Abstract
Climate change-induced global warming threatens the survival of key ecosystems including shallow water coral reefs. Elevated temperatures can disrupt the normal physiological functioning of photosynthetic organisms by altering the fluidity and permeability of chloroplast membranes that is defined and regulated by their lipid composition. Since the habitat-forming reef corals rely on the obligatory symbiosis with dinoflagellates of the family Symbiodiniaceae, their heat stress response can be expected to be strongly influenced by the symbiont's lipid metabolism. However, in contrast to the steady increase in the knowledge of the functioning of coral symbionts at the genomic and transcriptomic level, the understanding of their membrane lipid composition and regulation in response to temperature stress is lagging behind. We have utilised mass spectrometry-based lipidomic analyses to identify the key polar lipids that form the biological membranes of reef coral symbionts, comparing the thermotolerant species Durusdinium trenchii with the thermosensitive taxon Cladocopium C3, both hosted by Acropora valida. Our results indicate that the superior thermotolerance D. trenchii inside the host corals could be achieved through (1) the amount and saturation of sulfoquinovosyldiacylglycerols, in particular through putative photosystem II interactions, (2) the increased digalactosyldiacylglycerol to monogalactosyldiacylglycerol ratio with the potential to stabilise thylakoid membranes and integrated proteins, and (3) the chaperone-like function of lyso-lipids. Thereby, our study provides novel insights into the heat tolerance of coral symbionts, contributing to the understanding of the potential of coral reef ecosystems to respond and adjust to heat stress events that are becoming more frequent due to climate change. Finally, our identification of multiple mechanisms of heat tolerance in Symbiodiniaceae furthers the knowledge of the general stress physiology of photosynthetic organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is imposing significant stress on the physiological functioning of living organisms. In particular, episodes of elevated seawater temperatures exert a direct impact on the fluidity of biological membranes (Los and Murata 2004) and their melting point in poikilothermic organisms (Mansour et al. 2018). This disturbance can affect ion and solute transporters, induce changes in passive permeability and modify the function of membrane proteins via changes in their mobility and clustering (Hazel 1995; Welti et al. 2002). In photosynthetic cells, the effects of elevated temperature on the chloroplast membranes can cause inhibition of photosystem II (PSII) and result in a disruption of the electron transport chain and carbon fixation (Dörmann and Benning 2002).
Among the photosynthetic organisms that are severely endangered by temperature stress are dinoflagellates of the family Symbiodiniaceae (LaJeunesse et al. 2018). Members of different genera within this family form an obligatory symbiosis with reef corals responsible for building the calcareous framework of coral reef ecosystems thriving in tropical and subtropical regions around the globe.
Episodes of elevated seawater temperatures, exacerbated by light and unfavourable nutrient conditions, result often in the phenomenon of coral bleaching, the loss of the brownish-coloured symbionts that causes the white coral skeleton to shine through the transparent animal tissue (Brown 1997; Douglas 2003; Wiedenmann et al. 2013; Rosset et al. 2017). High-level mortality follows coral bleaching if the corals do not recover their symbionts within a relatively short period of time (Jokiel and Coles 1977; Glynn et al. 2001; Loya et al. 2001; Baird and Marshall 2002; Baker et al. 2008; Riegl et al. 2015; Hughes et al. 2018). Frequent mass bleaching events are occurring with increasing frequency as a consequence of global warming, threatening the survival of coral reefs (Donner et al. 2005; Baker et al. 2008; Hughes et al. 2018). Since ~ 25% of all marine species are directly or indirectly dependent on coral reefs, their demise will have a substantial impact on marine biodiversity and result in a loss of ecosystem services for human societies (Hughes et al. 2003; Pandolfi et al. 2003).
Although the cellular mechanisms that underpin heat stress-mediated coral bleaching are not fully understood, it is clear that exposure to light and high temperature impairs the functioning of photosynthesis in the symbiotic algae (Lesser and Farrell 2004), associated with excess production of reactive oxygen species (Warner and Suggett 2016) and/or the loss of structural integrity of the thylakoid membranes (Warner et al. 1999; Tchernov et al. 2004; Díaz-Almeyda et al. 2011; Mansour et al. 2018). Therefore, the potential of corals to adjust to rising seawater temperatures will strongly depend on the capability of their algal symbionts to maintain their physiological function under changing conditions.
Recently, genetic analysis revealed a great taxonomic diversity among coral symbionts (Hume et al. 2016; LaJeunesse et al. 2018), but experiments with different Symbiodiniaceae species and genera in culture suggest that physiological traits cannot be directly explained by the taxonomic background in general (Goyen et al. 2017; Suggett et al. 2017; Mansour et al. 2018). However, ecological studies have identified the prevailing presence of specific symbiont species in heat tolerant corals. For example, in the hottest coral reef environment of the world, the Southern Persian/Arabian Gulf, Symbiodinium thermophilum is the prevalent symbiont of a range of host species (D'Angelo et al. 2015; Hume et al. 2016). The symbiont species D. trenchii, previously named Symbiodinum trenchii or clade D1a, is widely distributed in the world’s oceans where its prevalence increased in the aftermath of heat stress anomalies, promoted by environmental degradation (LaJeunesse et al. 2014; Pettay et al. 2015). This species has the potential to increase the bleaching threshold of the host coral by ~ 1–1.5 °C (Berkelmans and van Oppen 2006; Baker et al. 2008; LaJeunesse et al. 2014; Silverstein et al. 2017).
At present, the mechanisms underlying the increased thermal tolerance of D. trenchii are not fully understood. Earlier work that found a significantly higher saturation of 18-C fatty acids in thermally tolerant Symbiodiniaceae strains (Tchernov et al. 2004) led to the hypothesis that fatty acid saturation might be responsible for the increased thermal tolerance of D. trenchii. However, while a decrease in the proportion of unsaturated 18-C fatty acids was measured in cultured Cladocopium C1 (formerly clade C1) and D. trenchii after exposure to increased temperatures, there were no indications for a faster or stronger response in the latter (Kneeland et al. 2013). Equally, a comparative analysis of fatty acid content, their degree of saturation and associated thylakoid membrane integrity did not support a straightforward relationship between the saturation degree of fatty acids and increased thermal tolerance in cultured Cladocopium C1 and Symbiodinium A1 (Díaz-Almeyda et al. 2011).
