Abstract
Anthropocene coral reefs are faced with increasingly severe marine heatwaves and mass coral bleaching mortality events. The ensuing demographic changes to coral assemblages can have long-term impacts on reef community organisation. Thus, understanding the dynamics of subtropical scleractinian coral populations is essential to predict their recovery or extinction post-disturbance. Here we present a 10-yr demographic assessment of a subtropical endemic coral, Pocillopora aliciae (Schmidt-Roach et al. in Zootaxa 3626:576–582, 2013) from the Solitary Islands Marine Park, eastern Australia, paired with long-term temperature records. These coral populations are regularly affected by storms, undergo seasonal thermal variability, and are increasingly impacted by severe marine heatwaves. We examined the demographic processes governing the persistence of these populations using inference from size-frequency distributions based on log-transformed planar area measurements of 7196 coral colonies. Specifically, the size-frequency distribution mean, coefficient of variation, skewness, kurtosis, and coral density were applied to describe population dynamics. Generalised Linear Mixed Effects Models were used to determine temporal trends and test demographic responses to heat stress. Temporal variation in size-frequency distributions revealed various population processes, from recruitment pulses and cohort growth, to bleaching impacts and temperature dependencies. Sporadic recruitment pulses likely support population persistence, illustrated in 2010 by strong positively skewed size-frequency distributions and the highest density of juvenile corals measured during the study. Increasing mean colony size over the following 6 yr indicates further cohort growth of these recruits. Severe heat stress in 2016 resulted in mass bleaching mortality and a 51% decline in coral density. Moderate heat stress in the following years was associated with suppressed P. aliciae recruitment and a lack of early recovery, marked by an exponential decrease of juvenile density (i.e. recruitment) with increasing heat stress. Here, population reliance on sporadic recruitment and susceptibility to heat stress underpin the vulnerability of subtropical coral assemblages to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine habitats are under increasing pressure in the Anthropocene due to cumulative stress from multiple local and global stressors (Pandolfi et al. 2003; Hughes et al. 2017a, b; Duarte et al. 2020). Progressive warming of marine habitats under climate change is affecting global biodiversity patterns due to altered species performances, but this issue is further compounded by increasingly common climatic extremes (Smale et al. 2019). As marine ecosystems are comprised of a diversity of taxa with species-specific environmental optima and tolerances, species-level population dynamics and interactions inevitably define high-level ecosystem trends. Predicting ecosystem trajectories relies on disassembling these multi-taxa communities into their distinct species populations (Capdevila et al. 2020).
Populations can react abruptly to stress events, and often follow the disturbance dynamics model (Gilmour 2004; Crabbe 2009; Leray et al. 2012): while some populations decline, others proliferate in the newly available ecological niche space. These dynamics are influenced by species-specific life history strategies and differential responses to stress events. Using advanced quantitative techniques, such as integral projection models, future population trajectories can be determined from basic demographic information on vital rates (survival, growth, reproduction, recruitment) (Cant et al. 2020; Capdevila et al. 2020). Moreover, short-lived changes to vital rates can drive transient population dynamics with long-term impacts on population persistence (Capdevila et al. 2020). Although long-term studies on population dynamics and vital rates are numerous in terrestrial ecology, they are lacking in marine and coral reef ecology (Edmunds and Riegl 2020; Pisapia et al. 2020). In the context of climate change impacts on coral reefs, addressing population dynamics of marine species is an important scientific and monitoring priority (Edmunds and Riegl 2020; Pisapia et al. 2020).
Short sharp periods of anomalous temperature known as marine heatwaves have caused unprecedented damage to marine habitats (Oliver et al. 2018; Holbrook et al. 2019), especially coral reefs (Ainsworth et al. 2016; Hughes et al. 2017b). As these events persist from several weeks to a few months, stress levels manifest in a cumulative way. Thermal stress events can be measured using the Degree Heating Week (DHW) metric by accumulating sea surface temperature (SST) anomalies exceeding an upper threshold of a species thermal tolerance over a multi-month time-window (Liu et al. 2003). Thus, stress magnitude based on DHW depends on both the duration and intensity of the event, and the threshold temperature (Supplementary Materials). Notably, DHW metrics have proven useful for evaluating coral bleaching (Heron et al. 2016; Kim et al. 2019). Excessive thermal stress stimulates a decoupling of the symbiosis between corals, the reef-building organisms on coral reefs, and their zooxanthellae, photoautotrophic symbiotic algae (Brown 1997). This results in the expulsion of zooxanthellae, coral bleaching, and can lead to mortality. The frequency, duration and scale of mass coral bleaching and mortality events are increasing (Hughes et al. 2017b; Smale et al. 2019) and have devastated numerous tropical coral reefs.
Responses of coral assemblages to thermal stress differ markedly between high and low latitudes, due to various reasons, including environmental variation, bleaching history, and taxonomic composition (West and Salm 2003). In the tropics, branching Acropora spp. are generally more vulnerable to thermal stress and bleaching, with slow-growing massive taxa (e.g. Porites spp.) more stress tolerant (Marshall and Baird 2000; McClanahan et al. 2020, but see Guest et al. 2012). In contrast, Acropora spp. in high-latitude reefs appear to be more resistant to thermal stress (Schleyer et al. 2008; Harrison et al. 2011; Dalton et al. 2020), and Porites spp. less resilient (Dalton and Carroll 2011; Harrison et al. 2011; Kim et al. 2019). While anomalous temperatures and resulting bleaching-induced mortality decimated tropical coral populations in the north and mid-Great Barrier Reef in 2016, 2017 and 2020 (Ainsworth et al. 2016; Hughes et al. 2017b; Hughes and Pratchett 2020), lesser bleaching impacts were recorded at high eastern Australian latitudes (Kim et al. 2019). Nonetheless, episodes of coral bleaching at high-latitudes are becoming increasingly severe, with localised minor events in the 1980s and 1990s (Cook et al. 1990; Celliers and Schleyer 2002; Schleyer et al. 2008; Hongo and Yamano 2013) superseded by more destructive events in the 2010s (Dalton and Carroll 2011; Harrison et al. 2011; Kim et al. 2019). These bleaching and mortality events have long-term implications on coral reef composition (Dalton and Carroll 2011; Hongo and Yamano 2013), population dynamics, and demographic processes (Cant et al. 2020).
The impact of low-magnitude thermal stress on coral populations (i.e. elevated temperatures below bleaching thresholds) remains poorly known, as it is difficult to detect in situ. Coral stress responses can be accurately quantified in laboratory experiments by measuring rates of calcification, photosynthesis or respiration to determine thermal optima (i.e. optimal and threshold temperatures for metabolic function) (Edmunds and Burgess 2018; Silbiger et al. 2019). However, unless bleaching occurs, these coral responses cannot be detected by visual census in the field. Evidence of high-magnitude thermal stress responses (i.e. coral bleaching and mortality) are more common (Crabbe 2009; Kim et al. 2019; McClanahan et al. 2020), highlighting the urgent need to quantify the role of low-magnitude thermal stress in driving coral population dynamics.
