Abstract
Knowledge on the early life history, ecology, and biology of marine species is crucial for future projections of the resilience of coral reef ecosystems and for adequate management strategies. A fundamental component of population dynamics is the recruitment of new individuals, and in some marine populations, this may be a limiting factor. Recruitment peaks of coral reef fishes commonly occur during the warmer months of the year in many subtropical and temperate locations worldwide. In the Red Sea, very little is known about the influence of temperature on reproductive patterns of coral reef fishes and studies on recruitment are missing. The Red Sea is one of the hottest and most isolated tropical seas in the world. We hypothesized that sea surface temperatures (SSTs) during the Red Sea’s hottest season may exceed the optimum for successful recruitment of some coral reef fishes, which therefore has to occur during other, cooler seasons, unlike recruitment among coral reef ecosystems around the world. We identified taxa among fish recruits by matching mitochondrial DNA sequences (using COI, commonly known as “barcoding”) and assessed potential biological and environmental drivers of recruitment. We studied three reefs located along a cross-shelf gradient for 12 consecutive months in the central Red Sea to capture seasonal changes in biotic and abiotic parameters along this gradient. Our results indicated that recruitment peaks did not occur during the hottest SSTs for most taxa, especially at the hottest inshore and mid-shelf reefs, and identified fish recruitment to be mainly and strongly correlated with the biomass of planktonic invertebrates. Moreover, temporal patterns of fish recruitment differed within and among taxonomic families among the reefs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of early life stages of coral reef fishes is crucial to understand population dynamics and ecosystem functioning. The incredibly high biodiversity among coral reef fishes and the extremely large number of recruiting fish larvae makes it a difficult field of research. Nonetheless, data on reproduction and the ecology and biology of early life stages of coral reef fishes are essential for the management of fish stocks (Sale 2004) and to study pathways of geneflow and connectivity between their populations. The maintenance of diverse populations and high biodiversity among coral reefs can only be assured by a plentiful, healthy, and consistent supply of recruiting larvae (Letourneur 1996), which may be harder to maintain in regions subject to fishing pressure and anthropogenic stressors, as well as in isolated seas at the periphery of coral reef ecosystems, where geneflow between populations may be further restricted.
Initially, coral reef fishes were thought to spawn year-round lacking clear seasonality in reproduction due to their distribution near the equator, in contrast to temperate marine species (Qasim 1956). Indeed, the seasonal changes in biomass of other marine biota such as zoo- and phytoplankton are relatively low in the tropics (Barry et al. 1995). However, censuses of recruiting fishes carried out daily (e.g., Brothers et al. 1983; Robertson et al. 1988), weekly (e.g., Milicich et al. 1992), monthly (e.g., Meekan et al. 1993; Robertson et al. 1993; Caselle and Warner 1996), or through seasonal snapshot sampling (e.g., Fowler et al. 1992; Williams et al. 1994) have revealed clear peaks of recruitment, which can be as intense as > 80% of recruitment happening in just 6 days in an entire year (Sponaugle and Cowen 1994). We defined recruitment as the phase of transition when coral reef fishes arrive at a reef site, commonly undergo metamorphosis, are ready to settle, and change from a pelagic to a more benthic life form. Known factors influencing successful development and recruitment of fish larvae are reproductive cycles of prey (Lasker et al. 1975) and predators (Hobson and Chess 1977; Hamner et al. 1988); site-specific physical oceanographic and atmospheric features (Cowen 2002), such as strength of monsoonal-influenced weather conditions (especially in the Pacific, Indo-Pacific, and Indian Ocean, e.g., Abesamis and Russ 2010 vs., e.g., the Caribbean, Robertson et al. 1990, 1999), wind strength and direction, and rainfall (Srinivasan and Jones 2006); and the timing of lunar phases and tides (e.g., Sponaugle and Cowen 1994). The influence of the moon directly dictates many of the reproductive patterns of marine species (Robertson et al. 1988; Cowen and Sponaugle 1997) and also influences tides, which can influence larval supply (e.g., Leis 1994). Hence, lunar cycles seem to ubiquitously drive spawning, recruitment, and settlement (Sponaugle and Cowen 1994, 2011; Cowen and Sponaugle 1997).
Furthermore, for most coral reef fishes, peaks in recruitment tend to occur during warmer months (Russell et al. 1977; Abesamis and Russ 2010; Sponaugle et al. 2012; Cure et al. 2015) in which primary production is highest (Russell et al. 1977; Talbot et al. 1978; Williams 1983). These productive warm months provide optimal conditions for efficient larval growth (Wellington and Victor 1992; Sponaugle et al. 2006), but even minor increases above optimal temperatures could hamper the development of larvae (Stevens 1996; Rankin and Sponaugle 2011). For instance, among fish larvae, in situ observations showed increased pre-settlement growth rates and decreased pelagic larval durations (PLD) with a seasonal temperature rise, but this correlation was nonlinear and both growth rates and PLD decreased at reefs with the highest sea surface temperatures (Takahashi et al. 2012). In vitro, simulations of potential climate change conditions also induced a decreased performance (i.e., slower development and particular low body conditions) of fish larvae at high settlement temperatures with low feeding regimes (McLeod et al. 2013).
The Red Sea has some of the hottest, most oligotrophic marine ecosystems where coral reefs are yet thriving. It is an enclosed, relatively isolated tropical sea, with little interannual fluctuation in its environmental conditions. The Red Sea system has very little atmospheric changes such as rainfall and cloudy days and lacks any significant tides (< 20 cm at its center; Pugh et al. 2019). Seasonality in the Red Sea is characterized by a hot and a cooler season and is mainly driven by monsoonal conditions (e.g., Raitsos et al. 2013; Churchill et al. 2014; Triantafyllou et al. 2014). Its relatively stable environment may have resulted in predictable patterns in biological lifecycles and recruitment, which still needs to be investigated. The Red Sea is also known for extreme high late-summer sea surface temperatures (SSTs), potentially exceeding tolerance limits for some larvae, and for its highly oligotrophic waters, which together represent poor conditions for larval development. Almost the entire eastern coast (> 2000 km) of the Red Sea belongs to Saudi Arabia. In Saudi Arabia, reefs have long been exploited by fishermen (Jin et al. 2012; Spaet and Berumen 2015), but relatively little research has been conducted (Berumen et al. 2013). However, there is some limited information available on seasonal reproductive patterns of invertebrates such as corals, sea urchins, and anemones (Benayahu and Loya 1983; Kramarsky-Winter and Loya 1998; Bouwmeester et al. 2011, 2016). For coral reef fishes, the studies on reproductive cycles are even more scarce and are restricted to a few reports of, for example, a conspicuous spring spawning aggregation of parrotfish in the southern Red Sea (Hipposcarus harid, March or April around the full moon; Gladstone 1996; Spaet 2013); and the tendency of spawning of various fish to predominantly occur during May to August, in the northern Red Sea (mostly in mangroves and/or over sea grasses, away from the vicinity of reefs; Abu El-Regal 2013). Moreover, information on fish recruitment in the northern Red Sea is only available from the northernmost region of the Gulf of Aqaba (Froukh et al. 2001; Ben-Tzvi et al. 2007), which may differ significantly from other parts of the Red Sea in several environmental and biotic aspects (Roberts et al. 1992; Fine et al. 2013), and to our knowledge, there are no available data on recruitment of fish assemblages for the central Red Sea. However, a study in the central Red Sea on parentage analysis of a clownfish could not find any newly recruiting fish during the warmest months (Nanninga et al. 2015). This is generally unusual for tropical fishes, which tend to recruit in the warmer months (Wellington and Victor 1992; Sponaugle et al. 2006), and suggested differences from the observations made in the northern Red Sea.