Finally, a transcriptomic analysis of D. trenchii assigned a prominent role to a fatty acid desaturase in the evolutionary history of Durusdinium (clade D) (Ladner et al. 2012), which may indicate that desaturation of fatty acids might be involved in the response to thermal stress.
In summary, in spite of the accumulating evidence supporting a role of biological membranes in shaping the heat tolerance of coral symbionts (Tchernov et al. 2004; Díaz-Almeyda et al. 2011; Kneeland et al. 2013; Goyen et al. 2017; Mansour et al. 2018), there is no clear picture on how the lipid complement and fatty acid saturation might modulate the heat stress tolerance of D. trenchii and whether other lipid-mediated mechanisms might be utilised as well. An alternative response may involve the glycolipid sulfoquinovosyldiacylglycerol (SQDG), a key component of photosynthetic membranes, that has been previously assigned a critical role in modulating the heat and light stress tolerance of nutrient-deprived coral symbionts (Wiedenmann et al. 2013; D’Angelo and Wiedenmann 2014).
Therefore, in this study, we aimed to increase our understanding of the role of the lipid complement in the thermotolerance of coral symbionts. We applied high pressure liquid chromatography coupled with electrospray ionisation and tandem mass spectrometry (HPLC/ESI–MS/MS) analyses (Welti et al. 2002; Popendorf et al. 2013) to provide a comprehensive profile of the polar lipid compositions of two species of symbiotic cells selected by their differential thermotolerance. We used laboratory-cultured strains of the staghorn coral Acropora valida harbouring either D. trenchii or Cladocopium C3 (LaJeunesse et al. 2014, 2018) as dominant symbiont for experimentation to capture the in hospite functioning that is most representative of the natural environment. We compared the polar lipid profile of the two species with different thermal tolerance in the presence or absence of heat stress, to test our hypothesis that the constitution and remodelling of the membrane lipids are key processes associated with the heat stress response of the coral symbionts.
Materials and methods
Coral culture
Corals of Fijian origin, produced in local aquaculture, were obtained through Tropical Marine Centre London in 2009. The dominant symbionts harboured by different strains of Acropora valida were identified as Symbiodiniaceae Cladocopium C3 and D. trenchii, respectively, by analysis of the ITS2 rDNA sequences (Hume et al. 2013). Since then, these strains were asexually propagated within the experimental mesocosm at the University of Southampton (D'Angelo and Wiedenmann 2012). Repeated phylotyping confirmed that the corals did not change their dominant symbiont over time. Corals were cultured side-by-side at a constant temperature of 25 °C and light intensity of 150 µmol s−1 m−2 with a 12/12h light/dark cycle delivered by a metal halide lamp fitted with a 250 W Aqualine 10,000 burner (13,000 Kelvin) (Aqua Medic Bissendorf, Germany). Details of the setup are described in (D'Angelo and Wiedenmann 2012). Key water parameters during the relevant period were oxygen concentrations ~ 6.7 mg/l, pH ~ 8.2, salinity 33 and nitrate and phosphate were kept at replete levels (Wiedenmann et al. 2013; Rosset et al. 2017). Water current was generated by Turbelle Nanostream pumps (Tunze, Penzberg, Germany) with a flow rate 2400 l/h. The corals were placed in the main current of the pumps to ensure comparable exposure to water flow. Separate compartments of this experimental system with a comparable technical setup were used for the heat stress treatment. The experimental units were connected to the same recirculating water body, to ensure that major water parameters apart from temperature remained comparable.
Heat stress experiment
Replicate colonies containing either D. trenchii (control n = 3, heat stress n = 4) or Cladocopium C3 were used (n = 3 for each condition). Corals were kept in separate compartments of the experimental aquarium supplied with recirculated water from the same waterbody (~ 6000 l). Flow rates, current and light conditions were kept identical for the experimental tanks. In the treatment tanks, the temperature was gradually ramped up to 30 °C over a period of eight days. This elevated temperature was maintained for another eight days prior to sampling. A Diving Pulse Amplitude Modulated (PAM) Fluorometry (Waltz Diving PAM, Germany) was used to measure the maximum quantum yield (Fv/Fm) of the algal symbionts as an indicator of photodamage (Warner et al. 1999). After dark recovery for 11 h, corals were acclimated to low light (~ 5 µmol photons * m−2 s−1) prior to taking the Fv/Fm measurements (Warner et al. 2010; Suggett et al. 2015). Great care was taken to keep the measuring probe at a comparable distance to the samples. By the end of the experiment, symbiont cell densities in the tissue of control and heat stress treated corals were determined as described below.
Isolation of symbiont cells
Symbiont cells were removed from defined areas of the coral colony by blasting the tissue using an airbrush. Algal cells were immediately separated from the host tissue homogenate by centrifugation at 2500×g (5 min, 4 °C). The pellets were then kept on ice. All samples were processed within a short timeframe (30 min), alternating the extraction of control and treatment samples. Cells were then re-suspended in ice-cold sterile seawater and an aliquot was removed for microscopic analysis. The remainder of the cells was again precipitated by centrifugation as described above. This wash step was repeated, and the cell pellets were immediately frozen at − 80 °C after removal of the supernatant. Cells in the separated aliquot were counted using a haemocytometer. To obtain cell density values for the corals, the determined cell numbers were normalised to the area of coral surface (cm2) from which they were extracted.
Lipid extraction
Cell pellets were standardised to contain an equal cell number before extracting total lipids by a modified Bligh and Dyer protocol (Bligh and Dyer 1959). Cell pellets were re-suspended in 800 µl Hank’s balanced salt solution (HBSS) followed by the sequential addition of 1 ml dichloromethane, 2 ml methanol, 1 ml dichloromethane and 1 ml of demineralised water with vigorous mixing after the addition of each solvent. Organic and water phases were separated by centrifugation at 1000 g for 10 min. The lower organic phase was transferred into a glass vial, dried under a stream of nitrogen gas at 37 °C, and stored at − 20 °C.