Coral size-frequency distributions provide valuable insights into population processes and the vital rates of survival, growth, and recruitment (Babcock 1991; Bak and Meesters 1998; Gilmour 2004; Smith et al. 2005; Adjeroud et al. 2007; Alvarado-Chacón and Acosta 2009; Crabbe 2009; Leray et al. 2012; Bauman et al. 2013; Anderson and Pratchett 2014; Zhao et al. 2014; Dietzel et al. 2020; Pisapia et al. 2020), because colony size is strongly associated to changes in other vital rates (Madin et al. 2020). In general, strong positive or negative skewness of size-frequency distributions can be indicative of greater recruitment processes, aging populations, or disproportionate mortality of either the small or large colonies (Bak and Meesters 1998; Adjeroud et al. 2007; Zhao et al. 2014). Temporal changes in mean coral size can reflect cohort growth or size-specific mortality, while changes to variance can indicate high mortality throughout size classes (Bak and Meesters 1998; Anderson and Pratchett 2014). These inferences can be used to assess spatial and interspecific variation in demographic processes (Dietzel et al. 2020), indicate population health (Smith et al. 2005), and identify limitations to population persistence (Precoda et al. 2018). However, disentangling the demographic drivers of given size-frequency distributions can be difficult (i.e. recruitment, growth or mortality). While assuming a constant supply of recruits can help interpret growth and mortality (Babcock 1991), such assumptions may be invalid in the subtropics (Harriott 1999b).
Temporal demographic assessments are key to capturing short-lived population dynamics. Most published coral size-structure assessments use once-off spatial surveys (e.g. Alvarado-Chacón and Acosta 2009; Anderson and Pratchett 2014), with very few temporal assessments exceeding 2 yr (Babcock 1991; Gilmour 2004; Crabbe 2009; Leray et al. 2012). These temporal studies emphasize greater stress responses in juveniles and smaller size classes, in line with the classification of corals as r-strategists (Loya 1976). Higher susceptibility of smaller size classes is often linked to the disturbance dynamics model, with causes including storms (Gilmour 2004; Crabbe 2009), sedimentation (Gilmour 2004), Crown-of-Thorns starfish outbreaks (Leray et al. 2012), and bleaching episodes (Crabbe 2009; Leray et al. 2012). Reinforcing this model, population size-frequency distributions often remain relatively stable during disturbance-free periods (Babcock 1991; Gilmour 2004). These studies demonstrate the valuable inference possible from temporal assessments of population size-frequency distributions. However, they all focus on tropical reefs, supporting the urgent need for equivalent long-term coral demographic studies in the subtropics to understand coral population trajectories in these vulnerable marginal environments.
Here we investigate the population dynamics of the subtropical endemic coral Pocillopora aliciae (Schmidt-Roach et al. 2013) in a region of rapidly changing climate in subtropical eastern Australia over a 10-yr period (2010–2019) during which this species suffered severe bleaching and mortality in response to heat stress (Kim et al. 2019; Cant et al. 2020). Specifically, we address three questions: (1) How do survival, growth, and recruitment vary temporally, as indicated by population size-frequency distributions? (2) How does mass coral bleaching affect long-term population trends, as inferred from coral densities? (3) Do coral population processes such as coral recruitment correlate with marine heatwave intensity, and does this effect vary spatially?
Methods
Study site and species
The present study was conducted at the Solitary Islands Marine Park (30° South) in subtropical eastern Australia. In situ sea water temperature and coral size-frequency distributions were assessed at 4 sites ranging from 2 to 11 km offshore (Fig. 1). The physical and chemical oceanography of this region is primarily influenced by the warm East Australian Current (EAC), which flows poleward approximately 50 km offshore following the continental margin at the 1500 m isobath (Malcolm et al. 2011). Accordingly, offshore islands are more strongly influenced by seasonal eddies and periodic warming, and have water temperatures that are more variable and on average 0.5 °C and 1.0 °C warmer than mid-shelf and inshore islands, respectively (Malcolm et al. 2011). This drives strong cross-shelf gradients in biotic communities, with distinct fish communities between offshore, mid-shelf and inshore islands (Malcolm and Ferrari 2019), and a greater prevalence of tropical species of coral and fish offshore (Harriott et al. 1994; Sommer et al. 2014; Malcolm and Ferrari 2019). In this study we surveyed 2 replicate offshore islands (North Solitary Island and South Solitary Island) and 2 replicate mid-shelf islands (Northwest Solitary Island and Southwest Solitary Island) in line with previously established cross-shelf island classifications (Malcolm et al. 2011; Malcolm and Ferrari 2019) (Fig. 1).
Map showing the Solitary Islands Marine Park, New South Wales, eastern Australia. Insets mark the sampling sites on the western side of the islands with the approximate location of transects (T1–T3) and temperature loggers (black crosses). Offshore islands: North Solitary Island, South Solitary Island; Mid-shelf islands: Northwest Solitary Island, Southwest Solitary Island
We use the subtropical endemic Pocillopora aliciae (Schmidt-Roach et al. 2013) as a model organism to understand demographic associations with thermal stress. In 2016, Pocilloporidae bleached more severely than other coral taxa at the Solitary Islands (Kim et al. 2019) and in other areas globally (Dalton et al. 2020; McClanahan et al. 2020). Pocillopora aliciae was recently separated from Pocillopora damicornis based on genetic analyses (Schmidt-Roach et al. 2013) and is the most common species of Pocilloporidae in the Solitary Islands. Pocillopora aliciae are facultative brooders, observed to release fully developed planulae following the December new moon (Schmidt-Roach et al. 2013), similar to closely related P. damicornis, which also have high localised self-recruitment (Figueiredo et al. 2014). Pocillopora aliciae is found between Cape Byron (28.6° S) and Sydney (33.8° S).
Coral data
Coral population surveys were conducted in the austral spring of 2010, 2012, 2016 (referred to as 2016a), 2018, and 2019, and during a coral bleaching event in April 2016 (referred to as 2016b) (Fig. S1). At each site, three consecutive haphazardly placed 30-m belt transects were run at 8–10 m water depth. Downward-facing photographs were taken every metre, forming a 1-m-wide belt, and each photograph included a 50 cm calibration stick (Sommer et al. 2011, 2014). Individual coral sizes were extracted from the photographs using ImageJ region of interest (ROI) tools and R (Version 1.2.1335), with any dead portions excluded and the incidence of partial mortality recorded, although very rare (Supplementary Materials). Bleaching severity was homogeneous across each colony and was recorded as normal, moderate, or severe whitening (< 25%, 26–75%, > 76% respectively). Pocillopora aliciae colonies were categorised as adults or juveniles, with a cut-off of 10cm2, the minimum planar area at onset of sexual maturity (10 cm2) for a closely related species, Stylophora pistillata (Hall and Hughes 1996) (Supplementary Materials). Demographic processes were inferred from size-frequency distributions, as individual coral colonies were not followed through time, preventing direct measurements of growth and mortality rates.