Our study focused on the assessment of the patterns of coral reef fish recruitment in the central Red Sea of Saudi Arabia over the course of a year. To collect recruiting fishes, we used commercial light traps, which capture a wide range of species but are also known to miss some species (Sponaugle et al. 2012; D’Alessandro et al. 2013). We examined coral reef fish recruitment at three reefs located along a cross-shelf gradient in the central Saudi Arabian Red Sea. Seasonal changes in biotic and abiotic parameters (mainly chlorophyll a and SST) varied along this gradient. Three main goals were of particular interest in this study: (1) to explore whether Red Sea fishes had a clear recruitment peak in the year, hypothesizing that this peak will not occur during the highest SSTs; (2) to collect baseline data on recruitment of coral reef fishes and its taxonomic composition in the central Red Sea and along the cross-shelf gradient; and (3) to use the data to assess which parameters are associated with peaks in biomass of invertebrates and with the abundance of fish recruits.
Materials and methods
Trials and first collections
The assessment of recruitment patterns of coral reef fishes in the central Red Sea was conducted in a yearlong study between February 2015 and January 2016. Prior to the final experimental design, the equipment, a Bellamare collapsible LED battery-powered light trap (500-micron mesh), and sampling method were tested multiple times starting in 2013. For more details on the trials and the equipment, please see Appendix S1.
Experimental design: study sites and the collection of environmental data
A set of three replicate light traps (nine, total; three per reef) were used to collect fish recruits in the central Red Sea. These were set at a reef inshore, one mid-shelf, and one at the shelf-edge off of the coast of Thuwal, Saudi Arabia (Fig. 1), to obtain information on recruitment for a range of reef types and capture the influence of the cross-shelf gradient. Previous studies have already targeted our selected reef sites to assess potential cross-shelf biological and environmental differences in the Saudi Arabian central Red Sea. Detailed environmental data from our study sites can be found in Roik et al. (2016) and details on the variation in their macrobiotic communities in Khalil et al. (2017). Regarding the macrobiota along the cross-shelf gradient, the major findings of the latter study were: (1) an increase in fish biomass (dominated by herbivores) and coral cover with distance to the shore, with a moderate decrease in algal cover; (2) significant differences in the fish and benthic communities of inshore reefs compared to mid-shelf and shelf-edge reefs; and (3) no significant differences between reefs in richness and diversity indices nor in commercial fish biomass. More details on the environmental differences between the three study sites are also presented in our results.
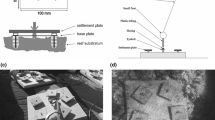
Sampling sites of recruiting fish larvae in the central Red Sea. a The study location at the Saudi Arabian coast, near the city of Thuwal, indicated with a white star. b Bathymetry (provided by M. Khalil) of the sampling region with color-coded stars at each of the studied reefs where three light traps were deployed to collect fish recruits: inshore (Abu Shusha) in yellow, mid-shelf (Al Fahal) in green, and at the shelf-edge (Shib Nazar) in blue. c The light trap and mooring system depicted as a sketch (top) and a photograph (bottom)
For our study, collection took place once per lunar month during five consecutive nights around the new moon for 12 months at fixed moorings. To reduce confounding variables arising from differences such as wave action, exposure, and depth, moorings were consistently deployed ~ 2 m below the surface, at the northern end of the sheltered (eastern) side of each reef, with a bottom depth of approx. 8–14 m. Prior to each sampling night, the rechargeable batteries of the light traps were replaced, and each light trap and its mooring were cleaned to assure the same starting conditions for each sampling period, avoiding sampling biases due to algal growth, the presence of other recruiting organisms on the moorings, reduced light intensity, or induced chemical cues. Additionally, to measure environmental differences at each reef, a current meter (2000 kHz frequency ADCP: acoustic Doppler current profiler, Nortek AS) was attached to the bottom of one of the moorings at each site, recording temperature (T, in °C), pressure (in m), current speed (in m s−1), and current direction (in beam coordinates) every 10 min, from which daily averages for the collection dates around the new moon were used. For some days of sampling, we lacked measurements of some parameters due to technical issues with the ADCP. In such cases, the monthly averages were used or in case these were also not available, the monthly averages from Roik et al. (2016) from the years 2012 and 2013 were chosen (since there is little interannual variation, and their measurements were taken from locations very close to ours and from the same reefs). Prior to merging measurements from Roik et al. (2016) into our dataset, both datasets were explored for correlations, which were always positive and the two datasets lacked significant differences (p > 0.05). This guaranteed little risk of biases by using the alternative data source to fill in our missing data. Visibility (in m) was measured daily by the same diver using a transect tape attached to one of the moorings, swimming away from the mooring until it was no longer visible (always in the same direction relative to the sun). This method was used instead of a Secchi disk, as the visibility at each site was greater than the total depth of the site. Weather conditions were also estimated by the same person each day using a 0–10 scale, defining choppiness/sea roughness with 0 representing a flat sea and 10 a very rough/choppy sea. Validated monthly averages of chlorophyll a concentrations (CHLA, in mg m−3) were provided by D. Raitsos for the region of the north central Red Sea (following the regional delineations of Raitsos et al. 2013). The data were obtained from 10-year high-resolution satellite remote sensing from the NASA Giovanni website (http://oceancolor.gsfc.nasa.gov; see Raitsos et al. (2013), for a detailed description of data processing). Environmental data were used to test for correlations with variation in recruitment biomass (FBM).
Collection of biological data
Biomass assessment
Clupeidae, Salpidae, and jellyfishes (Medusozoa) caught in the trap were immediately removed from the sample, as only coral reef fishes were considered in this study, and the weight and water content of Salpidae and jellyfishes would bias measurements of the biomass of the invertebrates (IBM, in g) collected. Coral reef fish recruits were separated out to assess the fish biomass (FBM, in g), their total number, and the taxon-specific fish abundance. Hence, the remaining biomass represented the IBM. Coral reef fish recruits were then preserved in 70% ethanol for later identification using mitochondrial DNA (mtDNA) sequencing, sometimes referred to as “barcoding.” In a few cases when the sample was too large (approx. > 400 g), a Folsom plankton splitter was used to split the sample into equal fractions (one to three times, depending on the sample size) and one of the fractions was used to extrapolate the FBM, total number of recruits, and taxon-specific fish abundance. Prior to this procedure, the sample was inspected for the presence of unique/unusual specimens that could get lost after fractioning the sample or be overrepresented in the faction used for measurements.