Lipid analysis
Chromatography conditions
Dried lipid extracts were reconstituted in 100% methanol and separated using an Agilent 1100 LC system (Agilent Technologies) with a diol column (PrincetonSPHERE 100 DIOL 100 Å 5 µ, 150 × 2.1 mm), maintained at 22 °C. Mobile phases consisted of A) n- hexane/isopropanol/formic acid: 25% aqueous ammonium hydroxide (800:200:0.9:0.36 v/v); and B) isopropanol/water/formic acid: 25% aqueous ammonium hydroxide (900:100:0.9:0.36 v/v), as previously described (Popendorf et al. 2013). Chromatographic separation was achieved using a gradient of 100% A to 48% A over 20 min, then kept isocratic at 48% A for another 10 min. Solvent flow was set to 0.4 ml min−1.
Mass spectrometry conditions and lipid analysis
Analysis of the lipids was performed using a triple quadrupole tandem mass spectrometer with electrospray ionisation interface (ESI-MS/MS) (Xevo, Waters, UK). Instrument parameters were set as follows: the source temperature was 150 °C, desolvation gas temperature was set at 200 °C, 3.22 kV to electrospray capillary, cone energy 50 V, argon was used as collision gas. The lipid classes monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), sulfoquinovosyldiacylglycerol (SQDG), diacylglyceryl-carboxyhydroxymethylcholine (DGCC), phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylglycerol (PG) were identified by the use of precursor or neutral loss scans specific to diagnostic head group fragments (Table S1) (Popendorf et al. 2013).
All chromatograms and mass spectra were processed using MassLynx software version 4.0 (Waters, UK). For each chromatogram and mass spectra, background noise was subtracted and the data was smoothed prior to analysis. Each scan of a particular lipid class produced a total ion current chromatogram representative of all lipid species within the given class. The total abundance of each given lipid class was measured by integration of the chromatogram peak areas. The composition of each lipid class was determined by analysis of the mass spectra. The relative abundance of individual lipid species within each class was calculated by integration of the spectral peak areas and determination of the percent contribution of each peak area to the total. A threshold of 2% was set, with peak areas above this threshold considered to represent significant components of a respective lipid class.
The unsaturation index (UI) for each lipid class was calculated using the following formula:
where y is every molecular lipid species belonging to the lipid class x.
MGDG and DGDG were quantified using lipid standards (Matreya LLC, PA, USA) dissolved in dichloromethane/methanol: 50 mM sodium acetate aqueous (300:665:35) using the precursor scan 243 ES+ (Welti et al. 2003). This was conducted by direct infusion with 5 µl/min flow rate and 58 V collision energy, using galactolipid standards as internal standards, allowing for direct comparison within the obtained mass spectra.
We compared the host lipid profiles with those of the symbionts using the same instrument settings and did not find indications of contaminations of the symbiont fraction above the detection threshold (data not shown).
Statistical analysis of data
The data is expressed as mean ± standard deviation (n = 3). Significant differences in lipid class abundance as well as in lipid class composition between the three samples were determined by two-way analysis of variance (ANOVA) followed by Tukey’s test for pairwise comparison (Sigmaplot). Differences were considered to be significant with P < 0.05.
Principal component analysis
Principle component analysis (PCA) was performed using PAST software. For each lipid class, only lipid species that contributed more or equal to four per cent of the total composition of the given lipid class were included in the analysis. The values of signal intensity for each lipid species were used for this analysis rather than the percentage data to represent both differences in the composition of a lipid class as well as in the total abundance of a given lipid class among the experimental treatments. Three PCA runs were performed: the first including all analysed lipid classes, the second including only glycolipids, and the third including only betaine and phospholipids. Lipid species with loading values of ≥ 0.2 or ≤ − 0.2 were considered to contribute prominently towards the principal components.
Results
In hospite response of symbiont species to heat stress
Replicate coral colonies of A. valida harbouring either D. trenchii or Cladocopium C3 as dominant symbionts were acclimated to the experimental aquarium conditions at 25 °C for < 6 months. The photosynthetic efficiency values (Fv/Fm) of ~ 0.65 characteristic for D. trenchii in hospite were higher compared to those of Cladocopium C3 (~ 0.55, Fig. S1a) at ambient temperature. In the high temperature treatment, only Cladocopium C3 symbionts showed a statistically significant reduction of Fv/Fm values compared to the control samples once 30 °C were reached after the 8 days of temperature ramping. Importantly, in temperature-treated Cladocopium C3 the lowered Fv/Fm values fell below 0.5 (Fig. S1a). In contrast, Fv/Fm values of D. trenchii never went below this value. The density of Cladocopium C3 zooxanthellae had dropped by ~ 50% at the end of the temperature treatment, whereas D. trenchii numbers remained essentially unaltered (Fig. S1b).
Differences in major lipid classes between the symbiont species
HPLC MS/MS analysis revealed the presence of lipid species belonging to the major polar lipid groups: glycolipids (MGDG, DGDG and SQDG), betaine lipids (DGCC), and phospholipids (PC, PG and PE). The minor class of phosphoserins (PS) and the betaine lipids diacylglyceryl-trimethylhomoserine (DGTS) and diacylglyceryl-hydroxymethyl-N,N,N-trimethyl-beta-alanine (DGTA) were not detected using standard protocols (Popendorf et al. 2013; Cañavate et al. 2016). The comparative analysis between the two symbiont species indicated a different lipid composition of the membranes both under control conditions and after exposure to high temperature (Fig. 1a). Heat stress resulted in a change of total lipid composition only in Cladocopium C3 cells. These patterns were reproduced when only the glycolipids are included in the analysis (Fig. 1b). In contrast, no changes were obvious when the betaine lipids and phospholipids were analysed (Fig. 1c).
