al. 2009; Williams et al. 2010), in one study the prevalence of white diseases was higher where butterflyfish abundances were lower (Williams et al. 2010). Parrotfish (family Scaridae) also had conflicting results, with studies suggesting both negative and positive correlations between disease prevalence and parrotfishes (family Scaridae, Fig. 2a, b) (Williams et al. 2010; Zaneveld et al. 2016; Ranith et al. 2017; Ezzat et al. 2020). The effect of damselfishes (family Pomacentridae) was also slightly mixed, although the majority of studies found that damselfish were positively correlated with disease (n = 4) and only one found a negative correlation (Fig. 2a) (Grober-Dunsmore et al. 2006; Casey et al. 2014; Vermeij et al. 2015; Greene et al. 2020). The context-dependent effect of fishes may partially be because in some cases fishes can promote infections through wounding and pathogen vectoring (see Sections 2 and 3) but in other cases their targeted feeding can slow disease progression by debriding infected tissue (see Section 4). The effect of fish predation may also change based on environmental conditions (see Section 6), which could create conflicting results when surveys are conducted under different conditions at different times in different locations.
Some of the variation in the patterns in Fig. 2 could also be attributed to the fact that: (1) some surveys examined a wide range of diseases, (2) a disease category like “white disease” really represents a group of diseases, and (3) some “diseases” diagnosed using macroscopic observation alone are likely misidentified (e.g., confusing brown band disease for white disease when ciliate population densities are low: Willis et al. 2004). Without effective ways to definitively identify unique diseases in the field, it will remain difficult to detect nuanced patterns between corallivores and individual diseases, which may be important for diseases influenced by fishes. Although we may be many years away from robust descriptions of many coral diseases, there may be functional groups of disease that behave in similar ways. For instance, diseases that can spread via direct transmission (i.e., contact between diseased and healthy tissue) may be particularly likely to be spread by fishes or other associated organisms that interact with disease lesions and move among coral colonies. Similarly, whether a pathogen, or group of pathogens, can infect corals without a breach in the coral tissue is important to understanding the effect that wounding should have on disease prevalence. Understanding these disease “behaviors,” and whether there are similar behaviors based on the phylogeny or environment of the pathogen, may be able to help us predict the relationship between corallivores and different forms of disease.
-
2.
Corallivores as drivers of disease
Six families of corallivores were shown to increase disease incidence or progression in controlled experiments: Muricidae (muricid snails), Amphinomidae (fireworms), Acanthasteridae (crown-of-thorns starfish), Cryptochiridae (cryptochirid gall crabs), Trinchesiidae (Phestilla sp.), and Chaetodontidae (butterflyfish) (Fig. 2c). We found eight experiments that demonstrated muricid snails could increase disease, which was the highest number of studies for any corallivore (Fig. 2c). The majority of studies showed that predatory snails increased white disease incidence or progression and two showed that they increased brown band disease incidence. Fireworms were the second most studied taxa, with three instances of fireworms increasing white disease incidence or progression and one instance of fireworms increasing bleaching incidence. Crown-of-thorns-starfish were observed to increase brown band disease incidence and progression, cryptochirid crabs to increase white disease incidence, Phestilla sp. to increase white disease incidence, and butterflyfish to increase black band disease incidence (Fig. 2c). There are multiple plausible mechanisms explaining why corallivores increase coral disease incidence. For one, corals direct considerable cellular resources to repair feeding wounds by corallivores, often at the expense of growth, reproduction, and bleaching resilience (Rice et al. 2019). These depleted resources, especially for chronically predated corals, also likely diminish a coral’s ability to fight infection (Mydlarz et al. 2006). Additionally, corallivory can destabilize the coral microbiome (Bettarel et al. 2018; Rice et al. 2019), which can allow opportunistic pathogens to colonize and potentially lead to disease (Zaneveld et al. 2017).
However, not all taxa that increased a disease increased every type of disease and there was variation among species within a corallivore family (Fig. 2e). For instance, although many studies found muricid snails increased disease incidence or progression, two studies found that muricid snails had no effect on disease incidence. In the first study, even though Drupella sp. were found to transmit brown band disease, Drupella sp. presence did not affect black band disease incidence (Nicolet et al. 2018b), suggesting the type of disease matters (Table 1, Electronic Supplementary Material). In the second study, researchers found that although C. galea (i.e., abbreviata) increased white disease incidence, C. caribea did not (Gignoux-Wolfsohn et al. 2012), suggesting species-level differences are also important. Similarly, the study that found no effect of damselfish studied lemon damselfish, Pomacentrus moluccensis (Nicolet et al. 2013), which are different ecologically than farming Stegastes damselfish, which were positively related to disease in correlative studies (Fig. 2a). Unlike P. moluccensis, Stegastes spp. remove coral tissue to create expansive algal gardens that they defend from other fishes, introducing algal microbial communities to their host coral as well as directly wounding them. Like the differing effect of C. caribea and C. galea, we would expect Stegastes damselfishes to have a different effect on disease than P. moluccensis. These species-level differences help explain some of the variation we see within a family.
The magnitude of corallivore effects—The subset of studies that were examined to determine the effect size of corallivores on disease incidence (see Methods) showed that corallivores could have a large effect on disease incidence, but that results were variable among studies (Fig. 3). Most studies involving muricid snails found that snails dramatically increased disease incidence, with some upper estimates suggesting that exposure to snails could increase disease incidence by ~ 15 times compared to controls (Fig. 3). Although most studies employing simulated wounding did not find strong results; one upper estimate suggested simulated wounding could increase pathogen colonization by ~ 25 times (Fig. 3). The two fish families (butterflyfish/Chaetodontidae and damselfish/Pomacentridae) had small effect sizes, with 3 out of 4 butterflyfish studies showing no effect and 1 of 1 damselfish studies showing no effect (Fig. 3). Crown-of-thorns starfish (family Acanthasteridae) consistently increased disease incidence, while fireworms (family Amphinomidae) increased disease incidence in 2 of 3 instances (Fig. 3). The mean effect was highest for crown-of-thorns starfish and fireworms (7.79 and 7.26, respectively), followed by muricid snails (5.60), wounding (5.47), cryptochirid crabs (5), butterflyfish (3.5), and damselfish (1). Although crown-of-thorns starfish and fireworms had the largest mean effect, they did not have the highest maximum effect (Wounding followed by Muricidae) and they also had low sample sizes (n = 2, n = 3, respectively). The low sample size gives a low confidence in the mean, although it is clear that crown-of-thorns starfish and fireworms can strongly increase disease prevalence. More research is needed to determine how the effects of crown-of-thorns starfish, fireworms, and other corallivores, vary spatiotemporally. The current variation in effect size (0–25) shows that how corallivores influence coral disease is context dependent, varying by pathogen, environment, and corallivore species.
Effect of corallivores or simulated wounding from studies that looked at disease incidence in corals exposed to predators versus unexposed controls. Points represent the risk a coral will develop disease signs compared to controls, where a risk ratio of one is no difference (dashed line), a ratio of less than one is a decrease in disease likelihood, and a ratio of greater than one is an increase in disease likelihood. Points have a Haldane-Anscombe correction to account for zeros. Each box represents the interquartile range of the data, with a line in the center for the median. The lines extending from the boxes stretch to the minimum and maximum of the data (the first or third quartile −/+ 1.5*interquartile range)
Corallivores as vectors of disease—A vector is an organism that transmits pathogens among hosts (Work et al. 2008). Corallivores acting as vectors cause disease by harboring infectious agents and transferring them to corals, maintaining a critical link in the spread of disease (e.g., Sussman et al. 2003). Corallivores can increase disease prevalence, incidence, or progression through weakening corals or disrupting their microbiomes without acting as vectors. However, vectors can be critical in the spread of infectious diseases. Potentially harmful parasites had previously been identified in corallivores (e.g., Aeby 1998), but it was not until 2003 that Sussman and colleagues provided the first full account of a coral disease vector. In their foundational study, they placed corallivorous fireworms (H. carunculata) infected with the coral-bleaching pathogen Vibrio shiloi in tanks with healthy coral fragments to find that 100% of coral fragments exposed to infected fireworms began to bleach, while all control corals remained healthy (Sussman et al. 2003).