Temperature data
To examine effects of cumulative thermal stress on coral colony density, we calculated thermal stress using an adaptation of the Degree Heating Weeks (DHW) metric for in situ temperature data (as opposed to remotely sensed SST), based on an 18-yr in situ temperature record and a 34-yr remotely sensed SST record (Supplementary Materials). This approach resulted in two in situ DHW metrics calculated using different temperature accumulation thresholds (DHW1C & DHW0C). Further information is in the Supplementary Materials. NOAA Coral Reef Watch (CRW) relate DHW values to bleaching alert levels: No Bleaching (DHW = 0 °C-weeks), Possible Bleaching (0 > DHW > 4 °C-weeks), Significant Bleaching Likely (4 > DHW > 8 °C-weeks), Severe Bleaching and Significant Mortality Likely (DHW > 8 °C-weeks). Since bleaching in this study was not observed below in situ DHW1C of 10 °C-weeks, we consider thermal stress < 10 °C-weeks as a low-magnitude thermal stress. The maximum thermal stress experienced by juvenile corals (up to approx. 2 yr old) was measured using the maximum juvenile thermal stress metric (DHW1C-max & DHW0C-max). This metric was calculated as the maximum in situ DHW experienced in the two pre-survey years and was computed by site and time-point. Juvenile corals in this study were up to approximately 2 yr old based on P. aliciae growth rates (Harriott 1999a). Notably, DHW trends were only retained in this manuscript for the DHW metric which provided best-fitting models based on the Akaike Information Criterion (c.f. next section).
Statistical analysis
How do survival, growth, and recruitment vary temporally, as indicated by population size-frequency distributions? Variation in size-frequency distributions were compared among years and sites based on probability density curves and the following descriptive statistics: mean colony area (i.e. size-frequency distribution mean), coefficient of variation, skewness, and kurtosis (Peterson and Carl 2020). All summary statistics were computed on log-transformed colony area measurements (Bak and Meesters 1998; Anderson and Pratchett 2014). We used colony planar area to estimate size as it relates to age and fecundity (Babcock 1991; Madin et al. 2020). Changes in mean colony area may indicate cohort growth but can be masked by high recruitment and mortality processes (Bak and Meesters 1998). Coefficient of variation (CV = σ/μ) can be used to compare populations with different mean colony sizes (Bak and Meesters 1998) and skewness to measure the asymmetry of size-frequency distributions, which can indicate recruitment and large-colony mortality processes (Bak and Meesters 1998; Grinyó et al. 2016). Specifically, positive skewness can reflect a high proportion of recruits/juveniles compared to large colonies, with the opposite true for negative skewness. High kurtosis can suggest slow growth transitions or low recruitment and large-colony survival (Anderson and Pratchett 2014), while low kurtosis describes a flat distribution undergoing fast growth transitions or high recruitment and large colony survival. Temporal variations in size-frequency distributions were tested among years (fixed effect) and among transects (random effect) using mixed effect ANOVAs paired to Tukey tests (Bates et al. 2015) for each site independently.
How does mass coral bleaching affect long-term population trends, as inferred from coral densities? Coral colony density (m−2) was calculated as the number of corals per photo for all size classes together, and again for adult and juvenile size classes separately (Fig. S1a, b), as juvenile coral density is closely linked to recruitment processes. Temporal variations in colony density among years (fixed effect) and among sites and transects (nested random effects: transect within site) were tested using Negative Binomial generalised linear mixed effect models (GLMM) paired to Tukey tests (see below for validation steps) (Bates et al. 2015). These tests were carried out independently for offshore and mid-shelf regions due to striking cross-shelf differences in P. aliciae abundance and known cross-shelf gradients in coral assemblage (Harriott et al. 1994; Sommer et al. 2014; Dalton and Carroll 2011).
Do coral population processes such as coral recruitment correlate with marine heatwave intensity, and does this effect vary spatially? The effect of maximum juvenile thermal stress (DHW1C-max, fixed effect) on coral density among years, sites, and transects (nested random effects: transect within site within year) was tested using GLMMs with a log link Negative Binomial variance structure suitable for zero-inflated count data (Fig. S8 & Fig. S9 for DHW and Temporal models respectively). Variations in the DHW1C-max effect between offshore and mid-shelf positions were tested using an interaction term (DHW1C-max * shelf position). Routine model validation steps included checking residuals versus fitted values, nonlinear residual patterns, overdispersion, and zero-inflation. Independent GLMMs were built for juvenile and adult coral density. Pseudo-R2 values were calculated to determine percentage of explained variation. The models using the 1 °C temperature threshold filter (DHW1C-max) outperformed models without the 1 °C filter (DHW0C-max) shown by lower AIC values (Chi-sq, χ = 0.5, P < 0.01; AIC0C–AIC1C = 2.0).
Results
Temperature
In situ water temperatures from the baseline period 2001–2009 ranged from 17.7 to 26.8 °C and 17.5–26.1 °C for offshore and mid-shelf sites, respectively (Fig. 2, grey points). The annual mean baseline temperature trend ranged from 19.0 ± 0.2 °C in August to 24.7 ± 0.2 °C in March. Throughout the year, difference in daily temperatures between offshore and mid-shelf sites varied by 0.5 ± 0.2 SD °C, with warmer islands offshore and cooler islands mid-shelf. Notably, variations in daily baseline temperatures were much higher between years (1.8 SD) than among sites (0.4 SD), supporting the decision to assess temperature trends as among-site averages.