Principal component analyses (PCA) of lipids identified in freshly isolated D. trenchii and Cladocopium C3 kept at 25 °C and after 30 °C treatment. a PCA of all lipid species identified, b PCA of glycolypids only, c PCA of betaine and phospholipids. The bottom graph of each panel show the major lipid species that contributed to the observed groupings in each particular analysis
Differential accumulation of glycolipids in hospite
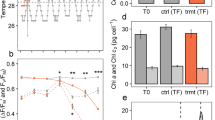
We compared the abundance of the chloroplast-located MGDG and DGDG lipids in the symbiont species under study. Cladocopium C3 symbionts accumulated slightly higher amounts of MGDG than D. trenchii both in the ambient controls and the temperature-treated samples (Fig. 2a). In contrast, the mean content of DGDG detected in Cladocopium C3 was lower compared to D. trenchii in both temperature conditions. This difference became statistically significant after exposure to high temperature (Fig. 2b). These patterns resulted in a significantly increased DGDG/MGDG ratio in D. trenchii as compared to Cladocopium C3 symbionts, both under control conditions as well as after the heat stress treatment (Fig. 2c).
Glycolipids in D. trenchii and Cladocopium C3 from A. valida corals kept at 25 °C and after 30 °C treatment. Content of MGDG (a) and DGDG (b) were calculated per million cells, quantification was performed using commercially available standards. c Ratio of DGDG to MGDG. d Amount of SQDG in symbiont cells expressed as the integrated HPLC chromatogram peak values. Bars display average values and error bars represent standard deviation. Lowercase letters indicate significant (a/b, P < 0.05) or nonsignificant (a/a, b/b) differences; P values from statistical analyses are given in Table S2
Compared to control conditions, C3 exposed to high temperature contained significantly lower amounts of SQDG. The level of these lipids in Cladocopium C3 increased in response to heat stress but reached only levels as low as in D. trenchii cells isolated from the control corals kept at 25 °C. In contrast, the accumulation of SQDG in D. trenchii was not affected by the 30 °C treatment (Fig. 2d).
Changes in betaine lipids and phospholipids
DGCC was the only betaine lipid detected in the A. valida symbionts. D. trenchii cells contained a significantly lower amount of this lipid as compared to Cladocopium C3, both under control conditions and after heat stress (Fig. 3a).
Betaine lipids and phospholipids in D. trenchii and Cladocopium C3 from A. valida corals after kept at 25 °C or after incubation at 30 °C. Amounts of DGCC (a), PC (b), PG (c) and PE (d) measured in symbiont cells are expressed as the integrated peak values of HPLC chromatograms. Bars display average values and error bars represent standard deviation. Lowercase letters indicate significant (a/b, P < 0.05) or nonsignificant (a/a, b/b) differences; P values from statistical analyses are given Table S2
Among the identified phospholipids in this study, Cladocopium cells accumulated higher levels of PC (Fig. 3b) and PG (Fig. 3c) than D. trenchii, whereas both species had comparable content of PE (Fig. 3d). The amount of detected phospholipids did not vary upon exposure to elevated temperature.
Higher content of lyso-lipids in S. trenchii symbionts
Lyso-forms of DGCC and PC were observed in both taxa in hospite. D. trenchii cells had a higher content of lyso-DGCC compared to Cladocopium C3 symbionts when corals where maintained under control conditions (Fig. 4a). Levels in D. trenchii dropped upon exposure to high temperature but remained significantly higher than in Cladocopium C3. Lyso-PC contents followed a comparable pattern, although differences were not statistically significant (Fig. 4b).
Lyso-lipids in D. trenchii and Cladocopium C3 from A. valida corals kept at 25 °C or after incubation at 30 °C. Amounts of lyso-DGCC (a), lyso-PC (b) in symbiont cells are expressed as the integrated peak values of HPLC chromatogram. Bars display average values and error bars represent standard deviation. Lowercase letters indicate significant (a/b, P < 0.05) or nonsignificant (a/a, b/b) differences; P values from statistical analyses are given Table S2
Varying unsaturation indices within lipid classes
MGDGs
The analysis of lipid species composition revealed significant differences in the main constituents of the MGDGs between the Symbiodinianaceae taxa (Fig. S2). Whereas ~ 50% of the MGDG species in Cladocopium contained 38C fatty acids both under control conditions and after temperature treatment, this value reached ~ 71% in D. trenchii. In both taxa, the fatty acids in this lipid class exhibited a high degree of unsaturation, characterised by 8, 9, or 10 double bonds per corresponding MGDG molecule. Three different MGDG species with 36-C fatty acids were characterised by a total of 7, 8, or 9 double bonds per molecule. They represented ~ 37% of the total MGDG content in Cladocopium C3 cells. In contrast, these values were significantly lower in D. trenchii (control: 22.0 ± 1.2, treatment: 18.3 ± 1.8 (mean ± SD)) (Fig. S2, Table S2). The different composition of MGDGs between the taxa resulted in a significantly higher unsaturation index (UI) in D. trenchii as compared to Cladocopium C3 (Fig. 5, Tables S2 and S3).
Unsaturation index (UI) from lipids identified in D. trenchii and Cladocopium C3 from A. valida corals kept under 25 °C or after incubation at 30 °C. Bars display average values and error bars are standard deviations. Lowercase letters indicate significant (a/b/c, P < 0.05) or nonsignificant (a/a, b/b, c/c) differences; P values from statistical analyses are given Table S2
DGDGs
The composition of DGDGs was similar in Cladocopium C3 and D. trenchii cells under control conditions. Out of all the DGDG molecules identified, ~ 20% contained 36-C and ~ 60% 38-C in their fatty acids in both species (Fig. S2). These major components did not vary after exposure to high temperature. The UI in Cladocopium cells kept at 25 °C was comparable to D. trenchii kept at 25 °C or after exposure to high temperature. Changes in the DGDG species composition of Cladocopium C3 upon heat stress exposure resulted in a significant decrease in the UI (Fig. 5, Table S2 and S4).
SQDGs
Three different species of SQDGs ([30:1], [30:0] and [32:0]) were identified in the Symbiodianaceae taxa in this study (Fig. S2). Out of the SQDG molecules identified in symbionts from corals kept under controlled conditions, 30.3 ± 7.4% (Cladocopium C3) and 25.7 ± 0.5% (D. trenchii) contained [30:1] fatty acids substituents (Table S5). Those values were significantly lower (21.0 ± 2.2% and 4.8 ± 2.0, respectively) when the corals were exposed to high temperature. A reversed pattern was observed for [32:0] molecules that were less abundant in symbionts from corals kept at 25 °C (Fig. S2, Table S5). The differences in the SQDG composition resulted in a significantly lower UI in D. trenchii cells as compared to Cladocopium C3 cells in both temperature conditions (Fig. 5, Table S2).
