Abstract
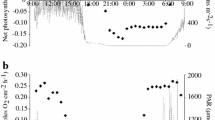
Although it is well established that different coral species have different susceptibilities to thermal stress, the reasons behind this variation are still unclear. In this study, 384 samples across five dominant coral species were collected seasonally between September 2013 and August 2014 at Luhuitou fringing reef in Sanya, Hainan Island, northern South China Sea, and their algal symbiont density and effective photochemical efficiency (Φ PSII) were measured. The results indicated that both the Symbiodinium density and Φ PSII of corals were subject to significant interspecies and seasonal variations. Stress-tolerant coral species, including massive Porites lutea and plating Pavona decussata, had higher symbiont densities but lower Φ PSII compared to the vulnerable branching species of Acropora over the course of all four seasons. Seasonally, coral symbiont densities were the lowest during winter, while during the same period, Φ PSII of corals was at the highest point. Further analysis suggested that dissolved inorganic nutrients and upwelling in the reef area were probably responsible for the observed seasonal variations in symbiont density. The fact that Porites lutea has the lowest Φ PSII during all four seasons is likely related to their symbionts’ lower capacity to provide required photosynthates for calcification. These results suggest that a coral’s thermal tolerance is primarily and positively dependent on its symbiont density and is less related to its effective photochemical efficiency.





Similar content being viewed by others
References
Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R (2013) Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci Rep 3:3486
Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct Ecol 23:539–550
Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2009) Coral bleaching: the role of the host. Trends Ecol Evol 24:16–20
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc Lond B Biol Sci 273:2305–2312
Brown BE, Dunne RP, Ambarsari I, Le Tissier MDA, Satapoomin U (1999) Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar Ecol Prog Ser 191:53–69
Browne NK, Tay JKL, Low J, Larson O, Todd PA (2015) Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Mar Environ Res 105:39–52
Cunning R, Baker AC (2013) Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:259–262
D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opinion Environ Sust 7:82–93
Dunn JG, Sammarco PW, LaFleur G Jr (2012) Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: a controlled experimental approach. J Exp Mar Bio Ecol 411:34–44
Fagoonee I, Wilson HB, Hassell MP, Turner JR (1999) The dynamics of zooxanthellae populations: a long-term study in the field. Science 283:843–845
Ferrier-Pagès C, Richard C, Forcioli D, Allemand D, Pichon M, Shick JM (2007) Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol Bull 213:76–87
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr 45:677–685
Furnas M, Mitchell A, Skuza M, Brodie J (2005) In the other 90%: phytoplankton responses to enhanced nutrient availability in the Great Barrier Reef Lagoon. Mar Pollut Bull 51:253–265
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12:1–17
Hill R, Takahashi S (2014) Photosystem II recovery in the presence and absence of chloroplast protein repair in the symbionts of corals exposed to bleaching conditions. Coral Reefs 33:1101–1111
Hinrichs S, Patten NL, Waite AM (2013) Temporal variations in metabolic and autotrophic indices for Acropora digitifera and Acropora spicifera — implications for monitoring projects. PLoS One 8:e63693
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoogenboom M, Beraud E, Ferrier-Pagès C (2010) Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29:21–29
Huang LM, Tan YH, Song XY, Huang XP, Wang HK, Zhang S, Dong JD, Chen RY (2003) The status of the ecological environment and a proposed protection strategy in Sanya Bay, Hainan Island, China. Mar Pollut Bull 47:180–186
Iluz D, Dubinsky Z (2015) Coral photobiology: new light on old views. Zoology 118:71–78
Jing ZY, Qi YQ, Hua ZL, Zhang H (2009) Numerical study on the summer upwelling system in the northern continental shelf of the South China Sea. Cont Shelf Res 29:467–478
Jones RJ, Ward S, Yang AA, Hoegh-Guldberg O (2000) Changes in quantum efficiency of photosystem II of symbiotic dinoflagellates of corals after heat stress, and of bleached corals sampled after the 1998 Great Barrier Reef mass bleaching event. Mar Freshw Res 50:839–866
Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW (2014) Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59:788–797
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
Lesser MP, Gorbunov MY (2001) Diurnal and bathymetric changes in chlorophyll fluorescence yields of reef corals measured in situ with a fast repetition rate fluorometer. Mar Ecol Prog Ser 212:69–77
Li S, Yu KF, Chen TR, Shi Q, Zhang HL (2011) Assessment of coral bleaching using symbiotic zooxanthellae density and satellite remote sensing data in the Nansha Islands, South China Sea. Chin Sci Bull 56:1031–1037
Li S, Yu K, Shi Q, Chen T, Zhao M, Zhao J (2008a) Interspecies and spatial diversity in the symbiotic zooxanthellae density in corals from northern South China Sea and its relationship to coral reef bleaching. Chin Sci Bull 53:295–303
Li S, Yu K, Shi Q, Chen T, Zhao M, Yan H (2008b) Experimental study of stony coral response to the high Temperature in Luhuitou of Hainan Island. Trop Geogr 28:534–539
Li XB, Liu S, Huang H, Huang LM, Jing ZY, Zhang CL (2012) Coral bleaching caused by an abnormal water temperature rise at Luhuitou fringing reef, Sanya Bay, China. Aquat Ecosyst Health Manag 15:227–233
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
McClanahan TR (2004) The relationship between bleaching and mortality of common corals. Mar Biol 144:1239–1245
Nir O, Gruber DF, Einbinder S, Kark S, Tchernov D (2011) Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30:1089–1100
Piniak GA, Brown EK (2009) Temporal variability in chlorophyll fluorescence of back-reef corals in Ofu, American Samoa. Biol Bull 216:55–67
Rodolfo-Metalpa R, Reynaud S, Allemand D, Ferrier-Pagès C (2008) Temporal and depth responses of two temperate corals, Cladocora caespitosa and Oculina patagonica, from the north Mediterranean Sea. Mar Ecol Prog Ser 369:103–114
Rosic NN, Braun C, Kvaskoff D (2015) Extraction and analysis of mycosporine-like amino acids in marine algae. Methods Mol Biol 1308:119–129
Rowan R (2004) Coral bleaching—thermal adaptation in reef coral symbionts. Nature 430:742–742
Sawall Y, Al-Sofyani A, Banguera-Hinestroza E, Voolstra CR (2014) Spatio-temporal analyses of Symbiodinium physiology of the coral Pocillopora verrucosa along large-scale nutrient and temperature gradients in the Red Sea. PLoS One 9:e103179
Shearer TL, Rasher DB, Snell TW, Hay ME (2012) Gene expression patterns of the coral Acropora millepora in response to contact with macroalgae. Coral Reefs 31:1177–1192
Singh SP, Kumari S, Rastogi RP, Singh KL, Sinha RP (2008) Mycosporine-like amino acids (MAAS): chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J Exp Biol 46:7–17
Smith DJ, Suggett DJ, Baker NR (2005) Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Glob Chang Biol 11:1–11
Stimson J (1997) The annual cycle of density of zooxanthellae in the tissues of field and laboratory-held Pocillopora damicornis (Linnaeus). J Exp Mar Bio Ecol 214:35–48
Stimson J, Sakai K, Sembali H (2002) Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21:409–421
Szmant A, Forrester A (1996) Water column and sediment nitrogen and phosphorus distribution patterns in the Florida Keys, USA. Coral Reefs 15:21–41
Thompson DM, van Woesik R (2009) Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc R Soc Lond B Biol Sci 276:2893–2901
Ulstrup KE, Hill R, van Oppen MJH, Larkum AWD, Ralph PJ (2008) Seasonal variation in the photo-physiology of homogeneous and heterogeneous Symbiodinium consortia in two scleractinian corals. Mar Ecol Prog Ser 361:139–150
Wang HK, Dong JD, Wang YS, Chen GH, Zhang YY (2005) Variations of nutrient contents and their transportation estimate at Sanya Bay. J Trop Oceanogr 24:90–95
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of coral reef: a novel approach. Plant Cell Environ 19:291–299
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci U S A 96:8007–8012
Wicks LC, Hill R, Davy SK (2010) The influence of irradiance on tolerance to high and low temperature stress exhibited by Symbiodinium in the coral, Pocillopora damicornis, from the high-latitude reef of Lord Howe Island. Limnol Oceanogr 55:2476–2486
Wiedenmann J, D’Angelo C, Smith EG, Hunt AN, Legiret FE, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:160–164
Wooldridge SA (2009) A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Mar Freshw Res 60:483–496
Wooldridge SA (2014) Differential thermal bleaching susceptibilities amongst coral taxa: re-posing the role of the host. Coral Reefs 33:15–27
Yan HQ, Yu KF, Shi Q, Tan YH, Liu GH, Zhao MX, Li S, Chen TR, Wang YH (2016) Seasonal variations of seawater pCO2 and sea- air CO2 fluxes in a fringing coral reef, northern South China Sea. J Geophys Res Oceans 121:998–1008
Yu KF (2012) Coral reefs in the South China Sea: their responses to and records on past environmental changes. Science China Earth Sciences 55:1217–1229
Yu KF, Zhao JX, Lawrence MG, Feng YX (2010) Timing and duration of growth hiatuses in mid Holocene massive Porites corals from the northern South China Sea. J Quat Sci 25:1284–1292
Zhao MX, Yu KF, Zhang QM, Shi Q, Price GJ (2012) Long-term decline of a fringing coral reef in the northern South China Sea. J Coast Res 28:1088–1099
Zhao MX, Yu KF, Zhang QM, Shi Q, Roff G (2014) Age structure of massive Porites lutea corals at Luhuitou fringing reef (northern South China Sea) indicates recovery following severe anthropogenic disturbance. Coral Reefs 33:39–44
Acknowledgements
This work was funded by the National Key Basic Research Program of China (Nos. 2013CB956102 and 2013CB956103), the Natural Science Foundation of China (Nos. 91428203 and 41025007) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA05080301). We thank two anonymous reviewers for constructive comments and Hainian Yu from Brisbane Boys College for English writing improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Anastazia Banaszak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, L., Yu, K., Li, S. et al. Interseasonal and interspecies diversities of Symbiodinium density and effective photochemical efficiency in five dominant reef coral species from Luhuitou fringing reef, northern South China Sea. Coral Reefs 36, 477–487 (2017). https://doi.org/10.1007/s00338-016-1532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-016-1532-y