Since this seminal work, studies have shown other corallivores are capable of vectoring disease or disease agents. Research has implicated: (1) the corallivorous snail C. galea (i.e., abbreviata) in the transmission of white pox (Williams and Miller 2005; Sutherland et al. 2010, 2011), white band disease (Gignoux-Wolfsohn et al. 2012), and white plague (Clemens and Brandt 2015); (2) the nudibranch Phestilla sp. in the transmission of tissue sloughing in Gorgonians (Dalton and Godwin 2006); (3) the snail Drupella sp. in the transmission of brown band disease (Nicolet et al. 2013, 2018b); (4) the fireworm H. carunculata in the transmission of general disease signs (Miller et al. 2014) and shut-down reaction (Antonius 1977); (5) the crown-of-thorns starfish Acanthaster planci in the transmission of brown band disease (Nugues and Bak 2009; Katz et al. 2014); (6) the multiband butterflyfish, Chaetodon multicinctus, in the transmission of trematodiasis (Aeby 1998); and (7) a chriptochirid gall crab in the transmission of white plague-like disease (Pratte and Richardson 2016). In perhaps the most straightforward case, predation by a corallivorous vector results in disease transmission, as in the case of fireworms and Vibrio (Sussman et al. 2003). In other cases, direct feeding by corallivores is not required for disease transmission. For instance, Aeby and Santavy (2006) found that Montastraea faveolata in aquaria with the foureye butterflyfish (Chaetodon capistratus) contracted black band disease, with feeding increasing the rate of transmission. However, direct contact between fish and M. faveolata was not necessary for disease signs to develop—the mere presence of the fish in the same tank facilitated disease incidence (Aeby and Santavy 2006), suggesting that some diseases are transmitted via mechanisms other than feeding, such as through the deposition of corallivore feces. Indeed, multiple studies have found pathogens in corallivore guts. For example, the corallivorous flamingo tongue snail, Cyphoma gibbosum, can pass the Aspergillosis pathogen, Aspergillus sydowii, through its digestive tract and excrete viable spores (Rypien and Baker 2009). Similarly, studies have found trematodes in butterflyfish (Aeby 1998, 2002; Martin et al. 2018), the white pox pathogen Serratia marcescens in C. galea (i.e., abbreviata) snails (Sutherland et al. 2011), and potentially pathogenic bacteria in surgeonfish feces, which were transferred to corals through fecal deposition (Ezzat et al. 2019). Further, corallivorous fishes were recently shown to disperse viable Symbiodiniaceae through their feces (Grupstra et al. 2021), which may mean corallivores can disperse other viable microbes like pathogens via the same process.
Pathogens found inside corallivores may also suggest that corallivores can act as biotic reservoirs for disease. Biological vectors that also provide refuge for pathogens in sub-ideal environments can facilitate disease recurrence by providing a suitable environment for pathogens until the conditions are right to colonize corals. At cold temperatures, for instance, the bleaching pathogen V. shiloi is unable to survive in its coral hosts but can be found in the fireworm H. carunculata (Sussman et al. 2003). The pathogen is in a viable-but-not-culturable state inside the fireworm, which allows it to infect corals once the temperature rises (Sussman et al. 2003), creating a disease cycle facilitated by the corallivore and favorable abiotic conditions.
Although studies to date suggest invertebrate corallivores have a stronger, more consistent influence on coral disease, most corallivorous fishes are considerably more mobile than coral-associated invertebrates. They, therefore, may play a more important role in pathogen spread across larger spatial scales when they are able to vector pathogens. Because of this mobility, they may also pose a greater threat to corals if a non-native coral pathogen is introduced and able to establish itself in a corallivorous fish. In terrestrial systems, devastating insect-facilitated tree diseases (e.g., Cypress Canker Disease, Dutch Elm Disease, Pine Wilt Disease) have occurred when a non-native pathogen took over the niche of a native, non-aggressive microbial agent in an insect that was closely associated with a tree species (Santini and Battisti 2019). For instance, in the case of Dutch Elm Disease, the Ascomycete fungus, Ophiostoma ulmi (and O. novo-ulmi), outcompeted the native non-pathogenic fungus Ophiostoma quercus, which had long been associated with elm bark beetles. Once O. ulmi was established in the beetle population, it was able to use the close relationship between elms and the beetles to become one of the most destructive plant diseases of all time (Santini and Battisti 2019). Although it appears that many of the microbes transmitted via corallivorous fishes are harmless under benign environmental conditions, if a novel pathogen is introduced—such as in the case of Serratia marcescens from human guts (Patterson et al. 2002)—and that pathogen is able to take advantage of a highly mobile fish vector, as it did with less mobile corallivorous snails (Sutherland et al. 2010), it may spread rapidly.
-
3.
Wounding and disease
A corallivore need not be a vector to facilitate disease spread. To be successful, a pathogen must both (a) spread to new hosts and (b) penetrate host tissues and mucus layers to cause infection. When a corallivore both harbors a pathogen and creates a point of entry that pathogen, such as in the case of a feeding lesion created by an infected corallivore, the vector can check both of these boxes. However, a corallivore does not need to be a vector to influence disease if the wound created by the corallivore provides an entryway for external pathogens present near the wound. For instance, some corallivores may have little effect on their own, but can promote disease when they feed near pathogen sources (e.g., Wolf and Nugues 2013). Similarly, there may be a baseline rate of infection in the environment, but having corallivore-created wounds could exacerbate the infection rate for predated corals. For example, in an aquaria-based experiment, 91% of corals exposed to C. galea (i.e., abbreviata) contracted white band disease, while corals not exposed to snails still contracted white band disease ~ 11% of the time (Gignoux-Wolfsohn et al. 2012). In cases like these, corallivores are not necessary for infection, but increase the rate of infection or disease progression when present. For this reason, the effect of wounding will likely be most important to disease spread when the pathogen is waterborne in the environment or is already present in the coral microbiome (i.e., an opportunistic pathogen).
Wounding does not have to immediately cause an infection to lead to disease. The physical damage from wounding can stress corals and alter their microbiome (Shaver et al. 2017), making corals more disease-prone and vulnerable to microbial colonization (Raymundo et al. 2016; Bettarel et al. 2018). Some wounds may promote secondary infections or cause heightened tissue loss under stressful conditions (Zaneveld et al. 2016), such as high nutrient conditions, high sedimentation, low pH, or elevated temperatures. Wounding may also attract opportunistic pathogens via decaying coral tissue (Nugues and Bak 2009; Katz et al. 2014), which suggests that wounding has, in the most severe case, the potential to: (1) create an opening in the tissue for infection, (2) reduce a colony’s ability to resist disease, and (3) attract potentially harmful pathogens.
Although the importance of wounding varies, wound creation clearly has the ability to facilitate coral diseases. Studies that simulate corallivory have found that wounding increases the incidence of white band (Gignoux-Wolfsohn et al. 2012), black band (Aeby and Santavy 2006), brown band (Nicolet et al. 2013), and skeletal eroding band (Page and Willis 2008) disease. Simulated wounding also shifts the microbial community toward opportunistic taxa (Maher et al. 2019) and can help opportunistic microbes to persist in corals (Ezzat et al. 2019). This may be because corals direct photoassimilates (i.e., products from photosynthesis) toward an injured area. These photoassimilates aid in healing coral tissue, but also indirectly provide resources to opportunistic microbes, which can ultimately lead to infection (Roff et al. 2006). That said, not all wounding experiments increased disease transmission—some studies found no wounding effect (white disease: Williams and Miller 2005; Caribbean yellow-band syndrome: Jordán-Garza and Jordán-Dahlgren 2011; general: van de Water et al. 2015; dark-spot syndrome: Randall et al. 2016; microbiome: Shirur et al. 2016; microbiome: Wright et al. 2017). However, those results may be specific to the disease (e.g., Randall et al. 2016 suggest dark-spot syndrome is not highly transmissible) and the exposure duration or depth of wound (e.g., Williams and Miller 2005).
-
4.
Corallivore feeding on disease fronts
Corallivores have been observed feeding along disease margins, but the reason for this lesion predation remains unclear. There are numerous accounts of corallivorous fishes biting disease lesions, often with a preference for diseased tissue over healthy tissue (Aeby 1992; McIlwain and Jones 1997; Cole et al. 2009; Chong-Seng et al. 2011; Slattery et al. 2013). Butterflyfishes were most frequently observed feeding on lesions, with well over 20 observations, most of which were for black band disease (Fig. 4). Damselfishes and wrasses (family Labridae) both had multiple observations for multiple diseases, whereas parrotfishes, gobies (family Gobiidae) blennies (family Blenniidae), filefishes (family Monacanthidae), and surgeonfishes (family Acanthuridae) each had a single observation (Fig. 4). Although individual invertebrate bites are rarely monitored, corallivorous invertebrates have been observed preferentially infesting diseased coral colonies (e.g., Fig. 1c) (Miller and Williams 2007; Pratchett et al. 2010; Pollock et al. 2013; Bettarel et al. 2018), and in trials actively chose diseased corals over healthy ones (Pollock et al. 2013). Drupella rugosa, for instance, is attracted to corals stressed by physical wounding (Morton et al. 2002; Tsang and Ang 2015) and in a choice experiment, C. galea (i.e., abbreviata) chose diseased or wounded corals over healthy corals (Bright et al. 2015). Although most explanations remain speculative, predators may be targeting lesions because the tissue is less defended (e.g., Slattery 1999) or because the microbial consortia in the lesion offers a nutritional benefit (e.g., trace elements: Sato et al. 2010).
Fish taxa observed to directly feed on diseased lesions, broken up by fish family and coral disease. If there were multiple fishes observed in a study, each fish was counted as an observation. If the study grouped fishes into families (e.g., “butterflyfishes”) then that family counted as one observation. Studies that surveyed multiple diseases and quantified disease prevalence as a sum of all observed diseases were termed “General” disease. BBD = black band disease and BrB = brown band disease
The effect of lesion predation on disease progression appears context dependent. Many (n = 17; Fig. 2c) studies show that corallivores increase disease incidence, perhaps by weakening corals via wounding, introducing pathogens, or by further destabilizing the microbiome. However, some studies (n = 8) have found little effect of feeding on disease progression (e.g., Nicolet et al. 2013, 2018a; Fig. 2e). In other cases (n = 3; Fig. 2d), corallivores feeding along disease fronts even appears to slow disease progression. For instance, not only did the furry coral crab, Cymo melanodactylus, choose to feed on diseased corals, but white syndrome-like signs also progressed three times faster on corals without crabs (Pollock et al. 2013), suggesting crabs may actually slow disease. Similarly, butterflyfish that fed along disease bands slowed the progression of black band disease on Acropora corals (Cole et al. 2009) and corallivore removal of trematode-infected polyps reduced disease coverage on coral colonies (Aeby 1991). Although our results show that corallivores most often increase disease incidence or progression, it is also worth understanding how certain taxa (e.g., butterflyfishes and xanthid crabs) may be able to decrease disease incidence or progression in some cases.
Corallivores may slow disease spread by debriding diseased coral tissue and removing pathogens. Experiments that remove infected tissue in the absence of a corallivore have found that it can slow disease progression (Dalton et al. 2010; Williams 2013; Miller et al. 2014; Beurmann et al. 2017). However, the effects of corallivory on disease are also likely dependent on the type of disease and whether the corallivore is a biological vector. When corallivores are carrying pathogens and are the main mechanism for disease transmission, feeding will likely increase disease prevalence. However, non-vector predators could benefit corals by removing infected areas without introducing new pathogens, particularly under benign conditions where corals are able to heal quickly. Even a mobile vector such as a butterflyfish could benefit an individual coral by removing diseased tissue, although they may then transfer the pathogen to another colony. Alternatively, when the disease is highly contagious (i.e., waterborne), wounding by even a non-vector predator may dramatically increase infection rates and outweigh the potential benefit of debridement.
Feeding behavior and corallivore biology also likely play a role in determining the outcome of a corallivore-disease relationship. Fishes, snails, crabs, and worms all have distinct feeding mechanisms that influence corals differently, even under identical conditions. It has been hypothesized that invertebrates have more negative effects on disease compared to fishes because invertebrates spend longer in one localized area and because they create deeper predation scars, at least when compared to polyp-feeding fishes (Nicolet et al. 2018b). While this may be true, not all invertebrates are the same. Corallivores like xanthid or trapezid crabs that scrape mucus or coral polyps rather than creating deep predation scars may be effective pathogen “cleaners” rather than serious wounders, which is consistent with accounts of xanthid crabs decreasing disease spread (Fig. 2d). Further, corallivore mucus may vary between taxa, which can affect pathogen storage and transmission. For instance, anti-microbial peptides have been found in some Brachyuran crabs and may be in the mucus of other Brachyurans, such as xanthid crabs (suggested in Pollock et al. 2013). Although debridement has been explored as an option for treating coral disease (e.g., Beurmann et al. 2017), context (e.g., type of wound, type of disease, level of abiotic stress) will be critical in determining whether it is appropriate in a given situation.
-
5.
Interactions among corallivores, disease, and non-corallivore species
Other species interactions besides corallivory, such as those between corals and algae, influence coral disease and can alter how corallivores impact disease. For instance, a study in the southern Caribbean found that fireworms alone would not kill corals, but that fireworms combined with the alga Halimeda opuntia kill corals at a greater rate than the alga in isolation (Wolf and Nugues 2013). This could be because fireworms create additional points of entry for algal-associated pathogens, which have been linked to the occurrence of white plague type II (Nugues et al. 2004). Even in cases where an alga is not harboring a specific pathogen, algae produce organic matter that encourages the invasion of opportunistic pathogens (Barott and Rohwer 2012) and could cause harmful secondary infections after a corallivore wound.
Many corallivores also kill sections of live coral, which promotes algal colonization by freeing up substrate (Shaver et al. 2017). This in turn could facilitate disease when algae harbor pathogens (e.g., Nugues et al. 2004; Sweet et al. 2013) or cause coral stress (e.g., Morrow et al. 2012). This interaction may create a positive feedback loop whereby corallivores allow algae to colonize; algae act as reservoirs for disease-causing agents; corallivores wound corals, allowing those pathogens to invade; coral disease creates more dead coral substrate; more dead substrate can increase algal colonization; and more algal colonization leads to more disease (Fig. 5). This algal-wounding relationship could explain why algal gardening Stegastes are strongly associated with disease prevalence (Casey et al. 2014), but non-gardening damselfish are not (Nicolet et al. 2013). Given the importance of multiple species interactions in determining disease outcomes, merely surveying the abundance of corallivores may fail to detect a relationship between corallivores and disease prevalence if other organisms, such as algae, modulate the impact of corallivory.
Potential feedbacks among multiple stressors, corallivory, and coral disease. (1) Rising temperatures stress corals, making them more prone to disease and making wounds slower to heal; (2) Increased temperatures disrupt the microbiome and increase the virulence of some pathogens; (3) Nutrients and temperature can interact non-additively to cause coral mortality; (4) Nutrients exacerbate effects of corallivores and increase disease prevalence; (5) Corallivores wound corals, allowing for microbial colonization; (6) Algae alter the microbiome, encouraging pathogenic microbes; (7) Dead coral creates room for algal colonization; (8) Nutrients can encourage algal growth, which in turn can increase coral disease prevalence; (9) Disease pathogens and opportunistic microorganisms infect corals, causing coral death
The predators of corallivores also affect corallivore-associated disease dynamics as they can affect the abundance of corallivores. In some areas, corallivores can be widespread (e.g., corallivorous snails present on up to 64% on preferred coral species in Hayes 1990), corallivory can be intense (e.g., heavy fish corallivory in Fig. 1d, over 100 bite scars per m2 on Pocillopora in Jayewardene et al. 2009), and corallivore outbreaks can reach high densities (e.g., 1500 Drupella individuals per 0.5 m2 in Moyer et al. 1982). However, there is considerable spatiotemporal variation in this predation pressure, with predation by some species increasing up to 238 times between regions (Bonaldo and Bellwood 2011). Although we do not know what controls all corallivore populations, some of this variation is likely due to changes in top-down pressure. When predators of corallivores are overfished, the populations of their prey (e.g., corallivores) can increase, which can lead to coral mortality (discussed in Shaver et al. 2017; Rice et al. 2019) and potentially to disease outbreaks, in a classic trophic cascade (Fig. 6). Indeed, a study in the Line Islands found that areas with more predators have fewer incidences of disease (Sandin et al. 2008), and a study in Fiji found that corallivorous snails were more abundant in overfished areas due to reduced predation (Clements and Hay 2018). Determining (1) how predators alter the spatiotemporal densities of corallivores; (2) how these changes in corallivore densities affect coral disease; and (3) whether there are thresholds of corallivore densities above which their effects on disease are particularly impactful, is critical to predicting corallivore impacts through space and time.
Marine protected areas may indirectly provide some resilience against disease by protecting corallivore predator populations, conserving biodiversity, reducing macroalgae, and limiting fishing activity. One study from the Philippines found that fish biodiversity was higher in protected areas, where disease prevalence was lower (Raymundo et al. 2009) and surveys in the Florida Keys found that protected areas had more diverse corallivore predator assemblages, which was correlated with decreased C. galea (i.e., abbreviata) abundance (Shaver et al. 2020). It may be that marine protected areas with more robust corallivore predator assemblages indirectly decrease disease prevalence by controlling corallivore populations (Fig. 6). Indeed, protected areas appear to make reef communities more resilient to disturbances like disease (Mellin et al. 2016) and may help protect other forms of diversity over time. If they foster diversity, protected areas may also indirectly conserve other important species, such as coral-associated gobies that reduce corallivory by fishes (Dirnwoeber and Herler 2013) and coral-associated hydrozoans that reduce both disease prevalence and predation on their coral hosts (Montano et al. 2017). Further, diverse coral communities are more robust and grow faster than monotypic communities (Fig. 6; Clements and Hay 2019), which may make them more resistant to disease. Initial research suggests protected areas can also affect the coral holobiont; for example, a study in Fiji found that corals in protected areas are better at defending themselves against the bleaching pathogen Vibrio coralliilyticus than their counterparts on fished, macroalgae-dominated reefs (Beatty et al. 2019). In addition to preserving biodiversity and improving microbial conditions, corals in protected areas have less physical damage associated with fishing, which is positively related to disease prevalence (Lamb et al. 2015) and may attract corallivores. Indeed, there is likely a suite of biological interactions that act alongside corallivory to influence disease, but more research is needed on how these factors (e.g., algal cover, predator abundance, diversity) interact with corallivory to affect disease outcomes and on how policy measures like marine protected areas impact these interactions.
-
6.
Interactions among corallivores, disease, and anthropogenic stressors
Corallivory can interact with anthropogenic stressors to shape disease dynamics (Fig. 5). For instance, both nutrients (Bruno et al. 2003; Vega Thurber et al. 2013) and sedimentation (Pollock et al. 2014) can increase disease prevalence, and there is some evidence that nutrient inputs from runoff may indirectly promote some corallivores by improving larval survival (Brodie et al. 2005). Thus, areas experiencing coastal runoff may be suffering from the cumulative impacts of increased disease prevalence, direct stress from sedimentation, direct stress from nutrient enrichment, and increased corallivory. These stressors may also increase the detrimental effect of corallivory itself. In one such case, parrotfish caused tissue loss in 92% of the corals they preyed on under high nutrient conditions, but caused tissue loss in just 7% of bitten corals under normal nutrient levels (Zaneveld et al. 2016). These findings suggest that increased nutrients can increase bacterial opportunism and shift the effect of parrotfish feeding from a relatively benign species interaction into one that ends in coral death. Although parrotfish bites alone can alter the coral microbiome by introducing new bacteria (Ezzat et al. 2020), these bites may only manifest in disease under stressful conditions, such as when nutrients hinder a coral’s ability to heal (Dougan et al. 2020). This context-dependent nature could explain conflicting results from parrotfish studies (e.g., both negative and positive correlations in Fig. 2) if under benign conditions parrotfish have little to no effect on coral health, but under stressful conditions they increase disease.
Other events that damage coral can exacerbate corallivory and, consequently, disease. Damaged coral tissues release mucus and primary metabolites (Hay 2009) that may attract corallivores (Kita et al. 2005). Corallivores may then attract other conspecifics, such as in the case of gregarious corallivores like C. galea and D. rugosa, which are attracted to feeding by other snails (Morton et al. 2002; Bright et al. 2015). Damage by SCUBA divers may also increase the prevalence of coral disease (Lamb et al. 2014) as well as increase predation by D. cornus and, ultimately, coral mortality (Guzner et al. 2010). Similarly, physical damage by storms (Brandt et al. 2013) and ship groundings (Raymundo et al. 2018) appear to increase disease prevalence and may be linked to corallivorous snail aggregations (Knowlton et al. 1990; Bright et al. 2016).
Temperature increases associated with climate change will also exacerbate coral disease prevalence and progression, although we know little about how increased temperatures will interact with corallivory to affect disease. Many disease pathogens grow faster and are more virulent at high temperatures. For instance, the suspected white plague II pathogen, the bacteria Aurantimonas coralicida, both grows faster at warm temperatures and is better able to tolerate stressful pH conditions, potentially improving its ability to colonize the acidic coral surface layer (Remily and Richardson 2006). Certain diseases are more prevalent under warmer conditions (e.g., Boyett et al. 2007; Bruno et al. 2007) and the effects of disease, such as the rate or amount of tissue loss, may also be greater at warmer temperatures (Dalton et al. 2010). Additionally, pathogens that are cold temperature-limited and seek refuge in non-coral hosts during cold months, such as V. shiloi, may be able to remain in corals for longer under a warmer climate, increasing the potential damage they can cause. On the whole, warmer temperatures will likely result in longer disease durations and faster disease transmission, but how temperature increases affect the coral-corallivory-disease relationship remains unknown. For instance, corallivores may actively target temperature stressed corals (e.g., Tsang and Ang 2015), which could lead to an increase in disease prevalence by stressing already weakened corals. Even routine corallivory on temperature stressed corals could become problematic given that temperature stressed corals have reduced energy reserves (Schoepf et al. 2015), which play a role in wound healing and fighting infection. Increased temperatures, as well as ocean acidification, may also alter the rate at which corallivores feed (shown in other marine organisms: Allan et al. 2017; Watson et al. 2017), although the effects of corallivores under different climate scenarios have largely been overlooked.
More research is needed to understand how climate change influences the corallivore-disease relationship and potential consequences for coral reefs of the future. For instance, climate change may alter corallivore communities by changing food availability (i.e., coral species and/or abundance), which in turn will influence remaining coral populations (Rice et al. 2019). In that case, corallivores may aggregate on remaining corals (e.g., Knowlton et al. 1981; Bruckner et al. 2017) creating consumer fronts (Silliman et al. 2013) that overwhelm corals and increase disease prevalence. Alternatively, the decrease in food resources could curtail corallivore populations over time, allowing corals to recover. Indeed, there is considerable uncertainty surrounding how stressors such as overfishing, increasing temperatures, acidifying waters, and increasing nutrients will influence corallivory and thus its interaction with disease.
Conclusion and future perspectives
This collection of 65 studies from 26 different ecoregions reveals that both invertebrate and vertebrate corallivores can affect disease by acting as vectors, feeding on disease margins, or creating entry points into damaged tissues. The abundance of most corallivores was positively correlated with disease prevalence (23 of 27 surveys) and the abundance of all invertebrate corallivores was positively correlated with disease prevalence (16 of 16 surveys). We found that muricid snails and fireworms increased disease incidence or progression in the greatest number of experimental studies (Fig. 2c) and increased the likelihood of a coral developing disease signs by over tenfold in some cases (Fig. 3). Observationally, muricid snails (e.g., Coralliophila and Drupella sp.), fireworms, damselfish (with the exception of one instance), and the flamingo tongue snail were positively correlated with disease prevalence in all observational studies and were found to harbor potential pathogens in their guts, suggesting they likely increase disease spread and/or severity. Crown-of-thorns starfish, cryptochirid crabs, and Phestilla sp. also increased disease incidence or progression but had fewer studies supporting the relationship. Butterflyfishes were heavily studied, with numerous findings documenting targeted feeding on disease lesions, correlations with disease occurrence in the field, the pathogen contents of their digestive tract, and their ability to transmit disease. Although they are well-shown to vector trematodes, they do not appear to spread other diseases directly through their feeding (i.e., four experimental studies with no effect, Fig. 2e), although their feces may increase disease in some cases. Additionally, their feeding appears to slow disease progression in the case of black band disease and, in the case of trematodiasis, butterflyfish feeding may reduce the number of diseased polyps on a coral colony, which may explain why surveys have found butterflyfish can be both positively and negatively correlated with disease prevalence (Fig. 2). The majority of corallivores increased disease, with just butterflyfish and xanthid crabs decreasing disease incidence or progression. This differential effect is likely because butterflyfish and some xanthid crabs have a relatively low-impact form of feeding (i.e., target mucus and polyps) that does not damage corals as intensively as other corallivores (e.g., excavators, fireworms, crown-of-thorns starfish) and may actually debride infected tissue. The effect of simulated wounding was variable, with one instance increasing the likelihood of disease by over 20-fold, but many instances reporting little or no effect (Fig. 3), suggesting wounding is highly context dependent.
These results provide needed clarity on how corallivores influence disease and hopefully will open the door for more research examining these patterns. More research is needed to understand: (1) how the type of corallivore (e.g., invertebrate/vertebrate, family, species, specialist/non-specialist, feeding type) influences how it affects disease spread; (2) whether coral-corallivore-pathogen relationships are consistent temporally and geographically; (3) how diseases are vectored across large spatial scales and at what spatial scales corallivores are important; (4) whether novel coral pathogens are able to exploit existing non-aggressive microbe-predator-host relationships, such as in the case with Dutch Elm Disease; (5) the role of corallivore density and consumer fronts in disease dynamics; and (6) how climate change and other forms of stress will impact the relationship between corallivores and coral disease. More targeted, manipulative experiments with larger sample sizes will help us understand how corallivores are influencing disease dynamics, as the effect of a corallivore on a coral is likely dictated by the disease, the method of transmission, the type of corallivore, and the environment.
This depth of understanding is critical for successful management of coral populations and targeted coral restoration. If certain corallivores can facilitate coral health through disease abatement, they should be incorporated into restoration designs along with non-corallivorous species that reduce coral disease prevalence through indirect pathways (e.g., herbivorous fishes that control disease-inducing macroalgae, predators that control harmful corallivores). Similarly, models that predict disease outbreaks or coral dynamics should take into consideration corallivore populations, as well as the potential ramifications of climate change and human resource use on corallivore-coral relationships. The insights synthesized here, in combination with future work that targets these knowledge gaps, will be integral to understanding disease dynamics and the future of coral health.
Data availability
The datasets generated during the current study and the code needed to generate the figures are available on GitHub, at: https://github.com/juliannajollyrenzi/Renzi_et_al_2022.
References
Aeby G (2002) Trade-offs for the butterflyfish, Chaetodon multicinctus, when feeding on coral prey infected with trematode metacercariae. Behav Ecol Sociobiol 52:158–165
Aeby GS (1991) Behavioral and Ecological Relationships of a Parasite and Its Hosts within a Coral Reef System. Pac Sci 45:7
Aeby GS (1992) The potential effect the ability of a coral intermediate host to regenerate has had on the evolution of its association with a marine parasite. Proc 7th Int Coral Reef Symp 2:809–815
Aeby GS (1998) A Digenean Metacercaria from the Reef Coral, Porites compressa, Experimentally Identified as Podocotyloides stenometra. J Parasitol 84:1259–1261
Aeby GS, Santavy D (2006) Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar Ecol Prog Ser 318:103–110
Allan BJ, Domenici P, Watson SA, Munday PL, McCormick MI (2017) Warming has a greater effect than elevated CO2 on predator–prey interactions in coral reef fish. Proc Royal Soc B 1857:20170784
Anscombe FJ (1956) On Estimating Binomial Response Relations. Biometrika 43:461–464
Antonius A (1977) Coral mortality in reefs: a problem for science and management. Proc 3rd Int Coral Reef Symp 617–623
Antonius A, Riegl B (1997) A Possible Link Between Coral Diseases and a Corallivorous Snail (Drupella cornus) Outbreak in the Red Sea. Atoll Res Bull 1–9
Aronson R, Precht W (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Baquero O (2019) ggsn: North Symbols and Scale Bars for Maps Created with “ggplot2” or “ggmap.”
Barott KL, Rohwer FL (2012) Unseen players shape benthic competition on coral reefs. Trends Microbiol 20:621–628
Beatty DS, Valayil JM, Clements CS, Ritchie KB, Stewart FJ, Hay ME (2019) Variable effects of local management on coral defenses against a thermally regulated bleaching pathogen. Sci Adv 5:eaay1048
Bettarel Y, Halary S, Auguet JC, Mai TC, Van NB, Bouvier T, Got P, Bouvier C, Monteil-Bouchard S, Christelle D (2018) Corallivory and the microbial debacle in two branching scleractinians. ISME J 12:1109–1126
Beurmann S, Runyon CM, Videau P, Callahan SM, Aeby GS (2017) Assessment of disease lesion removal as a method to control chronic Montipora white syndrome. Dis Aquat Organ 123:173–179
Bonaldo RM, Bellwood DR (2011) Parrotfish predation on massive Porites on the Great Barrier Reef. Coral Reefs 30:259–269
Bourne DG, Ainsworth TD, Pollock FJ, Willis BL (2015) Towards a better understanding of white syndromes and their causes on Indo-Pacific coral reefs. Coral Reefs 34:233–242
Boyett HV, Bourne DG, Willis BL (2007) Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar Biol 151:1711–1720
Brandt ME, Smith TB, Correa AMS, Vega-Thurber R (2013) Disturbance Driven Colony Fragmentation as a Driver of a Coral Disease Outbreak. PLoS ONE 8:e57164
Bright AJ, Cameron CM, Miller MW (2015) Enhanced susceptibility to predation in corals of compromised condition. PeerJ 3:e1239
Bright AJ, Rogers CS, Brandt ME, Muller E, Smith TB (2016) Disease Prevalence and Snail Predation Associated with Swell-Generated Damage on the Threatened Coral, Acropora palmata (Lamarck). Front Mar Sci 3:77
Brodie J, Fabricius K, De’ath G, Okaji K (2005) Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull 51:266–278
Bruckner AW, Coward G, Bimson K, Rattanawongwan T (2017) Predation by feeding aggregations of Drupella spp. inhibits the recovery of reefs damaged by a mass bleaching event. Coral Reefs 36:1181–1187
Bruno JF, Petes LE, Harvell CD, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056–1061
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal Stress and Coral Cover as Drivers of Coral Disease Outbreaks. PLoS Biol 5:e124
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute. URL: https://digitalarchive.worldfishcenter.org/handle/20.500.12348/1107
Bythell J, Pantos O, Richardson L (2004) White Plague, White Band, and Other “White” Diseases. In: Rosenberg E, Loya Y (eds) Coral Health and Disease. Springer, Berlin, Heidelberg, pp 351–365
Casey JM, Ainsworth TD, Choat JH, Connolly SR (2014) Farming behaviour of reef fishes increases the prevalence of coral disease associated microbes and black band disease. Proc Biol Sci 281:20141032
Chong-Seng KM, Cole AJ, Pratchett MS, Willis BL (2011) Selective feeding by coral reef fishes on coral lesions associated with brown band and black band disease. Coral Reefs 30:473–481
Clemens E, Brandt ME (2015) Multiple mechanisms of transmission of the Caribbean coral disease white plague. Coral Reefs 34:1179–1188
Clements CS, Hay ME (2018) Overlooked coral predators suppress foundation species as reefs degrade. Ecol Appl 28:1673–1682
Clements CS, Hay ME (2019) Biodiversity enhances coral growth, tissue survivorship and suppression of macroalgae. Nat Ecol Evol 3:178–182
Cole AJ, Chong Seng KM, Pratchett MS, Jones GP (2009) Coral-feeding fishes slow progression of black-band disease. Coral Reefs 28:965–965
Cole AJ, Pratchett MS, Jones GP (2008) Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 9:286–307
Costa AS (1976) Whitefly-transmitted plant diseases. Annu Rev Phytopathol 14:429–449
Dalton SJ, Godwin S (2006) Progressive coral tissue mortality following predation by a corallivorous nudibranch (Phestilla sp.). Coral Reefs 25:529
Dalton SJ, Godwin S, Smith SDA, Pereg L (2010) Australian subtropical white syndrome: a transmissible, temperature-dependent coral disease. Mar Freshwater Res 61:342–350
Dirnwoeber M, Herler J (2013) Toxic coral gobies reduce the feeding rate of a corallivorous butterflyfish on Acropora corals. Coral Reefs 32:91–100
Dougan KE, Ladd MC, Fuchs C, Vega Thurber R, Burkepile DE, Rodriguez-Lanetty M (2020) Nutrient Pollution and Predation Differentially Affect Innate Immune Pathways in the Coral Porites porites. Front Mar Sci 7:563865
Ezzat L, Lamy T, Maher RL, Munsterman KS, Landfield K, Schmeltzer ER, Gaulke CA, Burkepile DE, Thurber RV (2019) Surgeonfish feces increase microbial opportunism in reef-building corals. Mar Ecol Prog Ser 631:81–97
Ezzat L, Lamy T, Maher RL, Munsterman KS, Landfield KM, Schmeltzer ER, Clements CS, Vega Thurber RL, Burkepile DE (2020) Parrotfish predation drives distinct microbial communities in reef-building corals. Anim Microbiome 2:5
Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield Iii AE, Hanula JL, Eickwort JM, Miller DR (2008) A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis 92:215–224
García-Guzmán G, Dirzo R (2001) Patterns of leaf-pathogen infection in the understory of a Mexican rain forest: incidence, spatiotemporal variation, and mechanisms of infection. Am J Bot 88:634–645
Gignoux-Wolfsohn SA, Marks CJ, Vollmer SV (2012) White Band Disease transmission in the threatened coral, Acropora Cervicornis. Sci Rep 2:804
Greene A, Donahue MJ, Caldwell JM, Heron SF, Geiger E, Raymundo LJ (2020) Coral disease time series highlight size-dependent risk and other drivers of white syndrome in a multi-species model. Coral Reefs 7:1022
Grober-Dunsmore R, Bonito V, Frazer T (2006) Potential inhibitors to recovery of Acropora palmata populations in St. John, US Virgin Islands. Mar Ecol Prog Ser 321:123–132
Grupstra CGB, Rabbitt KM, Howe-Kerr LI, Correa AMS (2021) Fish predation on corals promotes the dispersal of coral symbionts. Anim Microbiome 3:25
Guzner B, Novplansky A, Shalit O, Chadwick NE (2010) Indirect impacts of recreational scuba diving: patterns of growth and predation in branching stony corals. Bull Mar Sci 86:727–742
Haldane JBS (1940) The mean and variance of the moments of chi-squared, when used as a test of homogeneity, when expectations are small. Biometrika 29:133–134
Hay ME (2009) Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Annu Rev Mar Sci 1:193–212
Hayes JA (1990) Distribution, movement and impact of the corallivorous gastropod Coralliophila abbreviata (Lamarck) on a Panamanian patch reef. J Exp Mar Biol Ecol 142:25–42
Howells EJ, Vaughan GO, Work TM, Burt JA, Abrego D (2020) Annual outbreaks of coral disease coincide with extreme seasonal warming. Coral Reefs 39:771–781
Jayewardene D, Donahue MJ, Birkeland C (2009) Effects of frequent fish predation on corals in Hawaii. Coral Reefs 28:499–506
Jordán-Garza A, Jordán-Dahlgren E (2011) Caribbean yellow-band syndrome on Montastraea faveolata is not transmitted mechanically under field conditions. Dis Aquat Org 96:83–87
Katz SM, Pollock FJ, Bourne DG, Willis BL (2014) Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs 33:705–716
Kellogg C, West A, Runyon C (2017) Predation by Acanthurus leucopareius on black-band disease in Kauai, Hawaii. Bull Mar Sci 93:
Kita M, Kitamura M, Koyama T, Teruya T, Matsumoto H, Nakano Y, Uemura D (2005) Feeding attractants for the muricid gastropod Drupella cornus, a coral predator. Tetrahedron Lett 46:8583–8585
Knowlton N, Lang JC, Christine Rooney M, Clifford P (1981) Evidence for delayed mortality in hurricane-damaged Jamaican staghorn corals. Nature 294:251–252
Knowlton N, Lang JC, Keller BD (1990) Case study of natural population collapse: post-hurricane predation on Jamaican staghorn corals. Smithson Contrib Mar Sci 31:1–22
Lamb JB, True JD, Piromvaragorn S, Willis BL (2014) Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol Conserv 178:88–96
Lamb JB, Williamson DH, Russ GR, Willis BL (2015) Protected areas mitigate diseases of reef-building corals by reducing damage from fishing. Ecology 96:2555–2567
Maher RL, Rice MM, McMinds R, Burkepile DE, Vega Thurber R (2019) Multiple stressors interact primarily through antagonism to drive changes in the coral microbiome. Sci Rep 9:6834
Martin SB, Sasal P, Cutmore SC, Ward S, Aeby GS, Cribb TH (2018) Intermediate host switches drive diversification among the largest trematode family: evidence from the Polypipapiliotrematinae n. subf. (Opecoelidae), parasites transmitted to butterflyfishes via predation of coral polyps. Int J Parasitol 48:1107–1126
McIlwain J, Jones G (1997) Prey selection by an obligate coral-feeding wrasse and its response to small-scale disturbance. Mar Ecol Prog Ser 155:189–198
Mellin C, Aaron MacNeil M, Cheal AJ, Emslie MJ, Julian Caley M (2016) Marine protected areas increase resilience among coral reef communities. Ecol Lett 19:629–637
Miller MW, Williams DE (2007) Coral disease outbreak at Navassa, a remote Caribbean island(67). Coral Reefs 26:97–101
Miller M, Marmet C, Cameron C, Williams D (2014) Prevalence, consequences, and mitigation of fireworm predation on endangered staghorn coral(66). Mar Ecol Prog Ser 516:187–194
Montano S, Fattorini S, Parravicini V, Berumen ML, Galli P, Maggioni D, Arrigoni R, Seveso D, Strona G (2017) Corals hosting symbiotic hydrozoans are less susceptible to predation and disease. Proc Biol Sci 284:20172405
Moreira APB, Chimetto Tonon LA, de Valle P, Pereira C, Alves N, Amado-Filho GM, Francini-Filho RB, Paranhos R, Thompson FL (2014) Culturable Heterotrophic Bacteria Associated with Healthy and Bleached Scleractinian Madracis decactis and the Fireworm Hermodice carunculata from the Remote St. Peter and St. Paul Archipelago, Brazil. Curr Microbiol 68:38–46
Morrow KM, Ritson-Williams R, Ross C, Liles MR, Paul VJ (2012) Macroalgal Extracts Induce Bacterial Assemblage Shifts and Sublethal Tissue Stress in Caribbean Corals. PLoS ONE 7:e44859
Morton B, Blackmore G, Kwok CT (2002) Corallivory and Prey Choice by Drupella Rugosa (Gastropoda: Muricidae) in Hong Kong. J Molluscan Stud 68:217–223
Moyer J, Emerson W, Ross M (1982) Massive destruction of scleractinian corals by the muricid gastropod, Drupella, in Japan and the Philippines. Nautilus 96:2
Muko S, Nadaoka K (2020) Ecological disturbances and their relative impacts on coral cover: analysis of monitoring data in Sekisei Lagoon (Okinawa, Japan). Bull Mar Sci 96:195–216
Müller K (2020) Here: A Simpler Way to Find Your Files. R package version 1.0.1. https://CRAN.R-project.org/package=here
Mydlarz LD, Jones LE, Harvell CD (2006) Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst 1:251–288
Nagelkerken I, Buchan K, Smith GW, Bonair K, Bush P, Garzón-Ferreira J, Botero L, Gayle P, Harvell CD, Heberer C, Kim K, Petrovic C, Pors L, Yoshioka P (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser 160:255–263
Neuwirth E (2014) RColorBrewer: ColorBrewer Palettes. R package version 1.1-2. https://CRAN.R-project.org/package=RColorBrewer
Nicolet K, Hoogenboom M, Pratchett M, Willis B (2018a) Selective feeding by corallivorous fishes neither promotes nor reduces progression rates of black band disease. Mar Ecol Prog Ser 594:95–106
Nicolet KJ, Chong-Seng KM, Pratchett MS, Willis BL, Hoogenboom MO (2018b) Predation scars may influence host susceptibility to pathogens: evaluating the role of corallivores as vectors of coral disease. Sci Rep 8:1–10
Nicolet KJ, Hoogenboom MO, Gardiner NM, Pratchett MS, Willis BL (2013) The corallivorous invertebrate Drupella aids in transmission of brown band disease on the Great Barrier Reef. Coral Reefs 32:585–595
Nugues MM, Bak RPM (2009) Brown-band syndrome on feeding scars of the crown-of-thorn starfish Acanthaster planci. Coral Reefs 28:507–510
Nugues MM, Smith GW, van Hooidonk RJ, Seabra MI, Bak RPM (2004) Algal contact as a trigger for coral disease. Ecol Lett 7:919–923
Onton K, Page C, Wilson S, Neale S, Armstrong S (2011) Distribution and drivers of coral disease at Ningaloo reef, Indian Ocean. Mar Ecol Prog Ser 433:75–84
Page CA, Willis BL (2008) Epidemiology of skeletal eroding band on the Great Barrier Reef and the role of injury in the initiation of this widespread coral disease. Coral Reefs 27:257–272
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW (2002) The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci U S A 99:8725–8730
Pebesma E (2018) Simple Features for R: Standardized Support for Spatial Vector Data. The R Journal 10:439–446
Pedersen T (2020) patchwork: The Composer of Plots. R package version 1.1.1. https://CRAN.R-project.org/package=patchwork
Petes LE, Harvell CD, Peters EC, Webb MA, Mullen KM (2003) Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 15:167–171
Pollock FJ, Katz SM, Bourne DG, Willis BL (2013) Cymo melanodactylus crabs slow progression of white syndrome lesions on corals. Coral Reefs 32:43–48
Pollock FJ, Lamb JB, Field SN, Heron SF, Schaffelke B, Shedrawi G, Bourne DG, Willis BL (2014) Sediment and Turbidity Associated with Offshore Dredging Increase Coral Disease Prevalence on Nearby Reefs. PLoS ONE 9:e102498
Pratchett MS, Graham NAJ, Sheppard CRC, Mayes B (2010) Are infestations of Cymo melanodactylus killing Acropora cytherea in the Chagos archipelago? Coral Reefs 29:941–941
Pratte Z, Richardson L (2016) Possible links between white plague-like disease, scleractinian corals, and a cryptochirid gall crab. Dis Aquat Org 122:153–161
Precht WF, Gintert BE, Robbart ML, Fura R, Van Woesik R (2016) Unprecedented disease-related coral mortality in Southeastern Florida. Sci Rep 10:1–1
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Randall CJ, Jordán-Garza AG, Muller EM, van Woesik R (2016) Does Dark-Spot Syndrome Experimentally Transmit among Caribbean Corals? PLoS ONE 11:e0147493
Ranith RP, Senthilnathan L, Machendiranathan M, Thangaradjou T, Sasamal S, Choudhury SB (2017) Sources and threats of chronic tissue loss on coral reefs in the Lakshadweep Islands, Indian Ocean. Mar Ecol 38:e12436
Raymundo L, Work T, Miller R, Lozada-Misa P (2016) Effects of Coralliophila violacea on tissue loss in the scleractinian corals Porites spp. depend on host response. Dis Aquat Org 119:75–83
Raymundo LJ, Halford AR, Maypa AP, Kerr AM (2009) Functionally diverse reef-fish communities ameliorate coral disease. Proc Natl Acad Sci USA 106:17067–17070
Raymundo LJ, Licuanan WL, Kerr AM (2018) Adding insult to injury: Ship groundings are associated with coral disease in a pristine reef. PLoS ONE 13:e0202939
Remily ER, Richardson LL (2006) Ecological Physiology of a Coral Pathogen and the Coral Reef Environment. Microb Ecol 51:345–352
Rice MM, Ezzat L, Burkepile DE (2019) Corallivory in the Anthropocene: Interactive Effects of Anthropogenic Stressors and Corallivory on Coral Reefs. Front Mar Sci 5:525
Richardson LL, Voss JD (2005) Changes in a coral population on reefs of the northern Florida Keys following a coral disease epizootic. Mar Ecol Prog Ser 1:147–156
Rodríguez-Villalobos JC, Rocha-Olivares A, Work TM, Calderon-Aguilera LE, Cáceres-Martínez JA (2014) Gross and microscopic pathology of lesions in Pocillopora spp. from the subtropical eastern Pacific. J Invertebr Pathol 120:9–17
Roff G, Hoegh-Guldberg O, Fine M (2006) Intra-colonial response to Acroporid “white syndrome” lesions in tabular Acropora spp. (Scleractinia). Coral Reefs 25:255
Ross AM (2014) Genet and reef position effects in out-planting of nursery-grown Acropora cervicornis (Scleractinia:Acroporidae) in Montego Bay, Jamaica. Rev Biol Trop 62:318–329
Rotjan R, Lewis S (2008) Impact of coral predators on tropical reefs. Mar Ecol Prog Ser 367:73–91