In situ seawater temperature trends for the Solitary Islands at 10 m depth. Each plot shows the same average DHW1C temperature anomaly threshold (flat dotted brown line), and the same 2001–2009 baseline temperature trend (mean ± 95% CI, dark grey line and ribbon) derived from daily baseline site temperatures (pale grey points). For each subsequent year (individual plots), mean daily temperatures across sites (red lines) are shown relative to the baseline and as among-site average DHW1C (blue lines). Average trends were shown due to high consistency among sites. Summative bleaching status from surveys/observations are also noted on each plot as Severe or None for years with survey data, and as Not Reported or Anecdotal (Malcolm personal comms) for years without survey data
Between 2009 and 2014, accumulated heat stress (DHW1C) was low, not exceeding 8 °C-weeks (Fig. 2). Across sites, mean DHW1C reached 12.2 °C-weeks in 2015 (14.1, 13.5, NA, and 8.6 °C-weeks for North, South, Northwest, and Southwest Solitary Islands, respectively) and 14.0 °C-weeks in 2016 (18.0, 13.5, 12.6, and 12.1 °C-weeks for North, South, Northwest, and Southwest Solitary Islands, respectively). The rate of onset of the heatwave in 2016 was considerably faster than in 2015, where high-level heat stress (> 10 °C-weeks among sites) was reached in 42 days, compared with 60 days. In 2017 and 2018, maximum DHW1C values were below 8 °C-weeks. However, in 2019, values were again higher, exceeding 9.3 °C-weeks. During 2016, when coral bleaching occurred at all study sites, 221 days were hotter than baseline mean temperatures but only 69 days were cooler (i.e. outside the 95% CI, Fig. 2). The heatwave persisted for 41 days (06/02/16–19/03/16) at daily temperature anomalies > 1 °C, 20 days of which were > 2 °C and with a maximum of 2.9 °C (27/02/16). Notably, all DHW values were scaled higher for DHW0C; however, the main trends remained the same as for DHW1C.
Demographic time series
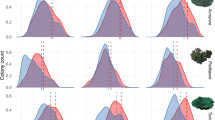
A total of 1558 images were annotated comprising 7196 P. aliciae colonies. The distribution of coral colony sizes ranged from 0.2 to > 2000 cm2 (circular diameter equivalent: 0.5 to > 50 cm) (Fig. S4). Given their small size, the smallest corals detected were re-examined; however, no mistakes were found from image analyses. Moreover, neither the removal of smallest corals from analyses (diameter < 1 cm) nor setting a lower juvenile-adult size cut-off (5 cm2) made qualitative changes to overall results (Fig. S7). Under a loge transformation, which standardised coral sizes and increased the resolution of highly abundant small colonies (Bak and Meesters 1998), the size-frequency distribution data ranged from -1.8 to 7.7 log-transformed colony size (cm2). For all sites, size-frequency distributions varied significantly through time (Fig. 3 & Table S1; LMM Tukey tests, P < 0.05). Combined with descriptive statistics (Table S1) and information on coral density for juveniles and adults (Fig. 4), five distinct patterns emerged from the time-series dataset, with distinct differences between offshore and mid-shelf coral populations. Notably, these population-level patterns are unlikely to be influenced by partial mortality, which was present in < 5% of surveyed coral colonies (Fig. S5).
Size-frequency distributions of log-transformed colony area for Pocillopora aliciae populations at offshore (red shading: North Solitary Island and South Solitary Island) and mid-shelf (blue shading: Northwest Solitary Island and Southwest Solitary Island) islands in austral spring, and during coral bleaching (2016a). The dashed line represents the juvenile–adult cut-off size. Histograms and probability densities were computed using 10 equal colony area bins. Common letters identify groups of years within which size-frequency distributions do not differ significantly based on site-specific GLMMs and Tukey tests (Table S1)
Temporal trends in coral density for a all Pocillopora aliciae corals, b juveniles only and c adults only from offshore (red shading) and mid-shelf (blue shading) regions, showing mean ± 95% CI. Common letters identify groups of years within which coral densities are not significantly different based on GLMMs and Tukey tests for offshore and mid-shelf areas independently (Table S2). Dashed arrows indicate significant consecutive temporal transitions. Points represent raw photo count data. Site-level means ± 95% CI are shown in Fig. S6
-
(1)
At offshore sites, coral densities were consistently higher than at mid-shelf sites (+ 3.5 ± 1.4 m−2; Mann–Whitney U, W = 0, P < 0.01).
-
(2)
Depending on shelf position, recruitment pulses were temporally isolated with higher skewness for offshore coral populations. Recruitment (inferred from juvenile coral densities) was likely the main driver of skewness offshore, shown by a positive linear relationship between both variables (Fig. S10). However, this was not the case for mid-shelf populations (Fig. S10). A bimodal size-frequency distribution biased toward juvenile corals (skew = 1.14) was detected at North Solitary Island in 2010, suggesting a considerable offshore recruitment pulse. This is supported by a similar but smaller peak at South Solitary Island (skew = 0.30). In contrast, no recruitment pulses were detected at mid-shelf populations in 2010 (Northwest Solitary Island skew = − 0.22, Southwest Solitary Island skew = 0.01). However, an increase in mid-shelf skewness (Northwest Solitary Island skew = 0.25, Southwest Solitary Island skew = 0.04) and juvenile coral density (+ 0.2 m−2) in 2012 suggests a marginal increase in recruitment during that period. As partial mortality was rare, it was unlikely that pulses in the density of juvenile size-class corals were caused by processes other than recruitment (Fig. S5).
-
(3)
Multiannual growth transitions from juvenile to adult stages were indicated by decreasing skewness and increasing mean colony size (Table 1) and adult colony density (Fig. 4) with time. This trend was striking for offshore size-frequency distributions following the offshore recruitment pulse (2010–2016). During this period, adult colony density increased from 2.2 to 4.7 m−2 (Fig. 4 & Table S2; GLMM Tukey, P > 0.05), suggesting cohort growth of juveniles and thus offshore recruitment success. However, there was a lack of cohort growth in mid-shelf populations. Despite the potential of increased recruitment in 2012 (Fig. 3 inferred from skewness and mean size), adult colony density had declined by 2016, 4 yr later.
-
(4)
Coral bleaching in 2016 (Fig. S1 & S3) caused substantial declines in Pocillopora populations across the Solitary Islands. Six months post-bleaching, juvenile density had declined by 88% compared to 2012 levels (3.2–0.4 m−2; Fig. 4 & Table S2; GLMM Tukey, P > 0.05), while adult colony density had declined by 47% compared to 2016a levels (Fig. 4 & Table S2; GLMM Tukey, P > 0.05). This represents a 51% decline in total coral density post-bleaching (5.9–2.9 m−2; Fig. 4 & Table S2; GLMM Tukey, P > 0.05). Coral bleaching in 2016 was recorded across all sites, but with higher incidences of severe bleaching in South and Southwest Solitary Islands (Fig. S3).
-
(5)
Early recovery of juvenile density post-bleaching (by 2018) was higher for offshore coral populations (42%; 0.4–0.7 m−2; Fig. 4 & Table S2; GLMM Tukey, P < 0.05) than for mid-shelf populations (23%; 0.13–0.16 m−2; Fig. 4 & Table S2; GLMM Tukey, P > 0.05). However, by the following year, offshore juvenile density had fallen back to the level immediately post-bleaching (Fig. 4 & Table S2; GLMM Tukey, P < 0.05).