DNA extraction, mtDNA sequencing, identification, and quantification of taxon-specific fish abundance
We developed a protocol to identify and measure the abundance of taxa among fish recruits as confidently as possible (see Appendix S2 for details on the identification protocol). In brief, recruits were grouped morphologically, and their groupings were cross-validated using sequence data for the mitochondrial gene, cytochrome oxidase subunit I (COI). Using the aforementioned protocol, the total number of recruits (Appendix S3) of the six most commonly present taxa and the taxon-specific fish abundances (Appendix S4) were calculated.
Statistical analysis and correlations
All statistical and correlation analyses were executed in R (version 0.99.903, © 2009-2016 RStudio, Inc.). Within sampling sites (i.e., within each reef), the catches among the three light traps were tested for significant differences using an ANOVA with the aov function to assure these were true replicates (in terms of biomasses and fish abundances) that could be pooled together. Data were checked for homogeneity of variance using the Bartlett’s test (Bartlett 1937) in the R function bartlett.test. To avoid problems associated with nonindependence of variables, scatter plots and a correlation matrix were used to detect whether any of the environmental variables were colinear prior to multiple regression analyses. Environmental data were tested for correlations using a correlation matrix from the cor function, and the symnum function was used to generate the matrix. Among environmental parameters with > 0.6 correlation, only one was chosen to be included in linear models, to avoid multicollinearity and/or overfitting due to the inclusion of a large number of parameters. Significant differences in environmental data among reefs were also assessed using the ANOVAs and Kruskal–Wallis rank sum statistics, in kruskal.test.
Lastly, the linear model lm function was used to test for linear correlations of (1) IBM, (2) FBM, or (3) sampling site with the environmental explanatory parameters: temperature, pressure, current speed, current direction, visibility, weather, and CHLA. For (1) and (2), the models also included the lunar day as a variable and were further run separately for data per site (i.e., from R1, R2, and R3, from inshore to shelf-edge, respectively). For (2), the four models (for all reefs and per reef) were also run with and without the additional inclusion of the biological measurement of IBM, because it may be a good proxy of food availability/productivity of the reef environment (since the IBM was mainly comprised of small copepods and other invertebrate larvae; see, e.g., Green and McCormick 2001; Østergaard et al. 2005; Sampey et al. 2007; Carassou et al. 2009; Llopiz 2013). For (3), all variables available (biological and environmental) were included to explore site-associated correlations.
For counts of total number of recruits (Appendix S4) and taxon-specific fish abundance (Appendix S4), we ran the general linear model glm function, using the Poisson distribution family and including the same environmental variables mentioned previously for (2), always including IBM, using data from all reefs as well as per reef, separately (see Appendix S4 for the matrix displaying all parameters included in each model).
To choose the best fitting model, a stepwise model inference was performed starting from a full model that included all suitable environmental parameters available. The model’s R2 and significance were inferred using the step function. The relative importance of each predictive parameter of the lms and glms was then calculated using calc.relimp (library relaimpo) and a bootstrapping of 1000.
Results
Our study sites were environmentally different and showed differences in the measured environmental and biological parameters. A graphical summary of our main findings can be found in Fig. 2.
Yearlong (months on the x-axes) profiles of the total number of recruiting coral reef fishes in the central Red Sea (TNR, in total numbers, right-hand, outer-y-axis values; continuous color-coded line: inshore reef in “yellow” (a), mid-shelf reef in “green” (b), and the reef at the shelf-edge in “blue” (c); invertebrate biomass profiles (IBM, in g, right-hand, inner-y-axis values; respectively, in dashed color-coded lines); and the site-specific sea surface temperature profiles in orange (SST, in °C, left-hand y-axes). The red arrows indicate hottest annual SSTs and the black arrows the peak of coral reef fish recruitment (i.e., max. TNR) at each location. Comparing graphs in the panels a–c, a delay in the recruitment peak increases in relation to the site-specific SST maximum, along the cross-shelf gradient, from the shelf-edge to the shore, and from lowest to highest maxima in SSTs. The months with the highest SSTs are highlighted with an orange shadow
Environmental data
Due to technical issues, our instruments/ADCPs sporadically failed to record measurements. We thus lacked data from May and June for the mid-shelf and inshore reefs, and from July 7 to the October 21 at the shelf-edge reef. To complete our dataset for all sites, averages from Roik et al. (2016) were included for the aforementioned periods and reef sites, as mentioned in the methodology. Using the completed dataset, we found highly significant (p < 0.001) differences among current speed, current direction, and visibility, among sites. Temperature did not differ significantly among sites but was consistently higher and had the largest range at the inshore site (25.28–32.83 °C; avg. 28.67 °C). The lowest temperature measurements with the smallest range of change were measured at the shelf-edge (25.33–30.70 °C; avg. 28.16 °C). Mid-shelf, temperature was 25.28–32.46 °C (avg. 28.41 °C) and similar to those inshore (see Fig. 3 and Appendix S3).
Boxplots representing the main distribution of the abiotic and biotic data collected at each of the three studied reefs (yellow: inshore/R1, green: mid-shelf/R2, and blue: shelf-edge/R2): visibility, current speed, current direction, sea surface temperature (SST), invertebrate biomass (IBM), and total number of fish recruits are displayed with their variance given by whiskers, their mean values indicated with an “x,” and outliers shown as colored dots. The upper and lower quartiles are given with boxes and the median with the line inside the box. Values used for the boxplots can be found in Appendix S3
At the shelf-edge, visibility and current speed were the highest and strongest and also had the largest range of variation (visibility: 24–52 m, median: 35 m; current speed: 0.016–0.082 m s−1, median: 0.033 m s−1). Inshore and mid-shelf measurements were more similar; for which the mid-shelf had the lowest visibility and the lowest current speed (Inshore: visibility: 13–37 m, median: 25 m; current speed: 0.015–0.059 m s−1, median: 0.027 m s−1; mid-shelf visibility: 24–52 m, median: 21.5 m; current speed: 0.013–0.053 m s−1, median: 0.023 m s−1). The predominant daily current direction was to the south (Fig. 4). However, at the inshore reef, currents were also measured in the opposite direction (northwest) and had the largest fluctuations. The mid-shelf reef showed the smallest fluctuations in current direction (see Figs. 3, 4, and Appendix S3). Fluctuations in sea level (i.e., pressure) ranged from 1 to 3 m and followed a shelf gradient trend with the largest changes at the shelf-edge site (R1: 1.1 m; R2: 1.5 m; and R3: 2.9 m), probably due to rough weather rather than tidal changes, which are absent in the Red Sea (Pugh et al. 2019). At the shelf-edge (R3), the current profilers failed to record in the period of July to October (see Fig. 4b).