DGCCs
In both symbiont taxa, the pool of DGCCs was characterised by their content of long-chain fatty acids with a high degree of unsaturation. However, the lipid species composition was markedly different (Fig. S2, Table S6). In D. trenchii, the three main representatives of the DGCC class were molecules with [38:6] (43.3 ± 9.6%), [44:12] (29.2 ± 6.5%), and [42:11] (11.5 ± 3.0%). In contrast, the three most abundant lipid species of this class in Cladocopium cells were [38:6] (52.3 ± 2.5%), [36:5] (23.0 ± 2.4%), and [44:12] (11.09 ± 3.9%). These differences result in a UI of DGCC that is significantly higher in D. trenchii cells, both under control conditions and after heat stress (Fig. 5, Table S2).
PCs, PGs and PEs
Two of the main species identified within DGCCs, namely [44:12] or [38:6], were also detected in PCs in both taxa (Fig. S2). Respectively, they contributed 59.1 ± 1.8% and 14.7 ± 1.7% to the total PC species in Cladocopium C3 and 49.6 ± 9.5% and 9.0 ± 1.8% in D. trenchii (Table S7). While the composition of PC species remained unaltered in D. trenchii in response to heat stress, in Cladocopium C3 the representation of individual PC molecules changed and resulted in a decrease of the UI to a value comparable to that of D. trenchii under control conditions (Fig. 5, Table S2).
Both under control and temperature stress conditions, a lower UI was calculated for PGs present in D. trenchii cells as compared to Cladocopium C3 (Fig. 5, Fig. S2, Tables S2 and S8). In contrast, PE was characterised by the same UI values in both taxa (Fig. 4g, Fig. S2, Tables S2 and S9). Among these lipids classes, only the PCs in Cladocopium C3 showed a significant reduction of the UI after exposure of the corals to high temperature.
Lyso-lipids
In D. trenchii, ~ 80% of the lyso-DGCCs contained [22:6] fatty acids as substituents (Fig. S2), whereas this species contributed only ~ 60% of lyso-DGCCs in Cladocopium C3 (Table S10). Thus, a higher UI characterised lyso-DGCCs in D. trenchii as compared to Cladocopium C3 cells (Table S2). No changes of this parameter were measured after the stress treatment (Fig. 5). In contrast, the UI of lyso-PC, also higher in D. trenchii than in Cladocopium C3 cells, decreased after exposure to elevated temperature in both symbiont taxa (Fig. 5). The most abundant lyso-PC species in both taxa contained the [22:6] fatty acid (Fig. S2, Table S11).
Discussion
Despite ample evidence demonstrating the higher thermal tolerance of corals when they are associated with D. trenchii (Berkelmans and van Oppen 2006; Baker et al. 2008; Pettay et al. 2015; Silverstein et al. 2017), the cellular mechanisms involved in the superior performance of this coral symbiont species under heat stress are not well understood. In this work, we used HPLC ESI–MS/MS to examine the polar lipid composition in symbiotic dinoflagellates comparing different species in hospite. Specifically, we used different strains of A. valida containing either thermotolerant D. trenchii or Cladocopium C3 which is representative of a thermosensitive taxon (LaJeunesse et al. 2003, 2014, 2018) for qualitative and quantitative analyses of the polar lipids of the dinoflagellate cells. The symbionts were extracted from host A. valida kept under control conditions and after exposure to heat stress. PCA of the key lipid classes reveal fundamental differences in the lipidomes of the two symbiont species already at ambient temperatures. Heat stress resulted in a change of total lipid composition only in Cladocopium C3 cells. The same patterns could be observed when the glycolipids were analysed in isolation but not when the betaine lipids and phospholipids were analysed by themselves. These data indicates that the observed changes in the total lipid complement are mostly driven by changes in glycolipids.
Here, we argue that the increased performance of the D. trenchii under study at elevated temperatures could result from several distinct strategies to modulate their membrane composition, and we propose lipid-mediated mechanisms of thermotolerance that have not yet been discussed for coral symbionts.
Heat stress response of corals harbouring D. trenchii and Cladocopium C3
The efficiency of PSII after dark recovery measured as Fv/Fm in vivo was used as an indicator for the degree of photodamage of the symbionts (Warner et al. 1999; Gorbunov et al. 2001; Smith et al. 2013). In the absence of heat stress, Fv/Fm values were notably higher for D. trenchii (~ 0.65) than for Cladocopium (~ 0.55), a difference that is expected between different species of Symbiodianacea (Suggett et al. 2015). However, while in heat-stressed Cladocopium C3 the Fv/Fm after dark recovery consistently dropped, reflecting a typical response of a stress sensitive species, the Fv/Fm of D. trenchii did not show a statistically significant change in response to the temperature treatment (Warner et al. 1999, 2010). At the same time, symbiont densities reduced by 50% in heat-stressed Cladocopium C3-containing corals, whereas no loss of symbionts was detected in animals harbouring D. trenchii. Our data suggests that the photosynthetic reaction centre pools of D. trenchii in hospite is less compromised under heat stress (Goyen et al. 2017) compared to those of Cladocopium C3. This may indicate that that D. trenchii is less affected by the temperature treatment and help to explain why symbiont numbers remain high despite the exposure to elevated temperatures. These findings are in line with increased bleaching tolerance reported for corals hosting D. trenchii from natural reefs (Berkelmans and van Oppen 2006; Jones et al. 2008; Silverstein et al. 2017).
Saturation of fatty acids in response to high temperature
Our study shows that out of the nine lipid classes detected in Symbiodiniaceae cells in hospite, five (MGDG, DGDG, SQDG, PC, lyso-PC) have a higher saturation of their fatty acids in the heat sensitive Cladocopium C3 after exposure to elevated temperature. This can be considered as a classical response of the cells in photosynthetic organisms to prevent leakiness of the biological membranes occurring at high temperatures (Gombos et al. 1994). The absence of a similar response in D. trenchii could indicate that the temperature stress used in our experiment was not severe enough to trigger a comparable mechanism in the thermotolerant symbiont or that the thermal acclimation response of this species relies on alternative strategies.