Demographic effect of thermal stress
Maximum juvenile thermal stress (DHW1C-max) and shelf position explained 60% of variation in P. aliciae recruitment, inferred by the exponential decay of juvenile coral density with increasing maximum juvenile thermal stress offshore (Fig. 5a). The effect size (β = slope) of maximum juvenile thermal stress (DHW1C-max) at offshore reefs (β = − 0.20) was double that of mid-shelf reefs (β = − 0.20 + 0.12 = − 0.08), shown by a significant interaction term (GLMM, term = DHW1C-max * shelf position, β = − 0.12, z = − 3.0, P < 0.005). This indicates that recruitment processes (i.e. larval settlement and growth to juvenile stages) were more successful offshore and at low levels of maximum juvenile thermal stress. There was no effect of maximum juvenile thermal stress on adult colony density (GLMM, term = DHW1C-max, z = − 1.4, P > 0.05), and only a marginal effect of shelf position (GLMM term, z = 1.9, P = 0.09).
Effects of maximum juvenile thermal stress (DHW1C-max) on: a juvenile coral density and b adult coral density based on GLMMs, for offshore (red shading) and mid-shelf (blue shading) regions. Fitted values (line ± 95% bootstrapped CI) are based on photo-level counts, while points show transect-level mean coral density ± 95% CI and site-level DHW1C-max values
Discussion
We investigated demographic patterns of a subtropical endemic coral over a 10-yr period and linked these patterns to in situ accumulated thermal stress. Together, our results reveal demographic processes and acute ontogenetic stage-specific associations with thermal stress for offshore coral populations at this high-latitude climate change hotspot. Pocillopora aliciae populations in the Solitary Islands are likely supported by large infrequent recruitment pulses that maintain population persistence and the high number of adult colonies recorded in our study (Fig. 3). The low density of P. aliciae at mid-shelf islands suggests that, although the populations may be viable, they rely heavily on sporadic recruitment from outside, likely nearby sources, congruent with the findings of Cant et al. (2020). Thermal stress associations were observed, whereby suppressed recruitment processes occurred during periods of high heat stress and even moderate levels of heat stress (Fig. 5) not normally considered detrimental for corals. We also recorded a 51% decline in P. aliciae colony density post-2016 and the most intense marine heatwave (in terms of DHW1C) at the Solitary Islands since at least 1985 (Fig. 3). The inherent reliance on sporadic recruitment pulses, combined with recruitment suppression under moderate heatwaves, has the potential to severely reduce this subtropical endemic population.
Population dynamics
Pocillopora alicae populations appear to have followed the disturbance dynamics model (Gilmour 2004; Crabbe 2009; Leray et al. 2012), which proposes that disturbances, such as storms, sedimentation and Crown-of-Thorns starfish outbreaks, can cause abrupt changes in coral populations and size-frequency distributions. In this study, abrupt changes to P. aliciae size structure were noted after severe bleaching during a marine heatwave in 2016; however, other changes in P. aliciae size structure could have been missed due to a data gap from 2012 to 2016. We demonstrate a strong association between heat stress and declines in P. aliciae population density and recruitment but cannot imply causation since other potential disturbances were not measured in this study. For instance, storm damage has impacted coral assemblages in eastern Australia (Harriott and Smith 2000; Sommer et al. 2018) and may be important in driving some observed patterns. The Coffs Harbour Waverider Buoy, located 16 km due south of South Solitary Island, recorded severe storm events with maximum wave heights exceeding 11 m twice during this study period, in January 2013 and June 2016 (Manly Hydraulics Laboratory, NSW Government).
Recovery dynamics for coral populations are under intensifying pressure due to the increasing frequency of marine heatwaves and mass coral bleaching events, which are forcing ever shorter recovery intervals (Hughes et al. 2017b; Oliver et al. 2018). Over several years, coral populations can naturally recover from disturbances including storms (Gilmour 2004; Crabbe 2009), sedimentation (Gilmour 2004), Crown-of-Thorns starfish outbreaks (Leray et al. 2012), and coral bleaching mortality (Babcock 1991; Guest et al. 2016). However, we observed no evidence for early recovery of P. aliciae populations in the Solitary Islands to pre-bleaching levels (2010–2016a) within the short post-bleaching timeframe of our study (3 yr). Even in 2019, juvenile and adult coral densities remained at the same levels recorded directly after the mass bleaching event in 2016. Pocillopora spp. are predominantly weedy species (Ward 1992; Darling et al. 2012; Edmunds 2017) and effective colonisers after disturbance, but P. aliciae in the Solitary Islands have not yet responded in this way. However, 3 yr of post-disturbance sampling is insufficient to comprehensively assess recovery patterns. The data presented here suggest that the persistence of P. alicae coral populations is likely hampered by sporadic recruitment and recruitment failure even under moderate thermal stress.
This study highlights the importance of sporadic recruitment pulses in sustaining subtropical coral populations. While typically there are stable patterns in coral demographic processes during disturbance-free periods (Babcock 1991; Gilmour 2004; Crabbe 2009; Leray et al. 2012), this study recorded highly variable recruitment and growth during such periods. Successful recruitment was indicated by strong positive-skewed size-frequency distributions in 2010 followed by concurrent cohort growth (Fig. 3). However, a lack of cohort growth in mid-shelf sites suggests recruitment failure that may have been influenced by colony breakage and mortality due to severe storms and wave heights in 2013. As partial mortality was so rare in this study, it is likely that the observed population size-structure trends were in part influenced by sporadic recruitment pulses. Notably, only one such pulse was observed over the 10 yr timeframe of this study (in 2010, Fig. 3), suggesting a strong reliance of high-latitude P. aliciae populations on such processes. This mirrors findings from other high-latitude coral populations that also are recruitment limited (e.g. Plesiastrea versipora, Precoda et al. 2018). The recruitment pulse was much larger at North Solitary Island than at South Solitary Island. Given similar thermal extremes (95th percentile temperatures) at both sites during the 2009–2011 period, the differences in recruitment may have been attributable to other factors, such as substrate availability, other physical oceanographic drivers, or localised self-recruitment typical of brooding corals (Ward 1992; Figueiredo et al. 2014).