Rosette plots representing the main current directions, speed, and dominance/prevalence of the currents at three reefs near Thuwal, Saudi Arabia, in the central Red Sea. Measurements were made using three current meters (2000 kHz frequency ADCP: acoustic Doppler current profiler, Nortek AS), one per sampling site, along the cross-shelf gradient. The increasing redness and size of the arrow represent the increase in speed and dominance/prevalence of the direction of the current flow, respectively. The plots are separated according to the periods of sampling: March to June 2015 (a); July to October 2015 (b); and October 2015 to March 2016 (c). The map in the middle is lacking measurements for the reef at the shelf-edge due to failure of the instrument throughout July to October
Biological data
The catches of the three replicate light traps within a reef site did not differ in terms of IBM, FBM, total number of recruits, or taxon-specific fish abundance measurements. Therefore, for each reef, the catches of these three light traps were pooled to assess recruitment peaks (Appendix S3).
Biomass assessment
Invertebrate biomass and FBM were both highest at the mid-shelf from September to November (green bars, Fig. 5). Inshore, the IBM peaks were even further away from the months with highest SST (i.e., from January to March; yellow bars, Fig. 5; Appendix S3). Inshore, FBM was also high in February. The IBM and FBM profiles at the shelf-edge reef generally had lower values but showed a similar pattern to those at the mid-shelf site.
Histograms of the total invertebrate biomass (IBM in g, top) and fish biomass (FBM in g; bottom) collected with light traps at each of three reefs in the central Red Sea [inshore (green), mid- shelf (yellow), and shelf-edge (blue)]. The value per reef is the sum of each the IBM and the FBM collected in three replicate samples for five nights each month (y-axis) in a yearlong sampling, from January to December. Collections from February to December took place in 2015. January was sampled in 2016
DNA extraction, mtDNA sequencing, identification, and quantification of taxon-specific fish abundance
A total of 5136 recruiting fishes were counted, from which 4855 were identified (95%). For the identification, the mitochondrial COI gene of about 1200 individuals was sequenced and aligned against our Red Sea databank (COI library, from Coker et al. 2018) and the currently available COI-BOLD sequences. However, there were still many species that remained unidentified due to the lack of reference COI sequences. Therefore, only conservative estimates of higher taxonomic units were reported, and recruits were grouped into six main fish categories/families for further analyses: wrasses, parrotfishes, gobies, blennies, damselfishes, and cardinalfishes (see Appendices S3 and S4). Parrotfishes and all other wrasses were treated separately due to the high abundance of parrotfishes in the catches and the generally high diversity of wrasses. Inshore, the total number of recruits was lowest but seemed to have the highest taxonomic richness. The estimated taxonomic composition of each reef is given in Table 1.
From these data, we were able to detect clear peaks in recruitment and FBM for most of the six families that most commonly entered the light traps (Figs. 5, 6). These peaks were most evident in wrasses and parrotfishes, both peaking in October and November. Gobies and blennies also showed peaks in those months, but additionally peaked in cooler months: December and May, respectively. Nonetheless, gobies also showed a peak in August at the mid-shelf site, which was the only strong peak recorded within the hottest part of the year. For blennies, the peak in May was the dominant peak at the shelf-edge but counts were generally low. Damselfishes were also generally caught in low numbers, but steadily throughout the year without any noticeable peak or trend. However, at the shelf-edge, the catches of damselfishes were slightly higher during the warm month of July, which was still cooler than at any of the other two reefs, inshore and at the shelf-edge. The shelf-edge site also had the lowest SSTs and was the only site where temperatures peaked in September and not in August. Overall, and especially in the case of the wrasses, peaks in recruitment occurred in the slightly cooler months of October, November, and December (Fig. 6).
Taxon-specific fish abundance a of the six most collected families of recruiting fish larvae caught in a light trap study in the central Red Sea. Families are listed with increasing abundance from top to bottom. The abundances for each month of sampling, displayed on the x-axis, are color-coded per reef site [inshore (green), mid-shelf (yellow), and shelf-edge (blue)]. The number of collected fishes is on the y-axis. The graph b to the right shows the total number of recruits sampled at each reef, grouped per month and color-coded as in a
Lastly, it is important to note the absence or rareness of some usually abundant coral reef fish taxa, such as surgeonfishes, which we only caught once (a sailfin tang, Zebrasoma desjardinii, at the shelf-edge reef on the September 14, 2015) during all our collection efforts since 2012 using light traps and plankton tows (e.g., Isari et al. 2017).
Statistical models
Invertebrate biomass (IBM)
The best fitting model for the IBM explained 30.73% of the variance. It included six regressors with the following relative importance for the main contributors: visibility (52%), temperature (18%), current speed (14%), lunar day, CHLA, and pressure. Inshore, the model explained 50.72% of the variance and included four regressors: CHLA (42%), visibility (26%), month (19%), and temperature. The model for the mid-shelf reef included five regressors and explained 61.7% variance: temperature (41%), pressure (21%), CHLA (18%), month, and visibility. At the shelf-edge, 36.21% variance was explained with four regressors: lunar day (49%), visibility (24%), pressure (14.1%), and weather.
Fish biomass (FBM)
Within all the FBM models, the IBM showed the strongest correlation. Over all sites, the best fitting model included three regressors explaining 16.6% of variance in the following proportion: IBM (74%), month (17.2%), and weather (8.4%). Inshore, five regressors accounted for 41% of variance: lunar day (46%), IBM (22%), month (17%), current direction, and pressure. At the mid-shelf, three regressors accounted for 26%: IBM (58%), weather (26%), and current direction (16%). And at the shelf-edge, five regressors accounted for 29%: IBM (60%), weather (14%), CHLA (13%), pressure, and temperature.
Without IBM as explanatory regressor, the explanatory power of the models was much lower with the following results: Over all sites, the best fitting model included three regressors only explaining 7.4% of variance: month, weather, and visibility. Inshore, four regressors explained 34.2% variance: lunar day, pressure, month, and current direction. Mid-shelf, three regressors explained 25.6% of variance: temperature, weather, pressure, and current direction. And at the shelf-edge, five regressors explained 21.3% of variance: temperature, weather, CHLA, pressure, and lunar day.
The reef site (REEF)
Along the cross-shelf gradient, significant differences between reefs were found for current direction, current speed, and visibility; and when modeling the factor REEF, six regressors (including the three previously mentioned) explained 37.43% variance in the best fitting model, as follows: visibility (59.4%), current direction (14.4%), current speed (12.6%), blennies, wrasses, and cardinalfishes.
In terms of biotic parameters, reefs differed significantly from one another in IBM, and the taxon-specific fish abundances of damselfishes, wrasses, and gobies. However, they did not differ significantly from one another in terms of FBM and the taxon-specific fish abundances of pipefishes, cardinalfishes, blennies, and parrotfishes.
Discussion
Our study provides fundamental data on coral reef fish recruitment in the Red Sea. Assessing three environmentally different reefs along a cross-shelf gradient, we found that peaks in recruitment consistently occurred slightly after sea surface temperature (SST) highs, toward cooler months, and recruitment peaks generally did not occur directly during the hottest season, as in other coral reefs worldwide (Russell et al. 1977; Abesamis and Russ 2010; Sponaugle et al. 2012; Cure et al. 2015). The abundance of fish recruits primarily covaried with the biomass of invertebrates, and the variation in the abundance, biomass, and taxonomic composition of fish recruits differed among reefs. Hence, different species were recruiting to different sites along the cross-shelf gradient, likely due to reef-specific features such as the habitat a reef may be providing according to its location along the cross-shelf gradient within the Red Sea (e.g., spatial differences in larval Isari et al. 2017 and adult fish stocks Khalil et al. 2017), or spatial environmental differences and current regimes among reefs (e.g., Roik et al. 2016; Coker et al. 2018).
Seasonal patterns in recruitment peaks
We interpret the misalignment of recruitment peaks and SST highs as a response to the very high SSTs characteristic for most of the Red Sea. These temperatures may exceed the performance optima of most coral reef fish larvae (e.g., Pankhurst and Munday 2011). Hence, in the Red Sea, recruitment timed to occur in the slightly cooler months may be an adaptive strategy for survival and success under extreme high temperatures. Interestingly, however, linear regressions did not identify temperature as the main driver of the abundance of fish recruits. Instead, the biomass of invertebrates (IBM, a potential indicator of food availability for fish larvae) was more significant in the best fitting models.
The importance of IBM as an explanatory variable can be related to the vital necessity to meet a larvae’s food demand in high temperatures (due to higher metabolic rates; Meekan et al. 2003; Green and Fisher 2004; Sponaugle et al. 2006) and in highly oligotrophic waters. Most of the invertebrates found in the IBM portion of our samples were copepods and could thus potentially be an important food source for the fish larvae (Green and McCormick 2001; Østergaard et al. 2005; Sampey et al. 2007; Carassou et al. 2009; Llopiz 2013). This may explain why the profiles of IBM and fish biomass (FBM) displayed an increase in IBM followed by a peak in FBM and recruitment. Similar observations have been described in the “match–mismatch” hypothesis, in which larvae hatch near the peak abundance of their zooplankton prey (Cushing 1973, 1990), and in the “biological pump” hypothesis. The latter describes a phenomenon in which a seasonal and cyclical peak in phytoplankton leads to a peak in zooplankton and a consequential decline in phytoplankton. Declining phytoplankton is followed by a decline in zooplankton, releasing pressure on the phytoplankton and initiating the cycle again (Longhurst and Harrison 1989; Longhurst 1995). The profiles of our plots of IBM and FBM resemble those from the “biological pump” of temperate seas, with covarying fluxes in abundances of IBM and FBM. In the Red Sea, coral reef fish larvae likely have increased metabolic rates due to the exceptionally warm environment. If so, elevated food requirements might explain why peaks of FBM were supported by peaks in IBM, and subsequently why IBM was the more significant variable in our models (as opposed to temperature). However, the correlation between IBM and FBM might also be due to the fact that, generally, favorable environmental conditions could simultaneously benefit both ichthyoplankton and other zooplankton. Seasonal increases in sea surface temperature and dissolved nutrients will increase primary production (i.e., phytoplankton), fueling the food web and feeding planktonic larvae (e.g., Thiel et al. 2007; Racault et al. 2015). Hence, the interplay of a variety of parameters and a cascade of physical and biological processes will ultimately determine the survival of coral reef fish recruits (e.g., Bergenius et al. 2005). We are unable to isolate a single explanation for the correlation between IBM and FBM, and thus, we caution the interpretation of a direct cause–effect relationship in this correlation.
Aside from the strong predictive power of IBM, weather and month were important regressors for recruiting FBM. Month as a regressor is indicative of the seasonality in catches of recruiting FBM among our study sites. The factor weather, however, may have a different explanation, linked to swimming abilities and weather-related swimming strategies of fish larvae. For instance, rough weather could make it more difficult for the larvae to swim in a precise direction (e.g., Lindquist and Shaw 2005) and enter the light trap.
Taxonomic differences in fish recruitment and spatial variation
When interpreting the results of taxon-specific fish abundances, we found differences among our reefs, which may indicate that unique reef-specific features are likely responsible for most of the taxonomic diversity caught at each site (e.g., Table 1, Fig. 6).
Regarding the taxon-specific temporal differences in our samples, clear peaks in recruitment were visible among the most abundant fish families, which were parrotfishes, other wrasses, and gobies (from lowest to highest abundance). Their recruitment peaks all occurred in October, November, and/or December, outside of the season with hottest SSTs. Oppositely, damselfishes were caught in relative low numbers, but throughout the year, for which we elude drawing major conclusions for this family. Furthermore, the higher abundances of some families over others should be interpreted with caution, since it may be linked to the type of light trap used. In the Great Barrier Reef, Plexiglas light traps are more commonly used and parrotfishes were more or less absent from the catches, while damselfishes were very common (Russell et al. 1977; Milicich et al. 1992; Thorrold 1992). Hence, we only discuss the relative seasonal changes in abundances within a family and not the family’s relative abundance within our study sites.
Among damselfishes in the central Red Sea, spawning in an anemonefish, with a very short larval stage of only a couple of weeks, is known to occur from January to March (Nanninga et al. 2015), for which most recruitment would subsequently also occur outside of the season with highest SSTs. However, from the few damselfishes (mostly Chromis spp.) collected in our study, recruitment seemed to take place year-round and most damselfish recruits were caught at the shelf-edge reef. At the shelf-edge, the highest numbers of damselfishes were caught in the warm month of July, during which recruitment of almost all other fish taxa was absent, but it was also at this site where SSTs peaked the latest, in September and not August (Figs. 2, 6). Yet, based on our limited data on damselfishes and their overall low abundances in the collections, it remains difficult to depict trends in their recruitment.
Recruitment peaks of parrotfishes occurred in October and November, when SSTs started to cool down, and the highest taxonomic diversity among parrotfishes was found at the shelf-edge. At the mid-shelf site, not only did parrotfish recruitment have lowest taxonomic diversity (mostly represented by Chlorurus sordidus and Hipposcarus harid), but the recruitment of wrasses was nearly absent (see Appendix S5 for more specific data on fish abundances). Among adult parrotfishes, the highest biomasses have also been reported for reefs at the shelf-edge, while they were lowest in reefs at the mid-shelf (Khalil et al. 2017), which may suggest a link between the mature population and the supply of larvae among parrotfishes in central Red Sea reefs. Among the wrasses, most recruiting larvae were also caught at the shelf-edge reef.