In line with the latter, major lipid classes (MGDGs, DGCC, lyso-DGCC and lyso-PCs) in D. trenchii revealed a higher degree of unsaturation as compared to Cladocopium C3, independently from the experimental temperature. In our study, only PCs, PGs and SQDGs were significantly more saturated in D. trenchii, both at ambient temperature and under heat stress.
An increased fatty acid saturation in D. trenchii in response to elevated temperature was detected only among SQDGs and lyso-PCs. These results suggest that the higher thermotolerance of D. trenchii in hospite is not accomplished by a generalised increased saturation of lipids in their biological membranes. At the same time, our findings suggest a central role of the saturation of SQDGs in the response to temperature stress as their unsaturation index is already reduced in D. trenchii at ambient temperature and the difference to Cladocopium C3 is further increased under temperature stress.
A key role of SQDGs in the thermotolerance of coral symbiont species
Fatty acids of SQDGs in free-living dinoflagellates and symbiotic forms from non-coral hosts include saturated or monounsaturated forms (Leblond and Chapman 2000; Awai et al. 2012; Garrett et al. 2013). In the coral symbiont species examined in this study, we have identified the SQDG molecules containing fatty acids with combined [C-chain lengths: double bonds] of [30:0], [30:1] and [32:0]. The decreased UI calculated for the SQDG class of D. trenchii after exposure of corals to high temperature is due to a reduced content of one species, [30:1], and the concomitant increase in the percent of [32:0] molecules. This type of modification, in which the single double bond is replaced to produce a fully saturated molecule, has been shown to have a disproportionately high effect on the environment of the molecule and on the lipid melting point, and thus the biophysical properties of the membrane (Quinn et al. 1989). Moreover, although they do not reach the values measured in D. trenchii, a reduced UI of SQDGs after the high temperature treatment was also detected in the heat sensitive Cladocopium C3 symbionts, pointing to a general response strategy in symbiotic dinoflagellates.
Notably, the present paper demonstrated marked differences between the two symbiont species not only regarding the degree of saturation, but also concerning the overall content of SQDG. We found that in the absence of heat stress D. trenchii contained lower amounts of these lipids as compared to Cladocopium C3. Upon exposure to elevated temperatures, Cladocopium C3 cells reduced SQDGs to levels comparable to D. trenchii. A previous study revealed a link between reduced thermal tolerance with a relative increase in the cellular SQDG content in coral symbionts in response to nutrient stress (Wiedenmann et al. 2013), suggesting the reduced SQDG content in D. trenchii, along with the higher saturation, might be a mediator of its thermal tolerance.
In plant cells, SQDGs are found exclusively in chloroplast membranes. They are bound to photosystem II-related complexes and contribute directly to the maintenance of structure and function of the photosynthetic apparatus (Sato et al. 2003). Structural disorder of the D1 protein within the PSII complex caused by the complete loss of SQDG has been suggested to interfere with the functioning of the oxygen-evolving complex and electron transfer (Frentzen 2004). Accordingly, the regular conformation of the PSII complex supported by the association with SQDG is considered to be indispensable for the heat and light stress tolerance of PSII in green algae (Minoda et al. 2002; Sato et al. 2003; Frentzen 2004). Importantly, PSII damage in Symbiodiniaceae and the resulting photoinhibition is a key determinant of coral bleaching (Warner et al. 1999; Warner and Suggett 2016). In this context, it has been proposed that among the various physiological impairment mechanisms operating at different extents across different taxa, heat stress may for instance aggravate photoinhibition by reducing the repair of PSII (Takahashi et al. 2004; Warner and Suggett 2016).
Alternative composition of thylakoid membranes as a strategy to cope with high temperatures
The galactolipids MGDG and DGDG are the main components of the thylakoid membranes of photosynthetic cells (Dörmann and Benning 2002; Harwood and Guschina 2009; Moellering and Benning 2011). Our results show that these lipids in D. trenchii and Cladocopium C3 in hospite are characterised mainly by their polyunsaturated fatty acids (PUFAs), in agreement with previous analyses of the lipid composition of dinoflagellates including Symbiodiniaceae (Leblond and Chapman 2000; Guella et al. 2003; Gray et al. 2009; Awai et al. 2012; Flaim et al. 2012; Leblond et al. 2015). Interestingly, we measured a significantly higher ratio of DGDG/MGDG in D. trenchii as compared to Cladocopium symbionts. This difference is remarkable due to the contrasting biophysical properties of these molecules, with DGDGs being bilayer-forming and MGDGs non-bilayer lipids (Quinn et al. 1989; Dörmann and Benning 2002). Consequently, the ratio of DGDG/MGDG in the membrane is crucial for proper physiological functioning and it is thus tightly regulated by photosynthetic cells (Bruce 1998). In S. microadriaticum, an increase in the DGDG/MGDG ratio was detected after exposure to elevated temperatures (Leblond et al. 2015). The critical effect of such a ratio change was demonstrated by the analysis of Arabidopsis mutants defective in the capacity to acquire thermotolerance (Chen et al. 2006). While wild-type plants survived heat stress exposure when they had previously been exposed to moderately high temperatures, the mutants died during comparable treatments. This lack of acquired thermotolerance was directly related to the incapability of the mutant to increase the DGDG/MGDG ratio in their chloroplasts in response to a pre-treatment with moderate heat stress (Chen et al. 2006).
The ratio of bilayer-forming to non-bilayer-forming lipids affects the insertion of proteins in the membrane and the intracellular protein trafficking (Dörmann and Benning 2002). Accumulating evidence indicates that this ratio might play a direct role in assisting the proper folding or renaturation of integral membrane proteins (Bogdanov and Dowhan 1999; Bogdanov et al. 2010). Therefore, our results suggest that the increased thermotolerance of D. trenchii in hospite could be directly related to the higher ratios of DGDG/MGDG even in the absence of heat stress. In contrast, the susceptibility of Cladocopium C3 could be related to the generally lower DGDG/MGDG ratios coupled to the incapability to remodel the galactolipid profiles of its chloroplasts in response to elevated temperatures. Future research should investigate to which extent the observed difference in the changes in the lipid profiles are an adaptive response to mitigate the impact of stress or an indicator of stress-induced damage to the organism.