Thermal stress and bleaching
In 2016, severe bleaching of P. aliciae occurred during an intense heat stress event. Although there was a heat stress event in 2015, the magnitude and rate of onset were lower (Fig. 2), and only low levels of bleaching were noted (Malcolm personal comms). Moreover, maximum juvenile thermal stress (DHW1C-max) was correlated with temporal variation in successful recruitment processes (exponential decay of juvenile coral density, Fig. 5). Slight increases in juvenile coral density at offshore sites post-bleaching (2018) were associated with reduced DHW1C-max (Fig. 2); however, further reductions in recruitment were observed in 2019 (Fig. 5), associated with increased thermal stress (Fig. 2). Comparable thermal stress associations between adult colony density and DHW1C-max were not evident, probably because adult corals likely experienced higher DHWs before the 2-yr pre-survey window used to calculate the DHW1C-max metric. Non-bleaching physiological responses to low-magnitude thermal stress (i.e. calcification, photosynthesis, or respiration) are similar for adult and juvenile corals, as determined from tank experiments (Edmunds and Burgess 2018). However, the effects of low-magnitude thermal stress are rarely reported from the field, in part due to the lack of an easily measurable response (i.e. bleached corals) (Edmunds 2005). Our study suggests that recruitment processes may be closely linked to thermal stress, providing important field observations of stage-specific coral associations to low-magnitude thermal stress. However, recruitment failure in the aftermath of major disturbances has also been attributed to declines in both the reproductive output of remaining adult corals and the size of the remaining population’s reproductive stock (Hughes et al. 2019). Given that we observed declines in both adult and juvenile density post-bleaching, such processes may also be influencing post-disturbance recruitment failure at the Solitary Islands.
Future outlook
Future thermal regimes for the Solitary Islands paint an uncertain picture for the persistence of the subtropical endemic P. aliciae, with likely future declines given its demographic responses to thermal stress (Cant et al. 2020). This uncertainty is driven by both acute thermal stress events and long-term climatological trends. In eastern Australia, the intensity, frequency, and duration of acute thermal stress events have been increasing over the past century (Oliver et al. 2018; Holbrook et al. 2019), with such trends predicted to continue into the coming century (Hughes et al. 2017b). Such marine heatwaves have already severely damaged local and regional endemic coral populations (Beger et al. 2014; Kim et al. 2019) and will continue to supress recovery processes (Hughes et al. 2019). Our demographic study is consistent with the finding that subtropical endemics and specialists were the hardest hit taxa from recent bleaching events in subtropical eastern Australia (Harrison et al. 2011; Kim et al. 2019; Dalton et al. 2020). For instance, Acropora spp., the dominant reef builders in the tropics, were not affected in the Solitary Islands (Kim et al. 2019). Thus, species will likely respond unevenly to acute thermal stress in this region (Kim et al. 2019). Assessing whether the demographic responses of other genera and species mirror our findings for P. alicae is a next step for this research, though it is likely that demographic responses will differ among taxa, much like the taxa-specific nature of bleaching responses (Kim et al. 2019). This single-species study highlights the urgent need to investigate the complex dynamics of high-latitude marine communities for multiple taxa, to assess the vulnerability of these ecosystems to climate change and future warming.
References
Adjeroud M, Pratchett MS, Kospartov MC, Lejeusne C, Penin L (2007) Small-scale variability in the size structure of scleractinian corals around Moorea, French Polynesia: patterns across depths and locations. Hydrobiologia 589:117–126. https://doi.org/10.1007/s10750-007-0726-2
Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W (2016) Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342. https://doi.org/10.1126/science.aac7125
Alvarado-Chacón EM, Acosta A (2009) Population size-structure of the reef-coral Montastraea annularis in two contrasting reefs of a marine protected area in the southern Caribbean Sea. Bull Mar Sci 85:61–76
Anderson KD, Pratchett MS (2014) Variation in size-frequency distributions of branching corals between a tropical versus sub-tropical reef. Mar Ecol Prog Ser 502:117–128. https://doi.org/10.3354/meps10697
Babcock RC (1991) Comparative demography of three species of scleractinian corals using age- and size-dependent classifications. Ecol Monogr 61:225–244. https://doi.org/10.2307/2937107
Bak RPM, Meesters EH (1998) Coral population structure: the hidden information of colony size-frequency distributions. Mar Ecol Prog Ser 162:301–306. https://doi.org/10.3354/meps162301
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bauman AG, Pratchett MS, Baird AH, Riegl B, Heron SF, Feary DA (2013) Variation in the size structure of corals is related to environmental extremes in the Persian Gulf. Mar Environ Res 84:43–50. https://doi.org/10.1016/j.marenvres.2012.11.007
Beger M, Sommer B, Harrison PL, Smith SDA, Pandolfi JM (2014) Conserving potential coral reef refuges at high latitudes. Divers Distrib 20:245–257. https://doi.org/10.1111/ddi.12140
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16:S129–S213. https://doi.org/10.1007/s003380050249
Cant J, Salguero-Gómez R, Kim SW, Sims CA, Sommer B, Brooks M, Malcolm H, Pandolfi JM, Beger M (2020) The projected degradation of subtropical coral assemblages by recurrent thermal stress. J Anim Ecol. https://doi.org/10.1111/1365-2656.13340
Capdevila P, Stott I, Beger M, Salguero-Gómez R (2020) Towards a comparative framework of demographic resilience. Trends Ecol Evol 35:776–786. https://doi.org/10.1016/j.tree.2020.05.001
Celliers L, Schleyer MH (2002) Coral bleaching on high-latitude marginal reefs at Sodwana Bay, South Africa. Mar Pollut Bull 44:1380–1387. https://doi.org/10.1016/S0025-326X(02)00302-8
Cook CB, Logan A, Ward J, Luckhurst B, Berg CJ (1990) Elevated temperatures and bleaching on a high latitude coral reef: the 1988 Bermuda event. Coral Reefs 9:45–49. https://doi.org/10.1007/BF00686721
Crabbe MJC (2009) Scleractinian coral population size structures and growth rates indicate coral resilience on the fringing reefs of North Jamaica. Mar Environ Res 67:189–198. https://doi.org/10.1016/j.marenvres.2009.01.003