The general variation we observed among taxon-specific fish among our three reef sites was particularly evident for gobies (Fig. 5). Gobies may be expected to be numerically dominant among near-reef larval assemblages (e.g., Isari et al. 2017) because of their reproductive habits and adult abundance (Brandl et al. 2019). The fact that the most abundant collected Gobiidae taxon differed among all reefs is also to be expected since gobies are the most biodiverse family of coral reef fishes (see e.g., Isari et al. 2017; Coker et al. 2018) and are generally very habitat- or site-specific (Munday et al. 1997). Moreover, gobies were the only fishes which showed a peak at highest SSTs (in August, at the mid-shelf site). This apparent tolerance to high SSTs and the exceptional temporal abundance of gobies may be another factor in the success and diversity of this family across tropical reefs.
Among the least abundant families, the blennies differed mainly in the number of genera caught at each reef and mostly belonged to the Tripterygiidae family; and no obvious trend was observed among the cardinalfishes, which were only sporadically collected. However, both of these taxa were completely absent during the hottest months of August and September except for some specimens collected at the shelf-edge, the coolest reef among all sites. These two families also were most abundant in May and then again in October and November, when temperatures had just begun to rise or were already decreased (Figs. 2, 6), supporting the overall trend observed among the aforementioned taxa.
Regarding the environmental variance defining the reef sites, our linear models predicted the main regressors to be visibility followed by current speed and current direction (e.g., Figure 3, and also Reidenbach et al. 2006). During sampling, these differences in turbidity and current speed among reefs were also remarkably noticeable. The mechanisms and development of currents are particularly complex in reef systems. Even though all sampling sites were located at the northern point of the sheltered site of the three reefs, the current regimes among sites differed a lot (Fig. 4). Regardless of well-defined large-scale current patterns in the Red Sea, the surrounding bathymetry as well as the reef topography can diverge and modify the currents once they approach the reef’s structure (e.g., Monismith 2007; Hench et al. 2008; Leichter et al. 2013). Accordingly, the reef’s inhabitants change and new fish recruits may be settling according to these emergent microhabitats shaped by distorted current regimes directly at the reef (e.g., distribution of Gobiodon species in Munday et al. (1997)). Current direction and speed dictate from where and how much water flows into the reef. Thus, it may define which type of recruits will arrive where and how many (e.g., depending on the location of larval supply around the reef; e.g., Isari et al. 2017). Currents may also dictate what kind of and how much food is available at a specific reef site, and according to this, for example, planktivorous fish may rather settle at a reef site with higher incoming pelagic waters, while benthic feeding fishes or herbivores may favor other spots with different current regimes or settle completely independent from oceanographic processes. Along these lines, visibility may also influence settlement, as visual predators may require other conditions as, for example, grazers, while some others may have evolved to have more plastic adaptive responses to their settlement location (e.g., D. abudafur in Robitzch et al. 2019).
Altogether, we stress the difficulty in identifying generalized drivers at a broad taxonomic level. Many fish families might have very species-specific recruitment patterns that cannot be resolved if clustered together with other species (at the family level), as we did. Thus, future studies in the Red Sea should focus on modeling single species (or ecologically similar groups) to increase resolution and accuracy in predictions. Studies in the Great Barrier Reef have suggested taxonomic consistency in patterns of interannual larval supply (Milicich and Doherty 1994), which also encourages further exploration of our data at the species level. While we acknowledge the logistical challenges of identifying larval fish collections to the species level, advances in sequencing technologies and growth in reference databases will continue to improve our knowledge in larval fish recruitment. Despite the associated challenges, recruitment studies deserve enhanced consideration among coral reef researchers, particularly in areas that lack baseline recruitment information, experience extreme environmental conditions, are highly vulnerable to climate change, or do not have management strategies for fish stocks. As this is the case for most of the Red Sea, a more generalized understanding of recruitment patterns would be of great value.
References
Abesamis RA, Russ GR (2010) Patterns of recruitment of coral reef fishes in a monsoonal environment. Coral Reefs 29:911–921
Abu El-Regal M (2013) Spawning seasons, spawning grounds and nursery grounds of some Red Sea fishes. Glob J Fish Aquat Res 6:105–125
Barry JP, Baxter CH, Sagarin RD, Gilman SE (1995) Climate-related, long-term faunal changes in a California rocky intertidal community. Science 5198(267):672–675
Bartlett MS (1937) Properties of sufficiency and statistical tests. Proc R Soc Lond A Math Phys Sci 160:268–282
Ben-Tzvi O, Kiflawi M, Gildor H, Abelson A (2007) Possible effects of downwelling on the recruitment of coral reef fishes to the Eilat (Red Sea) coral reefs. Limnol Oceanogr 52:2618–2628
Benayahu Y, Loya Y (1983) Surface brooding in the Red Sea soft coral Parerythropodjum Fulvum fulvum (Forskal, 1775). Biol Bull 165:353–369
Bergenius MAJ, McCormick MIM, Meekan MG, Robertson DR (2005) Environmental influences on larval duration, growth and magnitude of settlement of a coral reef fish. Mar Biol 147:291–300
Berumen ML, Hoey AS, Bass WH, Bouwmeester J, Catania D, Cochran JEM, Khalil MT, Miyake S, Mughal MR, Spaet JLY, Saenz-Agudelo P (2013) The status of coral reef ecology research in the Red Sea. Coral Reefs 32:737–748
Bouwmeester J, Berumen ML, Baird AH (2011) Daytime broadcast spawning of Pocillopora verrucosa on coral reefs of the central Red Sea. Galaxea, J Coral Reef Stud 13:23–24
Bouwmeester J, Gatins R, Giles EC, Sinclair-Taylor TH, Berumen ML (2016) Spawning of coral reef invertebrates and a second spawning season for scleractinian corals in the central Red Sea. Invertebr Biol 135:273–284
