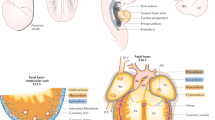
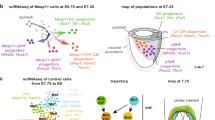
Abstract
In 2001, three independent groups reported the identification of a novel cluster of progenitor cells that contribute to heart development in mouse and chicken embryos. This population of progenitor cells was designated as the second heart field (SHF), and a new research direction in heart development was launched. Twenty years have since passed and a comprehensive understanding of the SHF has been achieved. This review provides retrospective insights in to the contribution, the signaling regulatory networks and the epithelial properties of the SHF. It also includes the spatiotemporal characteristics of SHF development and interactions between the SHF and other types of cells during heart development. Although considerable efforts will be required to investigate the cellular heterogeneity of the SHF, together with its intricate regulatory networks and undefined mechanisms, it is expected that the burgeoning new technology of single-cell sequencing and precise lineage tracing will advance the comprehension of SHF function and its molecular signals. The advances in SHF research will translate to clinical applications and to the treatment of congenital heart diseases, especially conotruncal defects, as well as to regenerative medicine.



Similar content being viewed by others
References
Anderson HE, Christiaen L (2016) Ciona as a simple chordate model for heart development and regeneration. J Cardiovasc Dev Dis 3(3):25
Andersen P, Tampakakis E, Jimenez DV, Kannan S, Miyamoto M, Shin HK, Saberi A, Murphy S, Sulistio E, Chelko SP et al (2018) Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat Commun 9(1):3140
Bagatto B, Francl J, Liu B, Liu Q (2006) Cadherin2 (N-cadherin) plays an essential role in zebrafish cardiovascular development. BMC Dev Biol 6:23
Baldini A, Fulcoli FG, Illingworth E (2017) Tbx1: transcriptional and developmental functions. Curr Top Dev Biol 122:223–243
Bertrand N, Roux M, Ryckebüsch L, Niederreither K, Dollé P, Moon A, Capecchi M, Zaffran S (2011) Hox genes define distinct progenitor sub-domains within the second heart field. Dev Biol 353(2):266–274
Briggs LE, Kakarla J, Wessels A (2012) The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation 84(1):117–130
Briggs LE, Phelps AL, Brown E, Kakarla J, Anderson RH, van den Hoff MJ, Wessels A (2013) Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ Res 112(11):1420–1432
Bryant DM, Mostov KE (2008) From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9(11):887–901
Buckingham M, Meilhac S, Zaffran S (2005) Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 6(11):826–835
Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S (2003) Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5(6):877–889
Caputo L, Witzel HR, Kolovos P, Cheedipudi S, Looso M, Mylona A, van Ijcken WF, Laugwitz KL, Evans SM, Braun T et al (2015) The Isl1/Ldb1 complex orchestrates genome-wide chromatin organization to instruct differentiation of multipotent cardiac progenitors. Cell Stem Cell 17(3):287–99
Catón J, Luder HU, Zoupa M, Bradman M, Bluteau G, Tucker AS, Klein O, Mitsiadis TA (2009) Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev Biol 328(2):493–505
Chen L, Fulcoli FG, Tang S, Baldini A (2009) Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res 105(9):842–851
Chen L, Fulcoli FG, Ferrentino R, Martucciello S, Illingworth EA, Baldini A (2012) Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet 8(3):e1002571
Chiapparo G, Lin X, Lescroart F, Chabab S, Paulissen C, Pitisci L, Bondue A, Blanpain C (2016) Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration. J Cell Biol 213(4):463–477
Choudhry P, Trede NS (2013) DiGeorge syndrome gene tbx1 functions through wnt11r to regulate heart looping and differentiation. PLoS ONE 8(3):e58145
Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP et al (2000) Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol 223(2):266–278
Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AF et al (2006) Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ Res 98(12):1555–1563
Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE (2007) Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest 117(7):1794–1804
Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE (2012) Wnt5a and Wnt11 are essential for second heart field progenitor development. Development 139(11):1931–1940
Colombo S, de Sena-Tomás C, George V, Werdich AA, Kapur S, MacRae CA, Targoff KL (2018) Nkx genes establish second heart field cardiomyocyte progenitors at the arterial pole and pattern the venous pole through Isl1 repression. Development 145(3):dev161497
Cota CD, Davidson B (2015) Mitotic membrane turnover coordinates differential induction of the heart progenitor lineage. Dev Cell 34(5):505–519
de la Cruz MV, Sánchez Gómez C, Arteaga MM, Argüello C (1977) Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123(Pt 3):661–686
de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J (2009) Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136(10):1633–1641
DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, Kathiriya IS, Hinson JT, Homsy J, Gray J, Pu W et al (2016) Single-cell resolution of temporal gene expression during heart development. Dev Cell 39(4):480–490
Desgrange A, Le Garrec JF, Bernheim S, Bønnelykke TH, Meilhac SM (2020) Transient nodal signaling in left precursors coordinates opposed asymmetries shaping the heart loop. Dev Cell 55(4):413-431.e6
Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG (2014) Early patterning and specification of cardiac progenitors in gastrulating mesoderm. Elife 3:e03848
Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor E (2015) A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520(7548):466–473
Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL (2004) Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131(16):3931–3942
Domínguez JN, Meilhac SM, Bland YS, Buckingham ME, Brown NA (2012) Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart. Circ Res 111(10):1323–1335
Dorn T, Goedel A, Lam JT, Haas J, Tian Q, Herrmann F, Bundschu K, Dobreva G, Schiemann M, Dirschinger R et al (2015) Direct nkx2-5 transcriptional repression of isl1 controls cardiomyocyte subtype identity. Stem Cells 33(4):1113–1129
Durocher D, Charron F, Warren R, Schwartz RJ, Nemer M (1997) The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. Embo j 16(18):5687–5696
Dyer LA, Kirby ML (2009a) The role of secondary heart field in cardiac development. Dev Biol 336(2):137–144
Dyer LA, Kirby ML (2009b) Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol 330(2):305–317
Engleka KA, Manderfield LJ, Brust RD, Li L, Cohen A, Dymecki SM, Epstein JA (2012) Islet1 derivatives in the heart are of both neural crest and second heart field origin. Circ Res 110(7):922–926
Epstein JA, Franklin H. Epstein Lecture (2010) Cardiac development and implications for heart disease. N Engl J Med 363(17):1638–1647
Francou A, Saint-Michel E, Mesbah K, Kelly RG (2014) TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field. Development 141(22):4320–4331
Francou A, De Bono C, Kelly RG (2017) Epithelial tension in the second heart field promotes mouse heart tube elongation. Nat Commun 8:14770
Fujii M, Sakaguchi A, Kamata R, Nagao M, Kikuchi Y, Evans SM, Yoshizumi M, Shimono A, Saga Y, Kokubo H (2017) Sfrp5 identifies murine cardiac progenitors for all myocardial structures except for the right ventricle. Nat Commun 8:14664
Fulcoli FG, Franzese M, Liu X, Zhang Z, Angelini C, Baldini A (2016) Rebalancing gene haploinsufficiency in vivo by targeting chromatin. Nat Commun 7:11688
Furtado MB, Biben C, Shiratori H, Hamada H, Harvey RP (2011) Characterization of Pitx2c expression in the mouse heart using a reporter transgene. Dev Dyn 240(1):195–203
Galli D, Domínguez JN, Zaffran S, Munk A, Brown NA, Buckingham ME (2008) Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 135(6):1157–1167
Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D (2001) Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol 235(1):62–73
Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K et al (2003) GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424(6947):443–447
Gaspar P, Tapon N (2014) Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol 31:74–83
Gessert S, Kühl M (2009) Comparative gene expression analysis and fate mapping studies suggest an early segregation of cardiogenic lineages in Xenopus laevis. Dev Biol 334(2):395–408
Gibbs BC, Damerla RR, Vladar EK, Chatterjee B, Wan Y, Liu X, Cui C, Gabriel GC, Zahid M, Yagi H et al (2016) Prickle1 mutation causes planar cell polarity and directional cell migration defects associated with cardiac outflow tract anomalies and other structural birth defects. Biol Open 5(3):323–335
Gibson WT, Veldhuis JH, Rubinstein B, Cartwright HN, Perrimon N, Brodland GW, Nagpal R, Gibson MC (2011) Control of the mitotic cleavage plane by local epithelial topology. Cell 144(3):427–438
Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J (2008) Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development 135(10):1887–1895
Grifone R, Kelly RG (2007) Heartening news for head muscle development. Trends Genet 23(8):365–369
Guo C, Sun Y, Zhou B, Adam RM, Li X, Pu WT, Morrow BE, Moon A, Li X (2011) A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J Clin Invest 121(4):1585–1595
Guris DL, Duester G, Papaioannou VE, Imamoto A (2006) Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell 10(1):81–92
Hami D, Grimes AC, Tsai HJ, Kirby ML (2011) Zebrafish cardiac development requires a conserved secondary heart field. Development 138(11):2389–2398
Harvey RP (2002) Patterning the vertebrate heart. Nat Rev Genet 3(7):544–556
He Z, Grunewald M, Dor Y, Keshet E (2016) VEGF regulates relative allocation of Isl1(+) cardiac progenitors to myocardial and endocardial lineages. Mech Dev 142:40–49
Heisenberg CP, Bellaïche Y (2013) Forces in tissue morphogenesis and patterning. Cell 153(5):948–962
High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA (2009) Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest 119(7):1986–1996
Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP (2009) sonic hedgehog is required in pulmonary endoderm for atrial septation. Development 136(10):1761–1770
Hoffmann AD, Yang XH, Burnicka-Turek O, Bosman JD, Ren X, Steimle JD, Vokes SA, McMahon AP, Kalinichenko VV, Moskowitz IP (2014) Foxf genes integrate tbx5 and hedgehog pathways in the second heart field for cardiac septation. PLoS Genet 10(10):e1004604
Hutson MR, Zeng XL, Kim AJ, Antoon E, Harward S, Kirby ML (2010) Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development 137(18):3001–3011
Huynh T, Chen L, Terrell P, Baldini A (2007) A fate map of Tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis 45(7):470–475
Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN (2006) Fgf8 is required for anterior heart field development. Development 133(12):2435–2445
Ivanovitch K, Temiño S, Torres M (2017) Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. Elife 6:e30668
Jahangiri L, Sharpe M, Novikov N, González-Rosa JM, Borikova A, Nevis K, Paffett-Lugassy N, Zhao L, Adams M, Guner-Ataman B et al (2016) The AP-1 transcription factor component Fosl2 potentiates the rate of myocardial differentiation from the zebrafish second heart field. Development 143(1):113–122
Jain R, Li D, Gupta M, Manderfield LJ, Ifkovits JL, Wang Q, Liu F, Liu Y, Poleshko A, Padmanabhan A et al (2015) HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science 348(6242):aaa6071
Jin H, Wang H, Li J, Yu S, Xu M, Qiu Z, Xia M, Zhu J, Feng Q, Xie J et al (2019) Differential contribution of the two waves of cardiac progenitors and their derivatives to aorta and pulmonary artery. Dev Biol 450(2):82–89
Kang J, Nathan E, Xu SM, Tzahor E, Black BL (2009) Isl1 is a direct transcriptional target of Forkhead transcription factors in second-heart-field-derived mesoderm. Dev Biol 334(2):513–522
Kappen C, Salbaum JM (2009) Identification of regulatory elements in the Isl1 gene locus. Int J Dev Biol 53(7):935–946
Kelly RG (2012) The second heart field. Curr Top Dev Biol 100:33–65
Kelly RG, Buckingham ME (2002) The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet 18(4):210–216
Kelly RG, Papaioannou VE (2007) Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene. Dev Dyn 236(3):821–828
Kelly RG, Brown NA, Buckingham ME (2001) The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1(3):435–440
Kelly RG, Buckingham ME, Moorman AF (2014) Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med 4(10):a015750
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S et al (2013) Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152(3):570–583
Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D (2009) A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol 11(8):951–957
Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M et al (2005) Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433(7026):647–653
Lazic S, Scott IC (2011) Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev Biol 354(1):123–133
Le Garrec JF, Domínguez JN, Desgrange A, Ivanovitch KD, Raphaël E, Bangham JA, Torres M, Coen E, Mohun TJ, Meilhac SM (2017) A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. Elife 6:e28951
Lescroart F, Kelly RG, Le Garrec JF, Nicolas JF, Meilhac SM, Buckingham M (2010) Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137(19):3269–3279
Lescroart F, Mohun T, Meilhac SM, Bennett M, Buckingham M (2012) Lineage tree for the venous pole of the heart: clonal analysis clarifies controversial genealogy based on genetic tracing. Circ Res 111(10):1313–1322
Lescroart F, Chabab S, Lin X, Rulands S, Paulissen C, Rodolosse A, Auer H, Achouri Y, Dubois C, Bondue A et al (2014) Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol 16(9):829–840
Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sànchez-Dànes A, Moignard V, Dubois C, Paulissen C, Kinston S et al (2018) Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359(6380):1177–1181
Li P, Pashmforoush M, Sucov HM (2010) Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell 18(3):480–485
Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR et al (2015) Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 521(7553):520–524
Li D, Sinha T, Ajima R, Seo HS, Yamaguchi TP, Wang J (2016a) Spatial regulation of cell cohesion by Wnt5a during second heart field progenitor deployment. Dev Biol 412(1):18–31
Li G, Xu A, Sim S, Priest JR, Tian X, Khan T, Quertermous T, Zhou B, Tsao PS, Quake SR et al (2016b) Transcriptomic profiling maps anatomically patterned subpopulations among single embryonic cardiac cells. Dev Cell 39(4):491–507
Liao J, Aggarwal VS, Nowotschin S, Bondarev A, Lipner S, Morrow BE (2008) Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev Biol 316(2):524–537
Liberatore CM, Searcy-Schrick RD, Vincent EB, Yutzey KE (2002) Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev Biol 244(2):243–256
Lien CL, McAnally J, Richardson JA, Olson EN (2002) Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol 244(2):257–266
Lin L, Bu L, Cai CL, Zhang X, Evans S (2006) Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol 295(2):756–763
Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R et al (2007) Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA 104(22):9313–9318
Lin CY, Lin CJ, Chen CH, Chen RM, Zhou B, Chang CP (2012) The secondary heart field is a new site of calcineurin/Nfatc1 signaling for semilunar valve development. J Mol Cell Cardiol 52(5):1096–1102
Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM (2005) Cardiac morphogenesis: matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn 233(3):739–753
Lioux G, Liu X, Temiño S, Oxendine M, Ayala E, Ortega S, Kelly RG, Oliver G, Torres M (2020) A second heart field-derived vasculogenic niche contributes to cardiac lymphatics. Dev Cell 52(3):350-363.e6
Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF (2002) Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129(21):5081–5091
Luo W, Zhao X, Jin H, Tao L, Zhu J, Wang H, Hemmings BA, Yang Z (2015) Akt1 signaling coordinates BMP signaling and β-catenin activity to regulate second heart field progenitor development. Development 142(4):732–742
Ma Q, Zhou B, Pu WT (2008) Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol 323(1):98–104
Marguerie A, Bajolle F, Zaffran S, Brown NA, Dickson C, Buckingham ME, Kelly RG (2006) Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovasc Res 71(1):50–60
Meilhac SM, Buckingham ME (2018) The deployment of cell lineages that form the mammalian heart. Nat Rev Cardiol 15(11):705–724
Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME (2004) The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6(5):685–698
Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR (2001) The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 238(1):97–109
Mommersteeg MT, Domínguez JN, Wiese C, Norden J, de Gier-de Vries C, Burch JB, Kispert A, Brown NA, Moorman AF, Christoffels VM (2010) The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc Res 87(1):92–101
Nachury MV (2014) How do cilia organize signalling cascades? Philos Trans R Soc Lond B Biol Sci 369(1650):20130465
Pandur P, Läsche M, Eisenberg LM, Kühl M (2002) Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418(6898):636–641
Pane LS, Zhang Z, Ferrentino R, Huynh T, Cutillo L, Baldini A (2012) Tbx1 is a negative modulator of Mef2c. Hum Mol Genet 21(11):2485–2496
Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM (2006) Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133(12):2419–2433
Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H et al (2007) An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128(5):947–959
Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO (1997) