Abstract
Benthic cyanobacterial mats (BCMs) have increased in abundance on coral reefs worldwide. However, their species diversity and role in nitrogen fixation are poorly understood. We assessed the cyanobacterial diversity of BCMs at four coral reef sites in Curaçao, Southern Caribbean. In addition, nitrogen fixation rates of six common mats were measured. Microscopic examinations showed 22 cyanobacterial species, all from the order Oscillatoriales. Species diversity was similar among sites despite differences in overall BCM abundance. Dominant mats were primarily composed of Hydrocoleum glutinosum, Oscillatoria bonnemaisonii or Lyngbya majuscula. However, some mats exhibited highly variable species composition despite consistent macroscopic appearance. 16S rRNA-based phylogeny revealed similar species as those identified by microscopy, with additional sequences of unicellular (Xenococcus and Chroococcidiopsis) and heterocystous (Rivularia and Calothrix) cyanobacteria. Vice versa, morphotypes of Tychonema, Schizothrix and Dichothrix were found by microscopy only. The detection of similar species at the same sites in a study conducted 40 years ago indicates that changes in environmental conditions over these years may have favored indigenous species to bloom, rather than facilitated the introduction and proliferation of invasive species. Nitrogen fixation rates of mats were 3–10 times higher in the light than in the dark. The highest areal nitrogen fixation rate (169.1 mg N m−2 d−1) was recorded in the cyanobacterial patch dominated by O. bonnemaisonii. A scale-up of nitrogen fixation at a site with 26% BCM cover at 7 m depth yielded an aerial rate of 13 mg N m−2 reef d−1, which exceeds rates reported in open ocean blooms of Trichodesmium in the Caribbean. Our results suggest that the Caribbean basin is not only a hotspot for planktonic nitrogen fixation, but also for benthic nitrogen fixation. Because BCMs fix vast amounts of nitrogen, their proliferation will strongly alter the nitrogen budget of coral reefs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are highly productive ecosystems despite being surrounded by nutrient-poor waters (Crossland et al. 1991). Dissolved inorganic nitrogen, next to phosphorus and iron, often limits the growth of phototrophic reef organisms (Lapointe 1997; Larned 1998; Den Haan et al. 2013). However, biological nitrogen fixation (diazotrophy) by cyanobacteria is an important source of nitrogen and sustains a substantial part of the reef primary production (Wiebe et al. 1975; Larkum et al. 1988; Charpy-Roubaud et al. 2001). Therefore, cyanobacteria have been suggested to play a key role in the nitrogen cycle of coral reefs (Wiebe et al. 1975; Hallock 2005; Charpy et al. 2012). Over the past two decades, benthic cyanobacterial mats (BCMs) have become a dominant component on many reefs worldwide (Thacker and Paul 2001; Albert et al. 2005; Paul et al. 2005; De Bakker et al. 2017; Ford et al. 2018). Future predictions suggest that BCMs will continue to increase in abundance, as they are favored by changes in environmental conditions associated with direct and indirect anthropogenic impacts (e.g., eutrophication and overfishing) and climate change (e.g., increase in water temperature) (Hallock 2005; Paerl and Paul 2012; Brocke et al. 2015; Ford et al. 2018). BCMs inhibit coral larvae settlement (Kuffner et al. 2006), act as coral pathogens (Carlton and Richardson 1995) and can produce toxins that lead to selective browsing by herbivorous fish (Nagle and Paul 1998, 1999). However, the impact of cyanobacterial mat proliferation on the reef’s nitrogen pool is virtually unknown.
BCMs have been reported to exhibit high nitrogen fixation rates on coral reefs in the Pacific (Wiebe et al. 1975; Larkum et al. 1988; Charpy et al. 2007, 2010; up to 71.7 mg N2 m−2 d−1) and Indian Ocean (Bauer et al. 2008; Casareto et al. 2008; up to 97.0 mg N2 m−2 d−1). Recently, Oscillatoria spp.-dominated BCMs in Curaçao (Southern Caribbean) were found to exhibit the highest nitrogen fixation rates reported to date (up to 166.8 mg N2 m−2 d−1) (Den Haan et al. 2014). It is, however, not surprising that Caribbean BCMs have relatively high nitrogen fixation rates, as the Caribbean basin is a known hotspot for pelagic nitrogen fixation (Luo et al. 2012). Saharan eolian dust provides an ample supply of iron to Caribbean diazotrophic organisms on a yearly basis (Roff and Mumby 2012). Since iron is an essential element in the nitrogenase enzyme responsible for nitrogen fixation, periodic eolian dust input is likely to fuel Caribbean nitrogen fixation and could be, at least in part, responsible for the relatively high nitrogen fixation rates reported in BCMs in the Caribbean.
Though quite a few studies have estimated the nitrogen fixation rates of benthic cyanobacteria on coral reefs (for example, see Table 5 in Den Haan et al. 2014), the reported rates are often expressed based on the macroscopic appearance of the cyanobacterial community. This is problematic, as the cyanobacterial community within a mat or even a solitary tuft can be highly diverse, and as a consequence, BCMs that have a similar macroscopic appearance might have very dissimilar nitrogen fixation rates. Here, we investigated the cyanobacterial diversity and nitrogen fixation rates of multiple BCMs on the coral reefs of Curaçao. We chose Curaçao, as BCMs can cover large areas of the seabed on this island, most likely as the result of increased nutrient runoff from urbanized areas (Brocke et al. 2015; Den Haan et al. 2016). In this study, we (1) compared the cyanobacterial diversity of BCMs at four reef sites that differed in total BCM abundance, (2) studied the species composition of six common BCMs that exhibited a distinctive macroscopic appearance and (3) investigated the nitrogen fixation rates of one patch of each of the six common BCMs over a diel cycle. Furthermore, we (4) estimated the total nitrogen fixation potential of BCMs at the reef-wide scale.
Materials and methods
Benthic surveys and sample collection
This study was conducted between October and December 2010 on the fringing reefs of the island of Curaçao, Southern Caribbean. We studied two sites with high BCM abundance, Pest Bay (PB) and Buoy 0 (BY), and two sites with low BCM abundance, Cap Malmeeuw (CM) and Spanish Waters (SW), all located along the south leeward coast (ESM Fig. 1). All sites are characterized by a ca. 100 m wide reef terrace that gradually slopes toward a drop-off at ca. 10 m depth. The reef slope (20°–30°) extends to a second (ca. 40 m depth) terrace. The island is exposed to all-year-around trade winds running from east to west (van Duyl et al. 2002). Oceanic currents generally flow westwards at ca. 50 cm s−1 (Gast et al. 1999). The island has a semiarid climate with a cold/dry season from February to September and a warm/rainy season from October to January (http://www.meteo.an). Seasonal fluctuations in water temperature are ca. 3 °C (~ 26–29 °C), with ca. 0.5 °C daily fluctuations (Brocke et al. 2015). The island is surrounded by a belt of Quaternary and Neogene limestone (Hippolyte and Mann 2011). Sediments are very permeable (ca. 2.4 × 10−10 m2 permeability; Brocke et al. 2015).
The coverage of BCMs and other major benthic components (i.e., corals, sand, algae, sponges and others) was determined at 3, 7, 10, 20 and 30 m depths using 25 quadrats of 1 m2 per depth at each site. Visual assessments were made to the nearest percentage with the help of cross-intersects forming a 5 × 5 grid inside the quadrat. Six BCMs, subsequently used in the nitrogen fixation experiment (see below), were recorded separately based on their color and structure (Fig. 1; macroscopic description in ESM). They were selected, as they were the most abundant at our sites and typically formed large-sized (> 200 cm2) patches (Fig. 1).
Macroscopic view of the BCMs analyzed for nitrogen fixation rates: a brown shade, b orange mat, c brown mat, d red mat, e red hairy, f purple tuft. Dominant BCM species in photographs: a Hydrocoleum glutinosum and Oscillatoria bonnemaisonii, b Hydrocoleum glutinosum and Lyngbya majuscula, c Oscillatoria bonnemaisonii, d Oscillatoria sp., e Lyngbya majuscula and Trichocoleus acutissimus, f Dichothrix sp.
All BCMs (i.e., distinct patches) within the first 12–15 quadrats at each depth and site were sampled by hand picking (1–2 cm2 of mat surface per sample) and placed inside individual Falcon tubes (60 ml). Their mat type was recorded (macroscopic description in ESM). Tubes were shaded and immediately transported back to the laboratory inside a cooling box filled with ambient seawater (~ 29 °C). In the laboratory, each sample was separated in two subsamples: (1) a subsample fixed with 4% formaldehyde (w:v) solution for morphological analysis and (2) a subsample frozen at − 20 °C for molecular analysis (see below).
Morphological analyses
Genus or species of each mat sample were identified using direct microscopy. The phenotypic identification was performed using a Zeiss Axioskop 40 microscope on which a Zeiss camera (AxicCam ICc1) was mounted. Using the computer program Axiovision (Carl Zeiss, Jena, Germany), we determined morphological features such as sheath, calyptras, end cell morphology, cell sizes (n = 50 per sample) and proportions and degree of constriction at cross-walls. Subsequent taxonomic identification of the cyanobacterial species was based on the identification keys of Komárek and Anagnostidis (2005) and Komárek and Hauer (2010). The relative abundance of each genus or species within each sample was scored on a semiquantitative scale (0: 0%, 1: 1–19%, 2: 20–39%, 3: 40–69%, 4: 70–89%, 5: 90–100%) after screening 3–5 subsamples.
Molecular analyses
Extraction of DNA was successfully performed from 62 mat samples according to Zhou et al. (1996). PCR amplification was done using the cyanobacteria-specific primers CYA359F and CYA781R (Nübel et al. 1997), with 40 nucleotide GC-clamps. DGGE was carried out on 16S rRNA gene amplicons according to the protocol as described by Muyzer and Smalla (1998). Amplified PCR products were loaded on a Dcode DGGE system (Bio-rad, Hercules, USA) with a linear gradient of 30–65% of urea and formamide. Electrophoresis was performed for 4 h at 200 V and 60 °C. Staining of nucleic acids was performed in 1% SYBRGold Nucleic Acid Stain (Molecular Probes, Eugene, USA), and dominant bands were excised and sequenced. DNA re-amplification for sequencing was performed using the initial primers without GC-clamp at the same cycling conditions. PCR products were purified from contaminants using the MiniElute PCR purification kit (Qiagen, Hamburg, Germany). Sequencing was performed using the BigDye® Terminator v3.1 Cycle sequencing kit (Applied Biosystems, Carlsbad, USA). Products from the sequencing reactions were purified using the Agencourt CleanSEQ kit (Beckman Coulter Genomics, Danvers, USA). Sequences were retrieved using a 3130× Genetic Analyzer (Applied Biosystems, Carlsbad, USA). Sequence data were processed using the CLC main workbench software version 6.0.2 (CLCbio, Aarhus, Denmark). Ambiguous bases in all sequences were resolved manually.
Obtained sequences were aligned and analyzed using the ARB software version 071207 (Ludwig et al. 2004) and the official SILVA database (http://www.arb-silva.de) for small subunit RNA sequences (SSURef_NR99_115_SILVA_20_07_13_opt.arb) (Pruesse et al. 2007). Complete cyanobacterial 16S rRNA gene sequences available from GenBank were imported and aligned to the sequences in the ARB database. To evaluate the consistency of computed tree topologies, subsets of data were analyzed using various algorithms as follows. A variety of single and multiple out-group sequences representing phylogenetically diverse organisms were included in the analysis. To assess the influence of the most variable nucleotide positions, they were excluded from some calculations by applying filters based on character frequency (ARB manual). The obtained cyanobacterial forward and the reverse complementary of the reverse sequences obtained were aligned against each other in order to obtain consensus sequences. These sequences were then aligned with the sequences in the ARB database using the alignment ARB tool. The alignment was corrected manually. Phylogenetic trees were calculated by applying the three different methods integrated in the ARB software namely maximum likelihood (ML) method, maximum parsimony and neighbor joining, based on long 16S rRNA gene sequences (> 1300 bp). The ML tree was finally selected for presentation because of its stability. Partial sequences obtained in this study were inserted into the tree using the parsimony ARB tool, while maintaining the overall tree topology without changes. The 16S rRNA gene sequence of E. coli was used as out-group.
Nitrogen fixation experiment
One large-sized (> 200 cm2) patch from each of the six mats (Fig. 1) was assessed for nitrogen fixation using the acetylene reduction assay (ARA). Patches were sampled at the site and depth where mats were most frequently encountered. Pieces of ca. 200 cm2 of mat surface were scooped with a spade, placed into individual large buckets, transported in the dark back to the laboratory and immediately used for experiments. Furthermore, we collected in situ seawater using Plexiglas tubes (5.3 L).
Back to the laboratory, the in situ seawater was filtered using 0.22 μm Whatman cellulose acetate membrane filters. Incubation experiments were simulated under natural conditions over a diel cycle according to Montoya et al. (1996). Nine pieces of 1 cm2 of mat surface were haphazardly stamped out of each patch and placed inside individual 38 ml gas-tight serum bottles. The bottles were incubated in a flow-through aquarium. This aquarium was connected to a water pump that provided continuous water flow to ensure a similar temperature in the aquarium as on the reef (27–29 °C). Light intensity and temperature during the incubations were recorded with a UA-002-08 data logger (Onset Computer Corporation, Pocasset, USA). Light intensities (lux) were converted to the availability of photosynthetically active radiation (PAR) using the equation: 1 mmol quanta (400–700 nm) m s−1 = 51.2 lx (sensu Valiela 1984). The average light intensities (± SEM) in the laboratory around noon (1150–1210 h; sampling intervals 1 min; averaged over all incubations) were 201.6 ± 27.9 µmol photons m−2 s−1 for the incubated cyanobacterial mats originating from 7 m depth and 90.9 ± 4.3 µmol photons m−2 s−1 for the mats originating from 20 m depth. These light intensities were similar to those reported during sunny days at 7 and 20 m depths at Buoy 0 by Den Haan et al. (2014).
Incubations lasted for 24 h, starting at 1830 h (sunset) and ending at 1830 h the next day. Due to constraints in the volume of gas that could be withdrawn from each bottle, three incubation sets for each mat were used. Each incubation set for each mat consisted of three 1 cm2 mat pieces (n = 3 replicates) and one control, with no biological material added to the seawater. 4.5 ml of high purity acetylene gas (Linde Gas, Willemstad, Curaçao) was added to the headspace (13 ml) at 1830 h with a gas-tight syringe. The first set was sampled during the night (1830–0630 h), while the second and a third kept in the dark were sampled during the day (0930–1800 h). A 1-ml gas sample was taken from each replicate and each control every 3 h with a gas-tight syringe. The gas then was injected into 6 ml Vaccuetes® (Greiner Bio-One, Frickenhausen, Germany) filled with a saturated sodium chloride solution in order to properly conserve the sample until further analysis. Ethylene production was measured by injecting 0.2 ml of headspace gas into a gas chromatograph with flame ionization detector (HRGC-4000 A, Konik, Sant Cugat del Vallès, Spain) (Stewart et al. 1967).
After the incubation experiment, chlorophyll a was extracted from the incubated patches with pure methanol and concentrations were subsequently measured spectrophotometrically according to Porra et al. (1989). Nitrogenase activity was calculated according to Capone (1993), and results are presented as rates normalized to chlorophyll a content (phototrophic biomass) as well as surface area (m2). Nitrogenase has a threefold–fourfold higher affinity toward acetylene in comparison with dinitrogen (Montoya et al. 1996); therefore, we used a conversion factor of 4 as described previously by Peterson and Burris (1976). In addition, genus or species of each patch (piece of 1 cm2) were sampled and identified using direct microscopy as described above.
Statistical analyses
Differences in species abundance in mat samples were formally tested using a one-way permutation-based analysis of variance (PERMANOVA, Anderson 2001) based on unrestricted permutations of the raw untransformed abundance score data pooled across all site × depth combinations, and mat type as a fixed factor. Given the low number of possible permutations in the individual pairwise tests, we used the Monte Carlo asymptotic p values. Principal coordinates analysis (PCO) was used to visualize the data in two dimensions. Species contributing to differences between mat types were identified based on the strength of their Spearman correlations with the PCO axes. Analyses were performed in PRIMER 6 and PERMANOVA + statistical package (Anderson et al. 2008).
Frequencies of morphotypes in mat samples were then averaged for each depth × site combination with frequency taken as the midpoint of the respective semiquantitative scale. The Shannon Index was used to determine whether the diversity of the cyanobacterial species was comparable between all studied sites. We used an analysis of covariance (ANCOVA) with BCM site abundance (i.e., high/low) as a fixed factor and depth as a covariate to determine whether species richness differed between sites with low and high mat cover, and whether species richness within the mats was affected by depth.
Since only one patch from each of the six mats was assessed for nitrogen fixation and the pieces of 1 cm2 of mat surface sampled from each patch constitute “pseudoreplicates” (sensu Hurlbert 1984), nitrogen fixation data were not amenable to statistical analysis.
Results
BCM general description and cover
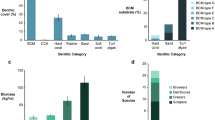
BCMs were detected on both hard substrates (incl. coral skeleton, turfs and macroalgae) and carbonate sediment patches. Mat structures ranged from soft gelatinous masses (ca. 3–6 mm thick) to firmer tufts (ca. 0.5–20 cm thick). BCMs exhibited a range of different colors, including brown and violet mainly in the upper 10 m of the reef (Fig. 1a, c, f), to orange or red deeper down (Fig. 1b, d, e). The sites PB and BY had the highest total BCM cover (15.7% and 10.8% of the quantified area, respectively, averaged across all five depths), whereas benthic mats at the sites CM and SW remained largely inconspicuous (< 1% at all five depths) (Fig. 2). At PB and BY, BCMs were more abundant in the upper 10 m of the reef than in the deeper parts of the reef (i.e., 20 and 30 m depths). Conversely, total coral cover, averaged across all depths, was higher at CM and SW (36.2 and 52.4%, respectively) than at PB and BY (13.5 and 12.4%, respectively) (Fig. 2). At PB, the light brown mat (referred to as ‘brown shade,’ Fig. 1a), the ‘orange mat’ (Fig. 1b) and the dark ‘brown mat’ (Fig. 1c) was dominant at 3 and 7 m depths (ESM Fig. 2). Deeper down (≤ 10 m), the ‘red mat’ (Fig. 1d) and the ‘red hairy’ mat (Fig. 1e) became predominant. At BY, trends were similar but the ‘orange mat’ was present deeper down (10 and 20 m). At the low BCM abundance sites, the mats that dominated the high BCM abundance sites were absent or rare. The ‘purple tuft’ mat was encountered at a relatively low abundance at all sites, except PB.
Morphological diversity
A total of 478 mat samples were morphologically characterized (ESM Table 1). Each sample was frequently dominated (> 70%) by a single cyanobacterial species, although other minor species were also observed. Pooling samples across all site x depth combinations, 22 different cyanobacteria was identified to genus or species level based on their microscopic features (Fig. 3). Samples were dominated by filamentous non-heterocystous cyanobacteria of the order Oscillatoriales. Major mat-building species belonged to the genera Hydrocoleum, Lyngbya, Phormidium, Symploca, Oscillatoria, Tychonema, Schizothrix, Pseudanabaena and Dichothrix. PERMANOVA indicated significant differences in species composition among visual types (Pseudo-F = 11.63, Pperm < 0.001; ESM Table 2; ESM Fig. 3). Almost all mats were significantly different from each other (Monte Carlo pairwise tests, p < 0.05; ESM Table 3). Three types were dominated by one cyanobacterial species. The ‘brown shade’ mat (Fig. 1a) was mainly composed of H. glutinosum (~ 70%) and O. bonnemaisonii (~ 30%). O. bonnemaisonii was the dominant cyanobacterium forming the thick dark ‘brown mat’ (Fig. 1c). The ‘red hairy’ mat (Fig. 1e) was consistently dominated by L. majuscula, but several other minor species were also present. In contrast, no dominant species were consistently detected in the ‘orange mat’ (Fig. 1b), the ‘red mat’ (Fig. 1d) and the ‘purple turf’ (Fig. 1f). These three mats displayed a high variability in species composition. The average cumulative number of species as a function of the number of samples for each visual mat type showed a plateau-shaped function (ESM Fig. 4), indicating that the number of samples was appropriate to document the absence or presence of each species.
Microscopic photographs of all found morphotypes: a Lyngbya majuscula, b Hydrocoleum glutinosum, c Phormidium formosum, d Phormidium nigroviride, e Lyngbya semiplena, f Trichocoleus acutissimus, g Dichothrix utahensis, h Pseudanabaena spp., i Schizothrix spp., j Lyngbya aestuarii, k Lyngbya spp., l Symploca hydnoides, m Lyngbya sordida, n Phormidium spp., o Symploca atlantica, p Trichocoleus spp., q Oscillatoria spp., r Oscillatoria bonnemaisonii, s Oscillatoria funiformis, t Tychonema sp.2, u Phormidium corium, v Tychonema sp.1
When abundance data were expressed as the midpoint of the respective semiquantitative scale and pooled across all site x depth combinations, the species Lyngbya majuscula was most frequently observed within the mat samples (15.5%), followed by Hydrocoleum glutinosum (12.7%) and Symploca hydnoides (11.2%). When data were averaged across each site × depth combination (Fig. 4), L. majuscula exhibited its highest abundance at 20 and 30 m depths at the sites PB and BY, whereas H. glutinosum was more abundant at 3 and 7 m depths. The abundance of the cyanobacterium S. hydnoides averaged across all depths was slightly higher at the CM and SW sites compared to PB and BY sites (16 and 15% vs. 9 and 5%, respectively). The cyanobacteria Oscillatoria spp., O. bonnemaisonii and Dichothrix utahensis could be detected at all sites, except the SW site. O. bonnemaisonii exhibited its highest abundance (14.7%) at the PB site at 7 m depth. The diversity of dominant cyanobacterial species was comparable at all sites (ESM Table 1), and no significant differences in species richness could be found between the sites with low and high mat cover (ANCOVA: F1,17 = 0.591, p = 0.452). Species richness within the mats was also unaffected by depth (ANCOVA: F1,14 = 0.947, p = 0.466).
Frequency of morphotypes as identified by microscopy (see Fig. 3 for full names) in mat samples at the four study sites (PB, Pest Bay; CM, Cap Malmeeuw; BY, Buoy 0; SW, Spanish waters) and five depths (3, 7, 10, 20, 30 m). Underlined names belong to most prominent species in cyanobacterial mats with a size class over 20 cm2
Phylogenetic diversity
DGGE in most cases displayed multiple bands (2–5), indicating the co-dominance of more than one cyanobacterial species in each sample (ESM Fig. 5). A total of 137 good quality sequences were obtained from DGGE bands, and these sequences were phylogenetically affiliated to different species of unicellular and filamentous cyanobacteria (Fig. 5). A large fraction of the sequences (total 33) was related to thin filamentous cyanobacteria of the genera Phormidium, Leptolyngbya and Pseudanabaena. These sequences were encountered in mats from all four sites. Sequences related to Symploca spp. constituted 13% of retrieved sequences and were only detected at BY and CM sites. Sequences belonging to unicellular cyanobacteria were detected at PB, BY and SW sites and were affiliated to the genera Xenococcus and Chroococcidiopsis. A single sequence closely related to Spirulina subsalsa was exclusively found at the BY site. Sequences related to known heterocystous cyanobacteria of the genera Rivularia and Calothrix were only encountered at the PB and BY, but not at the SW and CM sites. While 4 sequences obtained from the PB site fell phylogenetically close to Oscillatoria spongeliae, another 14 obtained from the other sites (i.e., BY, CM and SW) fell close to the sequence of Oscillatoria margaritifera. Hydrocoleum-related sequences were also retrieved from all sites, except the CM site, and were closely affiliated to sequences of the species H. lyngbyaceum and H. glutinosum. Mats from all sites generated sequences related to the genus Lyngbya.
Overall, the 16S rRNA phylogenetic affiliation with cyanobacterial taxa was in good agreement with microscopic identifications, especially for dominant phenotypes. Most of the cyanobacterial genera encountered by direct microscopy, such as Lyngbya, Oscillatoria, Hydrocoleum, Phormidium and Symploca, were consistently detected using 16S rRNA-DGGE. However, additional genera such as Leptolyngbya, Spirulina, Rivularia and Calothrix could only be detected using the molecular technique. Vice versa, morphotypes of Tychonema, Schizothrix and Dichothrix were found by microscopy only.
Nitrogen fixation
All incubated cyanobacterial patches fixed nitrogen with rates 3–10 times higher during light periods than in the dark (Table 1). The highest rates were measured in the morning between 0930 and 1230 h, except for the patch from the ‘brown mat’ (Fig. 1c), where the highest rate was detected at 1430 h (ESM Fig. 6). The estimated areal nitrogen fixation rate was highest for the patch from the ‘brown mat’ and was estimated at 169.1 mg N m−2 d−1. Next came the patches of the ‘orange mat’ (Fig. 1b) and ‘brown shade’ (Fig. 1a) with areal nitrogen fixation rates of 9.3 and 14.4 mg N m−2 d−1, respectively. It was ≤ 4.3 mg N m−2 d−1 for the remaining patches. When considering the chlorophyll a concentration for the calculation of nitrogen fixation rates, patches of the ‘brown shade’ and ‘orange mat’ had the highest values, with 166.8 and 66.6 nmol N2 µg chl a d−1, respectively.
Patches of the ‘brown mat’ and ‘brown shade’ were dominated by their expected species as identified by microscopy (ESM Table 2). Since these two mats were consistently dominated by one cyanobacterial species and made up > 90% of the total BCM cover at 7 m depth at PB (ESM Fig. 2), we multiplied the areal N2 fixation rates estimated from their patches by their respective cover and obtained an estimated total N2 fixation potential by BCMs of 13 mg N m−2 reef d−1 at this specific site and depth.
Discussion
The cyanobacterial mats of Curaçao harbored a large variety of cyanobacterial species, as revealed by microscopy and molecular tools. An advantage of the morphological analyses is that it enabled us to compare the current cyanobacterial diversity with that observed in the same coral reef more than 40 years ago (Van den Hoek et al. 1975; Vooren 1981). Interestingly, most of the identified cyanobacteria in our microbial mats were also identified in the previous studies as part of turf communities, including L. majuscula, L. sordida, Phormidium sp. and O. bonnemaisonii (Van den Hoek et al. 1975; Vooren 1981). This indicates that changes in environmental conditions over the past 40 years may have favored existing cyanobacterial species to bloom, rather than may have introduced invasive species of cyanobacteria into the ecosystem.
The 16S rRNA phylogenetic affiliation with cyanobacterial taxa did not always match with microscopic identifications and vice versa. This discrepancy between microscopic and molecular tools is well documented (Abed and Garcia-Pichel 2001; Palinska et al. 2012). The main reasons for that are: (1) cyanobacteria are notoriously difficult to identify using morphology; (2) the identity and distribution of the obtained sequences relies on names of mostly cultured organisms, or relate to uncultured and unnamed sequences; and (3) both microscopic survey and the molecular data based on studies of field populations are expected to represent microbial composition present at the moment of the sampling. However, the DNA data do not recognize whether these organisms were active or dormant nor whether the molecules are even autochthonous. Thus, the diversity of field populations based on DNA analyses may be a cumulative rather than an instantaneous record of a particular sampling site. In our study, we detected with molecular tools many sequences related to thin filamentous cyanobacteria (e.g., Phormidium, Leptolyngbya, and Pseudanabaena). These genera lack conspicuous morphological features and are often mis-identified by microscopy. In contrast, it will be comparatively more difficult to get DNA out of some of the easily morphologically detected cyanobacteria (like Hydrocoleum and Lyngbya) that are big in size, and this will give the chance to minor cyanobacteria to be amplified by PCR (i.e., preferential amplification; Palinska et al. 2012). This underlines the need to combine different techniques to study cyanobacterial diversity in order to circumvent the limitations associated with each technique (Abed and Garcia-Pichel 2001; Abed et al. 2003).
Most of the detected cyanobacterial species and phylotypes described in this study have also been documented in other coral reefs in the Pacific and Indian Ocean (Abed et al. 2003; Thacker and Paul 2004; Charpy et al. 2010, 2012). Interestingly, microbial mats from these coral reef systems as well as from Curaçao have often been dominated by a single cyanobacterium (Abed et al. 2003; Thacker and Paul 2004; Charpy et al. 2010, 2012). At the four studied sites, the large-sized patches were mainly dominated either by Hydrocoleum glutinosum, Oscillatoria bonnemaisonii or Lyngbya majuscula. In recent years, similar mass occurrences of Lyngbya, including L. majuscula, have been reported around the world (Thacker and Paul 2001; Albert et al. 2005; Paul et al. 2005; Charpy et al. 2012; Paerl and Paul 2012). Hydrocoleum spp. and O. bonnemaisonii have also been detected in high abundance in microbial mats from La Reunion and Tikehau Atoll (Charpy et al. 2007, 2010, 2012). O. bonnemaisonii and H. glutinosum are known to occur in shallow tropical coral reefs growing mostly on carbonate sediment (Charpy et al. 2010), as observed in this study. Both species have the ability to form multi-filamentous structures enabling them to be pioneer organisms of unstable sediments (Garcia-Pichel and Wojciechowski 2009). The filamentous cyanobacteria belonging to the genus Hydrocoleum are among the most common mat-forming cyanobacteria in tropical oceans (Abed et al. 2003, 2006). These cyanobacteria have been shown to be a major contributor to nitrogen fixation in tropical oceans and to share a common evolutionary origin with the planktonic Trichodesmium species (Abed et al. 2006; Charpy et al. 2010). Members of the genus Lyngbya and other benthic cyanobacteria have been intensively studied with regard to their production of toxins, which enables them to survive grazing pressure by herbivores (Thacker et al. 1997; Nagle and Paul 1999; Thacker and Paul 2004). Interestingly, the species composition analyzed by light microscopy did not always correlate with the macroscopic appearance of the mat. Some mats, although visually identical in color and structure, exhibited a high variability in species composition and dominant species. This may be due to a similar pigment composition across various species, which are exposed to similar environmental conditions, such as light and nutrient availability (Riethman et al. 1988).
All incubated cyanobacterial patches exhibited an ability to fix nitrogen at higher rates during the day than during the night. This indicates that most of the nitrogen fixation was performed by phototrophs, most likely cyanobacteria (Stal et al. 2010). The cyanobacteria O. bonnemaisonii, H. glutinosum. and L. majuscula, which dominated large-sized mats, have previously been shown to fix nitrogen in cultures or to be associated with a nitrogen-fixing microbial mat community (Elmetri and Bell 2004; Charpy et al. 2010, 2012). Non-heterocystous Oscillatoriales species are thought to separate the incompatible reactions of oxygenic photosynthesis and nitrogen fixation on a temporal basis, fixing mainly at night when oxygen concentrations are low (Bergman et al. 1997). However, studies on marine microbial mats demonstrated that nitrogen fixation is stimulated by light and phototrophic microorganisms are actively involved (Severin and Stal 2008, 2010). Stal (2012) described a Type III group of aerobic N-fixers that can combine photosynthesis and nitrogen fixation. This group comprises non-heterocystous filamentous and unicellular cyanobacteria and possesses the capacity of inducing nitrogenase and growing diazotrophically under fully aerobic conditions. In microbial mats, aerobic nitrogen-fixing non-heterocystous cyanobacteria belong predominantly to the genera Oscillatoria and Lyngbya. These genera are morphologically and phylogenetically closely related to Trichodesmium spp., which are known to fix nitrogen during the day (Capone et al. 1990). Our diurnal nitrogen fixation measurements are consistent with this hypothesis. This pattern has also been observed in cyanobacterial mats and turf communities in other coral reef systems (Wiebe et al. 1975; Charpy-Roubaud et al. 2001; Charpy-Roubaud and Larkum 2005; Charpy et al. 2007; Den Haan et al. 2014).
Our measured nitrogen fixation rates ranked among the highest reported so far, when compared to all published rates from other coral reef ecosystems (as a comparison, see Table 5 in Den Haan et al. (2014)). While a maximum nitrogen fixation rate of 169 mg N m−2 d−1 was measured in the patch of the ‘brown mat’ dominated by O. bonnemaisonii, most of the reported rates ranged between 0.1 and 5 mg N m−2 d−1. In the Caribbean region, there is a lack of recent information on nitrogen fixation rates and those last documented were from the 80 and 90 s, except for a recent study by Den Haan et al. (2014). Interestingly, using the same technique as in this study, Den Haan et al. (2014) investigated a brown mat also dominated by O. bonnemaisonii at another site in Curaçao in the summer of 2011 and found a similar nitrogen fixation rate: 166.8 mg N m−2 d−1. This supports the accuracy of our measurements. When the nitrogen fixation rates were scaled up at 7 m depth at PB, where the cyanobacterial mats had a homogeneous composition of O. bonnemaisonii and H. glutinosum (i.e., Mats C and A in Table 1), gross areal nitrogen fixation rates amounted to approximately 13 mg N m−2 reef d−1. This value is higher than what was estimated for the shallow lagoon of La Reunion Island heavily colonized by mats (2 mg N m−2 lagoon d−1) (Charpy et al. 2010), One Tree Reef in the southern Great Barrier Reef (2–4 mg N m−2 reef d−1) (Larkum et al. 1988), the lagoon of Tikehau atoll, French Polynesia (1–8 mg N m−2 lagoon d−1) (Charpy-Roubaud et al. 2001; Charpy-Roubaud and Larkum 2005) and the reef of Enewetak Atoll (0.5 mg N m−2 reef d−1) (Wiebe et al. 1975). The observed rate was even slightly higher than the areal nitrogen fixation measured in open ocean blooms of Trichodesmium in the Caribbean (2.59–9.78 mg N m−2 d−1) (Carpenter and Price 1977; Karl et al. 1997). In spite of that, it should be kept in mind that the nitrogen fixation rates used to produce reef-wide estimates of nitrogen fixation were calculated using only one patch per mat. We have no data on the variability between patches within a given mat; thus, these estimations should be treated with caution. Furthermore, nitrogen fixation rates will most likely vary throughout the year due to seasonal changes in light, temperature and nutrient/organic matter availability. Our study was conducted during the warm/rainy season. The combination of elevated temperature and high precipitation is likely to provide ideal growth conditions for the mats and increase their nitrogen fixation rates during this period. Thus, the rates found in this study might represent seasonal peaks for Curaçao.
On an annual basis, the Caribbean basin receives hundreds of millions of tons Saharan dust with bound iron particles (Jickells 1999; Petit et al. 2005). Roff and Mumby (2012) hypothesized that Saharan dust offsets the iron-limited productivity of benthic algae in the Caribbean, which may also hold true for benthic cyanobacteria. This may lead to higher nitrogen fixation rates, as iron is a vital constituent of the nitrogen enzyme responsible for atmospheric nitrogen fixation (Howard and Rees 1996; Rees et al. 2005). Similarly, the higher nitrogen fixation rates reported thus far in Curaçao could be, at least in part, explained by the eolian iron input. Another factor that may augment nitrogen fixation rates of BCMs in the Caribbean basin is coastal eutrophication. Charpy et al. (2012) reported that on the islands of Mayotte, Tulear and La Reunion, coastal eutrophication favored bloom-forming cyanobacterial mats that were capable of fixing vast amounts of atmospheric nitrogen. Continued cyanobacterial but also algal proliferation will further degrade coral reefs (Ford et al. 2018) and has been reported to increase the reef’s sediment with organic matter (Barott and Rohwer 2012). This in turn will fuel benthic cyanobacterial mat proliferation (Brocke et al. 2015; Ford et al. 2018) and augment its nitrogen fixation potential (Hanson and Gundersen 1976; O’Neil and Capone 1989; King et al. 1990). Ultimately, this could result in an undesirable positive feedback that will promote the proliferation of cyanobacterial mat abundance and consequently accelerate coral reef degradation.
References
Abed RMM, Garcia-Pichel F (2001) Long-term compositional changes after transplant in a microbial mat cyanobacterial community revealed using a polyphasic approach. Environ Microbiol 3:53–62
Abed RMM, Palinska KA, Camoin G, Golubic S (2006) Common evolutionary origin of planktonic and benthic nitrogen-fixing oscillatoriacean cyanobacteria from tropical oceans. FEMS Microbiol Lett 260:171–177
Abed RMM, Golubic S, Garcia-Pichel F, Camoin GF, Sprachta S (2003) Characterization of microbialite-forming cyanobacteria in a tropical lagoon: Tikehau Atoll, Tuamotu, French Polynesia. J Phycol 39:862–873
Albert S, O’Neil JM, Udy JW, Ahern KS, O’Sullivan CM, Dennison WC (2005) Blooms of the cyanobacterium Lyngbya majuscula in coastal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull 51:428–437
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth, UK
Barott KL, Rohwer FL (2012) Unseen players shape benthic competition on coral reefs. Trends Microbiol 20:621–628
Bauer K, Diez B, Lugomela C, Seppala S, Borg AJ, Bergman B (2008) Variability in benthic diazotrophy and cyanobacterial diversity in a tropical intertidal lagoon. FEMS Microbiol Ecol 63:205–221
Bergman B, Gallon JR, Rai AN, Stal LJ (1997) N2-fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev 19:139–185
Brocke HJ, Polerecky L, de Beer D, Weber M, Claudet J, Nugues MM (2015) Organic matter degradation drives benthic cyanobacterial mat abundance on Caribbean coral reefs. PLoS ONE 10:e0125445
Capone DG (1993) Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Florida, pp 621–631
Capone DG, O’Neil JM, Zehr J, Carpenter EJ (1990) Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol 56:3532–3536
Carlton RG, Richardson LL (1995) Oxygen and sulfide dynamics in a horizontally migrating cyanaobacterial mat - Black band disease of corals. FEMS Microbiol Ecol 18:155–162
Carpenter EJ, Price CC (1977) Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnol Oceanogr 22:60–72
Casareto BE, Charpy L, Langlade MJ, Ohba H, Suzuki Y (2008) Nitrogen fixation in coral reef environments. Proc 11th Int Coral Reef Symp 2: 896-900
Charpy-Roubaud C, Larkum AWD (2005) Dinitrogen fixation by exposed communities on the rim of Tikehau atoll (Tuamotu Archipelago, French Polynesia). Coral Reefs 24:622–628
Charpy-Roubaud C, Charpy L, Larkum A (2001) Atmospheric dinitrogen fixation by benthic communities of Tikehau Lagoon (Tuamotu Archipelago, French Polynesia) and its contribution to benthic primary production. Mar Biol 139:991–998
Charpy L, Alliod R, Rodier M, Golubic S (2007) Benthic nitrogen fixation in the SW New Caledonia lagoon. Aquat Microb Ecol 47:73–81
Charpy L, Palinska KA, Abed RMM, Langlade MJ, Golubic S (2012) Factors influencing microbial mat composition, distribution and dinitrogen fixation in three western Indian Ocean coral reefs. Eur J Phycol 47:51–66
Charpy L, Palinska K, Casareto B, Langlade MJ, Suzuki Y, Abed RM, Golubic S (2010) Dinitrogen-fixing cyanobacteria in microbial mats of two shallow coral reef ecosystems. Microb Ecol 59:174–186
Crossland CJ, Hatcher BG, Smith SV (1991) Role of coral reefs in global ocean production. Coral Reefs 10:55–64
De Bakker DM, van Duyl FC, Bak RPM, Nugues MM, Nieuwland G, Meesters EH (2017) 40 Years of benthic community change on the Caribbean reefs of Curaҫao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 36:355–367
Den Haan J, Huisman J, Dekker F, Ten Brinke JL, Ford AK, Van Ooijen J, Van Duyl FC, Vermeij MJA, Visser PM (2013) Fast detection of nutrient limitation in macroalgae and seagrass with nutrient-Induced fluorescence. PLoS ONE 8:e68834. https://doi.org/10.1371/journal.pone.0068834
Den Haan J, Visser PM, Ganase A, Gooren E, Stal L, van Duyl F, Vermeij MJA, Huisman J (2014) Nitrogen fixation rates in algal turf communities of a degraded versus less degraded coral reef. Coral Reefs 33:1003–1015
Den Haan J, Huisman J, Brocke HJ, Goehlich H, Latijnhouwers KRW, van Heeringen S, Honcoop SAS, Bleyenberg TE, Schouten S, Cerli C, Hoitinga L, Vermeij MJA, Visser PM (2016) Nitrogen and phosphorus uptake rates of different species from a coral reef community after a nutrient pulse. Sci Rep 6:28821
Elmetri I, Bell PRF (2004) Effects of phosphorus on the growth and nitrogen fixation rates of Lyngbya majuscula: implications for management in Moreton Bay, Queensland. Mar Ecol-Prog Ser 281:27–35
Ford AK, Bejarano S, Nugues MM, Visser PM, Albert S, Ferse SCA (2018) Reefs under siege—the rise, putative drivers, and consequences of benthic cyanobacterial mats. Front Mar Sci 5:18
Garcia-Pichel F, Wojciechowski MF (2009) The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 4:e7801. https://doi.org/10.1371/journal.pone.0007801
Gast GJ, Jonkers PJ, Van Duyl FC, Bak RPM (1999) Bacteria, flagellates and nutrients in island fringing coral reef waters: influence of the ocean, the reef and eutrophication. Bull Mar Sci 65:523–538
Hallock P (2005) Global change and modern coral reefs: new opportunities to understand shallow-water carbonate depositional processes. Sediment Geol 175:19–33
Hanson RB, Gundersen K (1976) Influence of sewage discharge on nitrogen fixation and nitrogen flux from coral reefs in Kaneohe Bay. Hawaii. Appl Environ Microb 31:942–948
Hippolyte J-C, Mann P (2011) Neogene-Quaternary tectonic evolution of the Leeward Antilles islands (Aruba, Bonaire, Curaçao) from fault kinematic analysis. Mar Petrol Geol 28:259–277
Howard JB, Rees DC (1996) Structural basis of biological nitrogen fixation. Chem Rev 96:2965–2982
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Jickells TD (1999) The inputs of dust derived elements to the Sargasso Sea: a synthesis. Mar Chem 68:5–14
Karl D, Letelier R, Tupas L, Dore J, Christian J, Hebel D (1997) The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388:533–538
King GM, Carlton RG, Sawyer TE (1990) Anaerobic metabolism and oxygen distribution in the carbonate sediments of a submarine-canyon. Mar Ecol-Prog Ser 58:275–285
Komárek J, Anagnostidis K (2005) Süsswasserflora von Mitteleuropa. Cyanoprokaryota 2. Teil: Oscillatoriales. Elsevier Spektrum Akademischer Verlag, München
Komárek J, Hauer T (2010) CyanoDB.cz - Online database of cyanobacterial genera. - Wordwide electronic publication, Univ. of South Bohemia & Inst. of Botany AS CR, http://www.cyanodb.cz
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol-Prog Ser 323:107–117
Lapointe BE (1997) Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol Oceanogr 42:1119–1131
Larkum AWD, Kennedy IR, Muller WJ (1988) Nitrogen fixation on a coral reef. Mar Biol 98:143–155
Larned ST (1998) Nitrogen- versus phosphorus-limited growth and sources of nutrients for coral reef macroalgae. Mar Biol 132:409–421
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüßmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Luo YW, Doney SC, Anderson LA, Benavides M, Bode A, Bonnet S, Boström KH, Böttjer D, Capone DG, Carpenter EJ, Chen YL, Church MJ, Dore JE, Falcón LI, Fernández A, Foster RA, Furuya K, Gomez F, Gundersen K, Hynes AM, Karl DM, Kitajima S, Langlois RJ, LaRoche J, Letelier RM, Maranon E, McGillicuddy DJ Jr, Moisander PH, Moore CM, Mourino-Carballido B, Mulholland MR, Needoba JA, Orcutt KM, Poulton AJ, Raimbault P, Rees AP, Riemann L, Shiozaki T, Subramaniam A, Tyrrell T, Turk-Kubo KA, Varela M, Villareal TA, Webb EA, White AE, Wu J, Zehr JP (2012) Database of diazotrophs in global ocean: abundances, biomass and nitrogen fixation rates. Earth Syst Sci Data Discuss 5:47–106
Montoya JP, Voss M, Kahler P, Capone DG (1996) A simple, high-precision, high-sensitivity tracer assay for N2-fixation. Appl Environ Microb 62:986–993
Muyzer G, Smalla K (1998) Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127–141
Nagle DG, Paul VJ (1998) Chemical defense of a marine cyanobacterial bloom. J Exp Mar Biol Ecol 225:29–38
Nagle DG, Paul VJ (1999) Production of secondary metabolites by filamentous tropical marine cyanobacteria: ecological functions of the compounds. J Phycol 35:1412–1421
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microb 63:3327–3332
O’Neil JM, Capone DG (1989) Nitrogenase activity in tropical carbonate marine sediments. Mar Ecol-Prog Ser 56:145–156
Paerl HW, Paul VJ (2012) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363
Palinska KA, Abed RMM, Wendt K, Charpy L, Lotocka M, Golubic S (2012) Opportunistic cyanobacteria in benthic microbial mats of a tropical lagoon, Tikehau Atoll, Tuamotu Archipelago: minor in natural populations, major in cultures. Fottea 12:127–140
Paul VJ, Thacker RW, Banks K, Golubic S (2005) Benthic cyanobacterial bloom impacts the reefs of South Florida (Broward County, USA). Coral Reefs 24:693–697
Peterson RB, Burris RH (1976) Conversion of acetylene reduction rates to nitrogen fixation rates in natural populations of blue-green algae. Anal Biochem 73:404–410
Petit RH, Legrand M, Jankowiak I, Molinié J, Asselin de Beauville C, Marion G, Mansot JL (2005) Transport of Saharan dust over the Caribbean islands: study of an event. J Geophys Res 110:1–19
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA Bioenergetics 975:384–394
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Rees DC, Tezcan FA, Haynes CA, Walton MY, Andrade S, Einsle O, Howard JB (2005) Structural basis of biological nitrogen fixation. Philos T Roy Soc A 363:971–984
Riethman H, Bullerjahn G, Reddy KJ, Sherman LA (1988) Regulation of cyanobacterial pigment-protein composition and organization by environmental factors. Photosynthesis Research 18:133–161
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27:404–413
Severin I, Stal LJ (2008) Light dependency of nitrogen fixation in a coastal cyanobacterial mat. ISME J 2:1077–1088
Severin I, Stal LJ (2010) NifH expression by five groups of phototrophs compared with nitrogenase activity in coastal microbial mats. FEMS Microbiol Ecol 73:55–67
Stal LJ (2012) Cyanobacterial mats and stromatolites. In: Whitton BA (ed) The Ecology of Cyanobacteria II: their diversity in space and time. Springer, Dordrecht, New York, London, pp 61–120
Stal LJ, Severin I, Bolhuis H (2010) The ecology of nitrogen fixation in cyanobacterial mats. In: Hallenbeck PC (ed) Recent advances in phototrophic prokaryotes. Springer, New York, pp 31–45
Stewart WD, Fitzgerald GP, Burris RH (1967) In situ studies on N2-fixation using the acetylene reduction technique. Proc Natl Acad Sci USA 58:2071–2078
Thacker RW, Paul VJ (2001) Are benthic cyanobacteria indicators of nutrient enrichment? Relationships between cyanobacterial abundance and environmental factors on the reef flats of Guam. Bull Mar Sci 69:497–508
Thacker RW, Paul VJ (2004) Morphological, chemical, and genetic diversity of tropical marine cyanobacteria Lyngbya spp. and Symploca spp. (Oscillatoriales). Appl Environ Microb 70:3305–3312
Thacker RW, Nagle DG, Paul VJ (1997) Effects of repeated exposures to marine cyanobacterial secondary metabolites on feeding by juvenile rabbitfish and parrotfish. Mar Ecol-Prog Ser 147:21–29
Valiela I (1984) Marine ecological processes. Springer, New York, NY
Van Duyl F, Gast G, Steinhoff W, Kloff S, Veldhuis M, Bak R (2002) Factors influencing the short-term variation in phytoplankton composition and biomass in coral reef waters. Coral Reefs 21:293–306
Van den Hoek C, Cortel-Breeman AM, Wanders JBW (1975) Algal zonation in the fringing coral reef of Curaçao, Netherlands antilles, in relation to zonation of corals and gorgonians. Aquat Bot 1:269–308
Vooren CM (1981) Photosynthetic rates of benthic algae from the deep coral reef of Curaçao. Aquat Bot 10:143–159
Wiebe WJ, Johannes RE, Webb KL (1975) Nitrogen fixation in a coral reef community. Science 188:257–259
Zhou JZ, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microb 62:316–322
Acknowledgements
Open access funding provided by Max Planck Society. This research was funded by the European Union Seventh Framework Programme (P7/2007–2013) under Grant Agreement No. 244161 (Future of Reefs in a Changing Environment). Financial support for genetic analyzes was provided by the PACES research program of the Alfred-Wegener-Institute Helmholtz-Zentrum für Polar- und Meeresforschung. MMN acknowledges support from the CNRS Chaire d’Excellence. We wish to thank the CARMABI foundation and the friendly staff for support during fieldwork, especially M. Vermeij, P. Stokkermans and C. Winterdaal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topic Editor Mark Vermeij
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brocke, H.J., Piltz, B., Herz, N. et al. Nitrogen fixation and diversity of benthic cyanobacterial mats on coral reefs in Curaçao. Coral Reefs 37, 861–874 (2018). https://doi.org/10.1007/s00338-018-1713-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1713-y