The polar lipid profile of Symbiodiniaceae and the role of lyso-lipids
At present, there is no comprehensive characterisation of the polar lipid profile of coral-hosted Symbiodiniaceae available. Our study therefore fills an important knowledge gap and complements reports on some lipid molecules characteristic in other symbiotic and non-symbiotic dinoflagellates (Gray et al. 2009; Awai et al. 2012; Flaim et al. 2012; Imbs et al. 2015; Leblond et al. 2015). With the exception of PEs, all lipids detected in our work exhibited pronounced differences in their cellular content or degree of saturation when comparing D. trenchii and Cladocopium. In contrast, the amount of these lipids remained essentially unaltered by the heat stress treatment in each species.
Interestingly, compared to Cladocopium, D. trenchii was characterised by higher levels of lyso-lipids, in particular by lyso-DGCC, showing also differences in their saturation degree. Lyso-lipids are upregulated in bacteria exposed to elevated temperature, where they facilitate the renaturation of proteins by a mechanism similar to that of molecular chaperones (Kern et al. 2001). The chaperon-like function of lyso-lipids could be confirmed by in vitro studies using synthetic model systems (Kern et al. 2001). Therefore, the increased accumulation of lyso-lipids may constitute a separate mechanism involved in the heat stress tolerance of D. trenchii.
While corals in nature can experience acute heat stress episodes comparable to our experimental treatment (Riegl et al. 2019), they are also frequently exposed to longer lasting chronic heat stress and therefore it would be important to extend these studies to evaluate the comparative responses of lipid remodelling between acute and chronic stress.
In summary, our work provides a comprehensive characterisation of the polar lipid content of dinoflagellate symbionts of reef-building corals in hospite, contributing knowledge urgently required to understand the physiological functioning of this association. We provide evidence that the lipid complement in hospite of the heat tolerant D. trenchii is profoundly different compared to the more susceptible Cladocopium C3.
The results of our study indicate that symbiotic algae can apply multiple strategies involving the lipid constitution of their membranes to cope with elevated temperature. These insights not only further our knowledge on the capacity of reef corals to adjust to the impact of climate change but also have a broad significance to understand heat stress responses in photosynthetic organisms.
References
Awai K, Matsuoka R, Shioi Y (2012) Lipid and fatty acid compositions of Symbiodinium strains. In: 12th international coral reef symposium
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc R Soc Lond B Biol Sci 273:2305–2312
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bogdanov M, Dowhan W (1999) Lipid-assisted protein folding. J Biol Chem 274:36827–36830
Bogdanov M, Heacock P, Guan Z, Dowhan W (2010) Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc Natl Acad Sci 107:15057–15062
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16(Supplement):S129–S138
Bruce BD (1998) The role of lipids in plastid protein transport. In: Soll J (ed) Protein trafficking in plant cells. Springer, New York, pp 223–246
Cañavate JP, Armada I, Ríos JL, Hachero-Cruzado I (2016) Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 124:68–78
Chen J, Burke JJ, Xin Z, Xu C, Velten J (2006) Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ 29:1437–1448
D'Angelo C, Wiedenmann J (2012) An experimental mesocosm for long-term studies of reef corals. J Mar Biol Assoc UK 92:769–775
D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opin Environ Sustain 7:82–93
D'Angelo C, Hume BCC, Burt J, Smith EG, Achterberg EP, Wiedenmann J (2015) Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J 9:2551–2560
Díaz-Almeyda E, Thomé P, El Hafidi M, Iglesias-Prieto R (2011) Differential stability of photosynthetic membranes and fatty acid composition at elevated temperature in Symbiodinium. Coral Reefs 30:217–225
Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Change Biol 11:2251–2265
Dörmann P, Benning C (2002) Galactolipids rule in seed plants. Trends Plant Sci 7:112–118
Douglas AE (2003) Coral bleaching—how and why? Mar Pollut Bull 46:385–392
Flaim G, Obertegger U, Guella G (2012) Changes in galactolipid composition of the cold freshwater dinoflagellate Borghiella dodgei in response to temperature. Hydrobiologia 698:285–293
Frentzen M (2004) Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: anionic membrane lipids and phosphate regulation. Curr Opin Plant Biol 7:270–276
Garrett TA, Schmeitzel JL, Klein JA, Hwang JJ, Schwarz JA (2013) Comparative lipid profiling of the cnidarian Aiptasia pallida and its dinoflagellate symbiont. PLoS ONE 8:e57975
Glynn PW, Mate JL, Baker AC, Calderon MO (2001) Coral bleaching and mortality in panama and ecuador during the 1997–1998 El Nino Southern Oscillation Event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Gombos Z, Wada H, Hideg E, Murata N (1994) The unsaturation of membrane lipids stabilizes photosynthesis against heat stress. Plant Physiol 104:563–567
Gorbunov MY, Kolber ZS, Lesser MP, Falkowski PG (2001) Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr 46:75–85
Goyen S, Pernice M, Szabó M, Warner ME, Ralph PJ, Suggett DJ (2017) A molecular physiology basis for functional diversity of hydrogen peroxide production amongst Symbiodinium spp. (Dinophyceae). Mar Biol 164:46
Gray CG, Lasiter AD, Li C, Leblond JD (2009) Mono-and digalactosyldiacylglycerol composition of dinoflagellates. I. Peridinin-containing taxa. Eur J Phycol 44:191–197
Guella G, Frassanito R, Mancini I (2003) A new solution for an old problem: the regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 17:1982–1994
Harwood JL, Guschina IA (2009) The versatility of algae and their lipid metabolism. Biochimie 91:679–684
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystroem M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hume B, D’Angelo C, Burt J, Baker A, Riegl B, Wiedenmann J (2013) Corals from the Persian/Arabian Gulf as models for thermotolerant reef-builders: Prevalence of clade C3 Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar Pollut Bull 72:313–322