Brandl SJ, Tornabene L, Goatley CHR, Casey JM, Morais RA, Côté IM, Baldwin CC, Parravicini V, Schiettekatte NMD, Bellwood DR (2019) Demographic dynamics of the smallest marine vertebrates fuel coral-reef ecosystem functioning. Science (80-) 364:1189–1192
Brothers EB, Williams DM, Sale PF (1983) Length of larval life in twelve families of fishes at “One Tree Lagoon”, Great Barrier Reef, Australia. Mar Biol 76:319–324
Carassou L, Le Borgne R, Ponton D (2009) Diet of pre-settlement larvae of coral-reef fishes: election of prey types and sizes. J Fish Biol 75:707–715
Caselle JE, Warner RR (1996) Variability in recruitment of coral reef fishes: the importance of habitat at two spatial scales. Ecology 77:2488–2504
Churchill JH, Bower AS, McCorkle DC, Abualnaja Y (2014) The transport of nutrient-rich Indian Ocean water through the Red Sea and into coastal reef systems. J Mar Res 72:165–181
Coker DJ, DiBattista JD, Sinclair-Taylor TH, Berumen ML (2018) Spatial patterns of cryptobenthic coral-reef fishes in the Red Sea. Coral Reefs 37:193–199
Cowen RK (2002) Oceanographic influences on larval dispersal and retention and their consequences for population connectivity. Coral Reef Fishes 149–170
Cowen RK, Sponaugle S (1997) Relationships between early life history traits and recruitment among coral reef fishes. In: Chambers CR, Trippel EA (eds) Early life history and recruitment in fish populations. Chapman and Hall, London, pp 423–450
Cure K, Hobbs JPA, Harvey ES (2015) High recruitment associated with increased sea temperatures towards the southern range edge of a Western Australian endemic reef fish Choerodon rubescens (family Labridae). Environ Biol Fishes 98:1059–1067
Cushing DH (1973) Dependence of recruitment on parent stock. J Fish Res Board Canada 30:1965–1976
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293
D’Alessandro EK, Sponaugle S, Cowen RK (2013) Selective mortality during the larval and juvenile stages of snappers (Lutjanidae) and great barracuda Sphyraena barracuda. Mar Ecol Prog Ser 474:227–242
Fine M, Gildor H, Genin A (2013) A coral reef refuge in the Red Sea. Glob Chang Biol 19:3640–3647
Fowler AJ, Doherty PJ, Williams DM (1992) Multi-scale analysis of recruitment of a coral reef fish on the Great Barrier Reef. Mar Ecol Prog Ser 82:131–141
Froukh TJ, Khalaf MA, Disi AM (2001) Studies on taxonomy and ecology of some fish larvae from the Gulf of Aqaba, M.Sc. Thesis. University of Jordan
Gladstone W (1996) Unique annual aggregation of longnose parrotfish (Hipposcarus harid) at Farasan Island (Saudi Arabia, Red Sea). Copeia 2:483–485
Green BS, Fisher R (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Bio Ecol 299:115–132
Green BS, McCormick MI (2001) Ontogeny of the digestive and feeding systems in the anemonefish Amphiprion melanopus. Environ Biol Fishes 61:73–83
Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DM (1988) Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull Mar Sci 42:459–479
Hench JL, Leichter JJ, Monismith SG (2008) Episodic circulation and exchange in a wave-driven coral reef and lagoon system. Limnol Oceanogr 53:2681–2694
Hobson ES, Chess JR (1977) Trophic relationships among fishes and plankton in the lagoon at Enewetak Atoll, Marshall Islands. Fish Bull 76:133–153
Isari S, Pearman JK, Casas L, Michell CT, Curdia J, Berumen ML, Irigoien X (2017) Exploring the larval fish community of the central Red Sea with an integrated morphological and molecular approach. PLoS One 12:1–24
Jin D, Kite-Powell H, Hoagland P, Solow A (2012) A bioeconomic analysis of traditional fisheries in the Red Sea. Mar Resour Econ 27:137–148
Khalil MT, Bouwmeester J, Berumen ML (2017) Spatial variation in coral reef fish and benthic communities in the central Saudi Arabian Red Sea. PeerJ 5:e3410
Kramarsky-Winter E, Loya Y (1998) Reproductive strategies of two fungiid corals from the northern Red Sea: environmental constraints? Mar Ecol Prog Ser 174:175–182
Lasker N, Burke JF, Patchefsky A, Haughey E (1975) Peritoneal reactions to particulate matter in peritoneal dialysis solutions. Trans Am Soc Artif Intern Organs 21:342–345
Leichter JJ, Alldredge AL, Bernardi G, Brooks AJ, Carlson CA, Carpenter RC, Edmunds PJ, Fewings MR, Hanson KM, Hench JL, Holbrook SJ, Nelson CE, Schmitt RJ, Toonen RJ, Washburn L, Wyatt ASJ (2013) Biological and physical interactions on a tropical island coral reef. Oceanography 26:52–63
Leis JM (1994) Coral sea atoll lagoons: closed nurseries for the larvae of a few coral reef fishes. Bull Mar Sci 54:206–227
Letourneur Y (1996) Dynamics of fish communities on Reunion fringing reefs, Indian Ocean. II. Patterns of temporal fluctuations. J Exp Mar Bio Ecol 195:31–52
Lindquist DC, Shaw RF (2005) Effects of current speed and turbidity on stationary light-trap catches of larval and juvenile fishes. Fish Bull 103:438–444
Llopiz JK (2013) Latitudinal and taxonomic patterns in the feeding ecologies of fish larvae: A literature synthesis. J Mar Syst 109–110:69–77
Longhurst A (1995) Seasonal cycles of pelagic production and consumption. Prog Oceanogr 36:77–167
Longhurst AR, Harrison WG (1989) The biological pump: Profiles of plankton production and consumption in the upper ocean. Prog Oceanogr 22:47–123
McLeod IM, Rummer JL, Clark TD, Jones GP, McCormick MI, Wenger AS, Munday PL (2013) Climate change and the performance of larval coral reef fishes: the interaction between temperature and food availability. Conserv Physiol 1:1–12
Meekan MG, Milicich MJ, Doherty PJ (1993) Spawning determines temporal patterns of replenishment in a coral reef fish. Mar Ecol Prog Ser 93:217–225
Meekan MG, Carleton JH, McKinnon AD, Flynn K, Furnas M (2003) What determines the growth of tropical reef fish larvae in the plankton: food or temperature? Mar Ecol Prog Ser 256:193–204
Milicich MJ, Doherty PJ (1994) Larval supply of coral reef fish populations: magnitude and synchrony of replenishment to Lizard Island, Great Barrier Reef. Mar Ecol Prog Ser 110:121–134
Milicich MJ, Meekan MG, Doherty PJ (1992) Larval supply: a good predictor of recruitment of three species of reef fish (Pomacentridae). Mar Ecol Prog Ser 86:153–166
Monismith SG (2007) Hydrodynamics of coral reefs. Annu Rev Fluid Mech 39:37–55
Munday P, Jones GP, Caley MJ (1997) Habitat specialisation and the distribution and abundance of coral-dwelling gobies. Mar Ecol Prog Ser 152:227–239
Nanninga GB, Saenz-Agudelo P, Zhan P, Hoteit I, Berumen ML (2015) Not finding Nemo: limited reef-scale retention in a coral reef fish. Coral Reefs 34:383–392
Østergaard P, Munk P, Janekarn V (2005) Contrasting feeding patterns among species of fish larvae from the tropical Andaman Sea. Mar Biol 146:595–606