Ruiz-Moreno D, Willis BL, Page AC, Weil E, Cróquer A, Vargas-Angel B, Jordan-Garza AG, Jordán-Dahlgren E, Raymundo L, Harvell CD (2012) Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis Aquat Organ 100:249–261
Rypien K, Baker D (2009) Isotopic labeling and antifungal resistance as tracers of gut passage of the sea fan pathogen Aspergillus sydowii. Dis Aquat Org 86:1–7
Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM, Konotchick T, Malay M, Maragos JE, Obura D, Pantos O, Paulay G, Richie M, Rohwer F, Schroeder RE, Walsh S, Jackson JBC, Knowlton N, Sala E (2008) Baselines and Degradation of Coral Reefs in the Northern Line Islands. PLoS ONE 3:e1548
Santini A, Battisti A (2019) Complex Insect-Pathogen Interactions in Tree Pandemics. Front Physiol 10:550
Sato Y, Willis BL, Bourne DG (2010) Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J 4:203–214
Schoepf V, Grottoli AG, Levas SJ, Aschaffenburg MD, Baumann JH, Matsui Y, Warner ME (2015) Annual coral bleaching and the long-term recovery capacity of coral. Proc Royal Soc B 1819:20151887
Scott CM, Mehrotra R, Hein MY, Moerland MS, Hoeksema BW (2017) Population dynamics of corallivores (Drupella and Acanthaster) on coral reefs of Koh Tao, a diving destination in the Gulf of Thailand. Raffles Bull Zool 65:68–79
Shaver EC, Renzi JJ, Bucher MG, Silliman BR (2020) Relationships between a common Caribbean corallivorous snail and protected area status, coral cover, and predator abundance. Sci Rep 10:16463
Shaver EC, Shantz AA, McMinds R, Burkepile DE, Vega Thurber RL, Silliman BR (2017) Effects of predation and nutrient enrichment on the success and microbiome of a foundational coral. Ecology 98:830–839
Shirur KP, Jackson CR, Goulet TL (2016) Lesion recovery and the bacterial microbiome in two Caribbean gorgonian corals. Mar Biol 163:238
Silliman BR, Newell SY (2003) Fungal farming in a snail. Proc Natl Acad Sci USA 100:15643–15648
Silliman BR, McCoy MW, Angelini C, Holt RD, Griffin JN, van de Koppel J (2013) Consumer fronts, global change, and runaway collapse in ecosystems. Annu Rev Ecol Evol 44:503–538
Slattery M (1999) Fungal pathogenesis of the sea fan Gorgonia ventalina: direct and indirect consequences. Chemoecology 9:97–104
Slattery M, Renegar DA, Gochfeld DJ (2013) Direct and indirect effects of a new disease of alcyonacean soft corals. Coral Reefs 32:879–889
South A (2017) rnaturalearth: World Map Data from Natural Earth. R package version 0.1.0. https://CRAN.R-project.org/package=rnaturalearth
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 57:573–583
Sussman M, Loya Y, Fine M, Rosenberg E (2003) The marine fireworm Hermodice carunculata is a winter reservoir and spring-summer vector for the coral-bleaching pathogen Vibrio shiloi. Environ Microbiol 5:250–255
Sutherland KP, Porter JW, Turner JW, Thomas BJ, Looney EE, Luna TP, Meyers MK, Futch JC, Lipp EK (2010) Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata: Likely human sewage source of white pox disease of elkhorn coral. Environ Microbiol 12:1122–1131
Sutherland KP, Shaban S, Joyner JL, Porter JW, Lipp EK (2011) Human Pathogen Shown to Cause Disease in the Threatened Eklhorn Coral Acropora palmata. PLoS ONE 6:e23468
Sweet MJ, Bythell JC, Nugues MM (2013) Algae as Reservoirs for Coral Pathogens. PLoS ONE 8:e69717
Tsang RHL, Ang P (2015) Cold temperature stress and predation effects on corals: their possible roles in structuring a nonreefal coral community. Coral Reefs 34:97–108
van de Water JAJM, Ainsworth TD, Leggat W, Bourne DG, Willis BL, van Oppen MJH (2015) The coral immune response facilitates protection against microbes during tissue regeneration. Mol Ecol 24:3390–3404
Vargas-Ángel B, Thomas JD, Hoke SM (2003) High-latitude Acropora cervicornis thickets off Fort Lauderdale, Florida, USA. Coral Reefs 22:465–473
Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, McMinds R, Zaneveld JR (2013) Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob Change Biol 20:544–554
Vermeij M, DeBey H, Grimsditch G, Brown J, Obura D, DeLeon R, Sandin S (2015) Negative effects of gardening damselfish Stegastes planifrons on coral health depend on predator abundance. Mar Ecol Prog Ser 528:289–296
Watson SA, Fields JB, Munday PL (2017) Ocean acidification alters predator behaviour and reduces predation rate. Biol Lett 2:20160797
Walton CJ, Hayes NK, Gilliam DS (2018) Impacts of a Regional, Multi-Year, Multi-Species Coral Disease Outbreak in Southeast Florida. Front Mar Sci 5:323
Weil E (2004) Coral Reef Diseases in the Wider Caribbean. In: Rosenberg E, Loya Y (eds) Coral Health and Disease. Springer, Berlin, Heidelberg, pp 35–68
Weil E, Smith G, Gil-Agudelo DL (2006) Status and progress in coral reef disease research. Dis Aquat Org 69:1–7
Weil E, Cróquer A, Urreiztieta I (2009) Yellow band disease compromises the reproductive output of the Caribbean reef-building coral Montastraea faveolata (Anthozoa, Scleractinia). Dis Aquat Org 16:45–55
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel P, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H (2019) Welcome to the tidyverse. Journal of Open Source Software 4:1686
Williams D, Miller M (2005) Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Mar Ecol Prog Ser 301:119–128
Williams G (2013) Contrasting recovery following removal of growth anomalies in the corals Acropora and Montipora. Dis Aquat Org 106:181–185
Williams GJ, Aeby GS, Cowie ROM, Davy SK (2010) Predictive Modeling of Coral Disease Distribution within a Reef System. PLoS ONE 5:e9264
Willis B, Page C, Dinsdale E (2004) Coral disease on the Great Barrier Reef. In: Rosenberg E, Loya Y (eds) Coral Disease and Health. Springer-Verlag, Berlin, Germany, pp 69–104
Wolf AT, Nugues MM (2013) Synergistic effects of algal overgrowth and corallivory on Caribbean reef-building corals. Ecology 94:1667–1674
Work TM, Richardson LL, Reynolds TL, Willis BL (2008) Biomedical and veterinary science can increase our understanding of coral disease. J Exp Mar Biol Ecol 7:63–70
Wright RM, Kenkel CD, Dunn CE, Shilling EN, Bay LK, Matz MV (2017) Intraspecific differences in molecular stress responses and coral pathobiome contribute to mortality under bacterial challenge in Acropora millepora. Sci Rep 7:1–13
Zaneveld JR, Burkepile DE, Shantz AA, Pritchard CE, McMinds R, Payet JP, Welsh R, Correa AMS, Lemoine NP, Rosales S, Fuchs C, Maynard JA, Thurber RV (2016) Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat Commun 7:11833
Zaneveld JR, McMinds R, Thurber RV (2017) Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat Microbiol 24:1–8
Acknowledgements
We would like to thank Christine Angelini, James Nifong, Qiang He, Stacy Zhang, and Joe Morton for comments on earlier versions of this manuscript. JJR was supported by the National Science Foundation’s Graduate Research Fellowship (DGE-1650114), Duke University, and the University of California, Santa Barbara. ECS was supported by the National Science Foundation’s Graduate Research Fellowship (DGE 1106401) and Duke University. BRS was supported by the National Science Foundation’s CAREER grant (BIO-OCE 1056980) and Duke University. DEB was supported by a grant from the National Science Foundation (BIO-OCE 2023701). We would also like to thank the anonymous reviewers who provided feedback and helped improve this manuscript during the review process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Renzi, J.J., Shaver, E.C., Burkepile, D.E. et al. The role of predators in coral disease dynamics. Coral Reefs 41, 405–422 (2022). https://doi.org/10.1007/s00338-022-02219-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02219-w