Dalton SJ, Carroll AG (2011) Monitoring coral health to determine coral bleaching response at high latitude eastern Australian Reefs: An applied model for a changing climate. Diversity 3:592–610. https://doi.org/10.3390/d3040592
Dalton SJ, Carroll AG, Sampayo E, Roff G, Harrison PL, Entwistle K, Huang Z, Salih A, Diamond SL (2020) Successive marine heatwaves cause disproportionate coral bleaching during a fast phase transition from El Niño to La Niña. Sci Total Environ 715:136951. https://doi.org/10.1016/j.scitotenv.2020.136951
Darling ES, Alvarez-Filip L, Oliver TA, Mcclanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386. https://doi.org/10.1111/j.1461-0248.2012.01861.x
Dietzel A, Bode M, Connolly SR, Hughes TP (2020) Long-term shifts in the colony size structure of coral populations along the Great Barrier Reef. Proc R Soc B 287:20201432. https://doi.org/10.1098/rspb.2020.1432rspb20201432
Duarte CM, Agusti S, Barbier E, Britten GL, Castilla JC, Gattuso JP, Fulweiler RW, Hughes TP, Knowlton N, Lovelock CE, Lotze HK, Predragovic M, Poloczanska E, Roberts C, Worm B (2020) Rebuilding marine life. Nature 580:39–51. https://doi.org/10.1038/s41586-020-2146-7
Edmunds PJ (2005) The effect of sub-lethal increases in temperature on the growth and population trajectories of three scleractinian corals on the southern Great Barrier Reef. Oecologia 146:350–364. https://doi.org/10.1007/s00442-005-0210-5
Edmunds PJ (2017) Unusually high coral recruitment during the 2016 El Nino in Mo’orea French Polynesia. PLoS ONE 12(10):e0185167. https://doi.org/10.1371/journal.pone.0185167
Edmunds PJ, Burgess SC (2018) Size-dependent physiological responses of the branching coral Pocillopora verrucosa to elevated temperature and PCO2. J Exp Biol 221:3896–3906. https://doi.org/10.1242/jeb.194753
Edmunds P, Riegl B (2020) Urgent need for coral demography in a world where corals are disappearing. Mar Ecol Prog Ser 635:233–242. https://doi.org/10.3354/meps13205
Figueiredo J, Baird AH, Harii S, Connolly SR (2014) Increased local retention of reef coral larvae as a result of ocean warming. Nat Clim Chang 4:498–502. https://doi.org/10.1038/nclimate2210
Gilmour JP (2004) Size-structures of populations of the mushroom coral Fungia fungites: the role of disturbance. Coral Reefs 23:493–504. https://doi.org/10.1007/s00338-004-0427-5
Grinyó J, Gori A, Ambroso S, Purroy A, Calatayud C, Dominguez-Carrió C, Coppari M, Lo Iacono C, López-González PJ, Gili JM (2016) Diversity, distribution and population size structure of deep Mediterranean gorgonian assemblages (Menorca Channel, Western Mediterranean Sea). Prog Oceanogr 145:42–56. https://doi.org/10.1016/j.pocean.2016.05.001
Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7:1–8. https://doi.org/10.1371/journal.pone.0033353
Guest JR, Low J, Tun K, Wilson B, Ng C, Raingeard D, Ulstrup KE, Tanzil JTI, Todd PA, Toh TC, McDougald D, Chou LM, Steinberg PD (2016) Coral community response to bleaching on a highly disturbed reef. Sci Rep 6:1–10. https://doi.org/10.1038/srep20717
Hall VR, Hughes TP (1996) Reproductive strategies of modular organisms: comparative studies of reef- building corals. Ecology 77:950–963. https://doi.org/10.2307/2265514
Harriott VJ (1999a) Coral growth in subtropical eastern Australia. Coral Reefs 18:281–291. https://doi.org/10.1007/s003380050195
Harriott VJ (1999b) Coral recruitment at a high latitude Pacific site: a comparison with Atlantic reefs. Bull Mar Sci 65:881–891
Harriott VJ, Smith SDA (2000) Coral population dynamics in a subtropical coral community, Solitary Islands Marine Park, Australia. In: Proceedings 9th International Coral Reef Symposium, pp 1–9
Harriott VJ, Smith SDA, Harrison PL (1994) Patterns of coral community structure of subtropical reefs in the Solitary Islands Marine Reserve, eastern Australia. Mar Ecol Prog Ser 109:67–76. https://doi.org/10.3354/meps109067
Harrison PL, Dalton SJ, Carroll AG (2011) Extensive coral bleaching on the world’s southernmost coral reef at Lord Howe Island. Australia Coral Reefs 30:775. https://doi.org/10.1007/s00338-011-0778-7
Heron SF, Johnston L, Liu G, Geiger EF, Maynard JA, De La Cour JL, Johnson S, Okano R, Benavente D, Burgess TFR, Iguel J, Perez DI, Skirving WJ, Strong AE, Tirak K, Eakin CM (2016) Validation of Reef-Scale Thermal Stress Satellite Products for Coral Bleaching Monitoring. Remote Sens 8(59):1–16. https://doi.org/10.3390/rs8010059
Holbrook NJ, Scannell HA, Sen Gupta A, Benthuysen JA, Feng M, Oliver ECJ, Alexander LV, Burrows MT, Donat MG, Hobday AJ, Moore PJ, Perkins-Kirkpatrick SE, Smale DA, Straub SC, Wernberg T (2019) A global assessment of marine heatwaves and their drivers. Nat Commun 10:1–13. https://doi.org/10.1038/s41467-019-10206-z
Hongo C, Yamano H (2013) Species-specific responses of corals to bleaching events on anthropogenically turbid reefs on Okinawa Island, Japan, over a 15-year period (1995–2009). PLoS ONE 8(4):e60952. https://doi.org/10.1371/journal.pone.0060952
Hughes TP, Pratchett MS (2020) We just spent two weeks surveying the Great Barrier Reef. What we saw was an utter tragedy. The Conversation. https://theconversation.com/we-just-spent-two-weeks-surveying-the-great-barrier-reef-what-we-saw-was-an-utter-tragedy-135197
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, Van De LIA, Lough JM, Morrison TH, Palumbi SR, Van NEH, Scheffer M (2017a) Coral reefs in the Anthropocene. Nature 546:82–90. https://doi.org/10.1038/nature22901
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs JPA, Hoogenboom MO, Kennedy EV, Kuo CY, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017b) Global warming and recurrent mass bleaching of corals. Nature 543:373–377. https://doi.org/10.1038/nature21707
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M, Kerswell A, Madin JS, Mieog A, Paley AS, Pratchett MS, Torda G, Woods RM (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390. https://doi.org/10.1038/s41586-019-1081-y
Kim SW, Sampayo EM, Sommer B, Sims CA, del Gómez-Cabrera MC, Dalton SJ, Beger M, Malcolm HA, Ferrari R, Fraser N, Figueira WF, Smith SDA, Heron SF, Baird AH, Byrne M, Eakin CM, Edgar R, Hughes TP, Kyriacou N, Liu G, Matis PA, Skirving WJ, Pandolfi JM (2019) Refugia under threat: mass bleaching of coral assemblages in high-latitude eastern Australia. Glob Chang Biol 00:1–14. https://doi.org/10.1111/gcb.14772
Leray M, Béraud M, Anker A, Chancerelle Y, Mills SC (2012) Acanthaster planci outbreak: decline in coral health, coral size structure modification and consequences for obligate decapod assemblages. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0035456
Liu G, Strong AE, Skirving W (2003) Remote sensing of sea surface temperatures during 2002 barrier reef coral bleaching. Eos (Washington DC) 84:2002–2004. https://doi.org/10.1029/2003EO150001
Loya Y (1976) The Red Sea coral Stylophora pistillata is an r strategist. Nature 259:1975–1977. https://doi.org/10.1038/259478a0
Madin JS, Baird AH, Baskett ML, Connolly SR, Dornelas MA (2020) Partitioning colony size variation into growth and partial mortality. Biol Lett 16(1):20190727. https://doi.org/10.1098/rsbl.2019.0727
Malcolm HA, Ferrari R (2019) Strong fish assemblage patterns persist over sixteen years in a warming marine park, even with tropical shifts. Biol Conserv 232:152–163. https://doi.org/10.1016/j.biocon.2019.02.005
Malcolm HA, Davies PL, Jordan A, Smith SDA (2011) Variation in sea temperature and the East Australian Current in the Solitary Islands region between 2001–2008. Deep Res Part II Top Stud Oceanogr 58:616–627. https://doi.org/10.1016/j.dsr2.2010.09.030
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163. https://doi.org/10.1007/s003380000086
McClanahan TR, Darling ES, Maina JM, Muthiga NA, D’Agata S, Leblond J, Arthur R, Jupiter SD, Wilson SK, Mangubhai S, Ussi AM, Guillaume MMM, Humphries AT, Patankar V, Shedrawi G, Pagu J, Grimsditch G (2020) Highly variable taxa-specific coral bleaching responses to thermal stresses. Mar Ecol Prog Ser 648:135–151. https://doi.org/10.3354/meps13402
Oliver ECJ, Donat MG, Burrows MT, Moore PJ, Smale DA, Alexander LV, Benthuysen JA, Feng M, Sen Gupta A, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Straub SC, Wernberg T (2018) Longer and more frequent marine heatwaves over the past century. Nat Commun 9:1–12. https://doi.org/10.1038/s41467-018-03732-9
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958. https://doi.org/10.1126/science.1085706
Peterson BG, Carl P (2020) PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis. R package version 2:4
Pisapia C, Edmunds PJ, Moeller HV, Riegl BM, McWilliam M, Wells CD, Pratchett MS (2020) Chapter Two - Projected shifts in coral size structure in the Anthropocene. In: Riegl BM (ed) Advances in marine biology, vol 87, pp 31–60. https://doi.org/10.1016/bs.amb.2020.07.003
Precoda K, Baird AH, Madsen A, Mizerek T, Sommer B, Su SN, Madin JS (2018) How does a widespread reef coral maintain a population in an isolated environment? Mar Ecol Prog Ser 594:85–94. https://doi.org/10.3354/meps12537
Schleyer MH, Kruger A, Celliers L (2008) Long-term community changes on a high-latitude coral reef in the Greater St Lucia Wetland Park, South Africa. Mar Pollut Bull 56:493–502. https://doi.org/10.1016/j.marpolbul.2007.11.011
Schmidt-Roach S, Miller KJ, Andreakis N (2013) Pocillopora aliciae: a new species of scleractinian coral (Scleractinia, Pocilloporidae) from subtropical Eastern Australia. Zootaxa 3626:576–582. https://doi.org/10.11646/zootaxa.3626.4.11
Silbiger NJ, Goodbody-Gringley G, Bruno JF, Putnam HM (2019) Comparative thermal performance of the reef-building coral Orbicella franksi at its latitudinal range limits. Mar Biol. https://doi.org/10.1007/s00227-019-3573-6
Smale DA, Wernberg T, Oliver ECJ, Thomsen M, Harvey BP, Straub SC, Burrows MT, Alexander LV, Benthuysen JA, Donat MG, Feng M, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Sen Gupta A, Payne BL, Moore PJ (2019) Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat Clim Chang 9:306–312. https://doi.org/10.1038/s41558-019-0412-1
Smith LD, Devlin M, Haynes D, Gilmour JP (2005) A demographic approach to monitoring the health of coral reefs. Mar Pollut Bull 51:399–407. https://doi.org/10.1016/j.marpolbul.2004.11.021
Sommer B, Harrison PL, Brooks L, Scheffers SR (2011) Coral community decline at Bonaire, southern Caribbean. Bull Mar Sci 87:541–565. https://doi.org/10.5343/bms.2010.1046
Sommer B, Harrison PL, Beger M, Pandolfi JM (2014) Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 95:1000–1009. https://doi.org/10.1890/13-1445.1
Sommer B, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2018) Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography 41:478–490. https://doi.org/10.1111/ecog.02986
Ward S (1992) Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis. Mar Biol 112:641–646. https://doi.org/10.1007/BF00346182
West JM, Salm RV (2003) Resistance and Resilience to Coral Bleaching: Implications for Coral Reef Conservation and Management. Conserv Biol 17:956–967. https://doi.org/10.1046/j.1523-1739.2003.02055.x
Zhao MX, Yu KF, Zhang QM, Shi Q, Roff G (2014) Age structure of massive Porites lutea corals at Luhuitou fringing reef (northern South China Sea) indicates recovery following severe anthropogenic disturbance. Coral Reefs 33:39–44. https://doi.org/10.1007/s00338-013-1109-y
Acknowledgements
Funding supporting this research was provided by the Natural Environment Research Council’s ONE Planet Doctoral Training Partnership (NE/S007512/1) and the European Commission’s Erasmus+ Traineeship to LL, the Australian Research Council Centre of Excellence for Coral Reef Studies (CE140100020) to JMP and others, the Australian Research Council Centre of Excellence for Environmental Decisions (CE110001014), the Royal Geographical Society’s Ralph Brown Expedition Grant, and the Winifred Violet Scott Charitable Trust to MB, and a University of Sydney Fellowship to BS. This project has further received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant agreement TRIM-DLV-747102 to MB. The authors would like to thank Dr. Steve Dalton, Dr. Sun Kim, Bob Edgar, K-Le Gomez-Cabrera, Carrie Sims, Nicole Kyriacou, Katie Cook, Paloma Matis, and Dive Quest for their invaluable assistance with fieldwork, and Dr. James Guest, Prof. Morgan Pratchett and reviewers for their invaluable recommendations. The collection of field data was supported by the Solitary Islands Marine Park branch and the NSW Department of Primary Industries, who provided research permits (SIMP 2010/001, SIMP 2016/002v2, P10/0024-1.0, and P10/0024-2.0).
Author information
Authors and Affiliations
Contributions
The manuscript was prepared by LL, MB, and BS, with review and comments given by JH, JC, HAM, and JMP. Data analyses, figures and image analyses were conducted by LL, and JH. Datasets were curated by MB and BS (images), and HAM (in situ temperature). Field surveys were conducted by BS, MB, JC, and LL.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lachs, L., Sommer, B., Cant, J. et al. Linking population size structure, heat stress and bleaching responses in a subtropical endemic coral. Coral Reefs 40, 777–790 (2021). https://doi.org/10.1007/s00338-021-02081-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-021-02081-2