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62:1015–1026
Pugh DT, Abualnaja Y, Jarosz E (2019) The Tides of the Red Sea. In: Rasul N.M.A., Stewart I.C.F. (eds) Oceanographic and Biological Aspects of the Red Sea. Springer Nature Switzerland, pp 11–40
Qasim SZ (1956) Time and duration of the spawning season in some marine teleosts in relation to their distribution. J du Cons Int pour l’Exploration la Mer 21:144–155
Racault M-F, Raitsos DE, Berumen ML, Brewin RJW, Platt T, Sathyendranath S, Hoteit I (2015) Phytoplankton phenology indices in coral reef ecosystems: Application to ocean-color observations in the Red Sea. Remote Sens Environ 160:222–234
Raitsos DE, Pradhan Y, Brewin RJW, Stenchikov G, Hoteit I (2013) Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS One 8:e64909
Rankin TL, Sponaugle S (2011) Temperature influences selective mortality during the early life stages of a coral reef fish. PLoS One 6:e16814
Reidenbach MA, Monismith SG, Koseff JR, Yahel G, Genin A (2006) Boundary layer turbulence and flow structure over a fringing coral reef. Limnol Oceanogr 51:1956–1968
Roberts CM, Shepherd ARD, Ormond RFG (1992) Large-scale variation in assemblage structure of Red Sea butterflyfishes and angelfishes. J Biogeogr 19:239–250
Robertson DR, Green DG, Victor BC (1988) Temporal coupling of production and recruitment of larvae of a Caribbean reef fish. Ecology 69:370–381
Robertson DR, Petersen CW, Brawn JD (1990) Lunar reproductive cycles of benthic-brooding reef fishes: reflections of larval biology or adult biology? Ecol Monogr 60:311–329
Robertson DR, Schober UM, Brawn JD (1993) Comparative variation in spawning output and juvenile recruitment of some Caribbean reef fishes. Mar Ecol Prog Ser 94:105–113
Robertson DR, Swearer SE, Kaufmann K, Brothers EB (1999) Settlement vs. environmental dynamics in a pelagic-spawning reef fish at Caribbean Panama. Ecol Monogr 69:195–218
Robitzch V, Chen CT, Sturaro N, Lepoint G, Berumen ML, Frédérich B (2019) “Homemade”: the phenotypic diversity of coral reef damselfish populations is driven by the local environment. Biol J Linn Soc 127:361–376
Roik A, Röthig T, Roder C, Ziegler M, Kremb SG, Voolstra CR (2016) Year-long monitoring of physico-chemical and biological variables provide a comparative baseline of coral reef functioning in the central Red Sea. PLoS One 11:1–34
Russell BCC, Anderson GRV, Talbot FH (1977) Seasonality and recruitment of coral reef fishes. Aust J Mar Freshw Res 28:521–528
Sale PF (2004) Connectivity, recruitment variation, and the structure of reef fish communities. Integr Comp Biol 44:390–399
Sampey A, McKinnon AD, Meekan MG, McCormick MI (2007) Glimpse into guts: overview of the feeding of larvae of tropical shorefishes. Mar Ecol Prog Ser 339:243–257
Spaet JLY (2013) Predictable annual aggregation of longnose parrotfish (Hipposcarus harid) in the Red Sea. Mar Biodivers 43:179–180
Spaet JLY, Berumen ML (2015) Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish Res 161:356–364
Sponaugle S, Cowen RK (1994) Larval durations and recruitment patterns of two Caribbean gobies (Gobiidae): contrasting early life histories in demersal spawners. Mar Biol 120:133–143
Sponaugle S, Cowen RK (2011) Early life history traits and recruitment patterns of Caribbean wrasses (Labridae). Ecol Monogr 67:177–202
Sponaugle S, Grorud-Colvert K, Pinkard D (2006) Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys. Mar Ecol Prog Ser 308:1–15
Sponaugle S, Paris C, Walter KD, Kourafalou V, D’Alessandro EK (2012) Observed and modeled larval settlement of a reef fish to the Florida Keys. Mar Ecol Prog Ser 453:201–212
Srinivasan M, Jones GP (2006) Extended breeding and recruitment periods of fishes on a low latitude coral reef. Coral Reefs 25(4):673–682
Stevens GC (1996) Extending Rapoport’s Rule to Pacific marine fishes. J Biogeogr 23:149–154
Takahashi M, McCormick MI, Munday PL, Jones GP (2012) Influence of seasonal and latitudinal temperature variation on early life-history traits of a coral reef fish. Mar Freshw Res 63:856–864
Talbot FH, Russell BC, Anderson GRV (1978) Coral reef fish communities: unstable, high-diversity systems? Ecol Monogr 48:425–440
Thiel M, Macaya E, Acuña E (2007) The Humboldt Current system of northern-central Chile: oceanographic processes, ecological interactions and socioeconomic feedback. Oceanogr Mar Biol 45:195–344
Thorrold SR (1992) Evaluating the performance of light traps for sampling small fish and squid in open waters of the central Great Barrier Reef lagoon. Mar Ecol Prog Ser 89:277–285
Triantafyllou G, Yao F, Petihakis G, Tsiaras KP, Raitsos DE, Hoteit I (2014) Exploring the Red Sea seasonal ecosystem functioning using a three-dimensional biophysical model. J Geophys Res Ocean 119:1791–1811
Wellington GM, Victor BC (1992) Regional differences in duration of the planktonic larval stage of reef fishes in the eastern Pacific Ocean. Mar Biol 113:491–498
Williams DM (1983) Daily, monthly and yearly variability in recruitment of a guild of coral reef fishes. Mar Ecol Prog Ser 10:231–237
Williams DM, English S, Milicich MJ (1994) Annual recruitment surveys of coral reef fishes are good indicators of patterns of settlement. Bull Mar Sci 54:314–331
Acknowledgements
For logistic and fieldwork support, we thank the Coastal and Marine Resources Core Laboratory at the King Abdullah University of Science and Technology (KAUST), particularly Haitham, Ramzi, Abdullah, Mohammed, Assam, Whaled, and Ghazi Aljahdali, as well as Amr Gusti from KAUST’s Red Sea Research Center. We thank the KAUST Bioscience Core Laboratory for their assistance with Sanger sequencing. We also acknowledge Katherine Rowe’s help in the field and sorting fishes in the laboratory, as well as many other fieldwork volunteers. We further acknowledge Sabrina Vettori for statistical and modeling advice; Colleen Campbell for processing and plotting current profiler (ADCP) data as well as for her assistance with Fig. 4; John Pearman, Darren Coker, and Joseph DiBattista for sharing their teleost mtDNA COI libraries for BLASTing procedures; and Pablo Saenz-Agudelo for constructive feedback on the manuscript. This research was supported by KAUST baseline research funds to MLB and Xabier Irigoyen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robitzch, V., Berumen, M.L. Recruitment of coral reef fishes along a cross-shelf gradient in the Red Sea peaks outside the hottest season. Coral Reefs 39, 1565–1579 (2020). https://doi.org/10.1007/s00338-020-01985-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01985-9