Hume BC, Voolstra CR, Arif C, D’Angelo C, Burt JA, Eyal G, Loya Y, Wiedenmann J (2016) Ancestral genetic diversity associated with the rapid spread of stress-tolerant coral symbionts in response to Holocene climate change. Proc Natl Acad Sci 113:4416–4421
Imbs A, Rybin V, Kharlamenko V, Dang L, Nguyen N, Pham K, Pham L (2015) Polyunsaturated molecular species of galactolipids: markers of zooxanthellae in a symbiotic association of the soft coral Capnella sp. (Anthozoa: Alcyonacea). Russ J Mar Biol 41:461–467
Jokiel PL, Coles SL (1977) Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar Biol 43:201–208
Jones AM, Berkelmans R, Van Oppen MJH, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc Lond B 275:1359–1365
Kern R, Joseleau-Petit D, Chattopadhyay MK, Richarme G (2001) Chaperone-like properties of lysophospholipids. Biochem Biophys Res Commun 289:1268–1274
Kneeland J, Hughen K, Cervino J, Hauff B, Eglinton T (2013) Lipid biomarkers in Symbiodinium dinoflagellates: new indicators of thermal stress. Coral Reefs 32:923–934
Ladner JT, Barshis DJ, Palumbi SR (2012) Protein evolution in two co-occurring types of Symbiodinium: an exploration into the genetic basis of thermal tolerance in Symbiodinium clade D. BMC Evol Biol 12:217
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53:305–319
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28(2570–2580):e2576
Leblond JD, Chapman PJ (2000) Lipid class distribution of highly unsaturated long chain fatty acids in marine dinoflagellates. J Phycol 36:1103–1108
Leblond JD, Khadka M, Duong L, Dahmen JL (2015) Squishy lipids: temperature effects on the betaine and galactolipid profiles of a C18/C18 peridinin-containing dinoflagellate, Symbiodinium microadriaticum (Dinophyceae), isolated from the mangrove jellyfish, Cassiopea xamachana. Phycol Res 63:219–230
Lesser MP, Farrell JH (2004) Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23:367–377
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta BBA Biomembr 1666:142–157
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Mansour JS, Pollock FJ, Díaz-Almeyda E, Iglesias-Prieto R, Medina M (2018) Intra-and interspecific variation and phenotypic plasticity in thylakoid membrane properties across two Symbiodinium clades. Coral Reefs 37:841–850
Minoda A, Sato N, Nozaki H, Okada K, Takahashi H, Sonoike K, Tsuzuki M (2002) Role of sulfoquinovosyl diacylglycerol for the maintenance of photosystem II in Chlamydomonas reinhardtii. Eur J Biochem 269:2353–2358
Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16:98–107
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC (2015) Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc Natl Acad Sci 112:7513–7518
Popendorf KJ, Fredricks HF, Van Mooy BA (2013) Molecular ion-independent quantification of polar glycerolipid classes in marine plankton using triple quadrupole MS. Lipids 48:185–195
Quinn P, Joo F, Vigh L (1989) The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol 53:71–103
Riegl B, Glynn PW, Wieters E, Purkis S, D'Angelo C, Wiedenmann J (2015) Water column productivity and temperature predict coral reef regeneration across the Indo-Pacific. Sci Rep 5:8273
Riegl B, Glynn PW, Banks S, Keith I, Rivera F, Vera-Zambrano M, D’Angelo C, Wiedenmann J (2019) Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern Galapagos during 2015/2016 ENSO. Coral Reefs. https://doi.org/10.1007/s00338-019-01787-8
Rosset S, Wiedenmann J, Reed AJ, D'Angelo C (2017) Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar Pollut Bull 118:180–187
Sato N, Aoki M, Maru Y, Sonoike K, Minoda A, Tsuzuki M (2003) Involvement of sulfoquinovosyl diacylglycerol in the structural integrity and heat-tolerance of photosystem II. Planta 217:245–251
Silverstein RN, Cunning R, Baker AC (2017) Tenacious D: Symbiodinium in clade D remain in reef corals at both high and low temperature extremes despite impairment. J Exp Biol 220:1192–1196
Smith EG, D’Angelo C, Salih A, Wiedenmann J (2013) Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 32:463–474
Suggett DJ, Goyen S, Evenhuis C, Szabó M, Pettay DT, Warner ME, Ralph PJ (2015) Functional diversity of photobiological traits within the genus S ymbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytol 208:370–381
Suggett DJ, Warner ME, Leggat W (2017) Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends Ecol Evol 32:735–745
Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H (2004) Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol 45:251–255
Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, Haggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci 101:13531–13535
Warner ME, Suggett DJ (2016) The photobiology of Symbiodinium spp.: linking physiological diversity to the implications of stress and resilience. In: Goffredo S, Dubinsky Z (eds) The Cnidaria, past, present and future. Springer, New York, pp 489–509
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci 96:8007–8012
Warner ME, Lesser MP, Ralph PJ (2010) Chlorophyll fluorescence in reef building corals. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 209–222. https://doi.org/10.1007/978-90-481-9268-7_10
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar C, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277:31994–32002
Welti R, Wang X, Williams TD (2003) Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Anal Biochem 314:149–152
Wiedenmann J, D'Angelo C, Smith EG, Hunt AN, Legiret F-E, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Change 3:160–164
Acknowledgements
The study was funded by the Natural Environment Research Council Grant NE/K00641X/1 and the European Research Council under the European Union’s Seventh Framework Programme Grant FP7/2007-2013/ERC Grant Agreement 311179. S.R. was funded by studentship awards of the Southampton Marine and Maritime Institute (SMMI) and the Vice Chancellor Studentship Award scheme of the University of Southampton. We thank Dr. David Suggett for his insightful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Simon Davy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rosset, S., Koster, G., Brandsma, J. et al. Lipidome analysis of Symbiodiniaceae reveals possible mechanisms of heat stress tolerance in reef coral symbionts. Coral Reefs 38, 1241–1253 (2019). https://doi.org/10.1007/s00338-019-01865-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01865-x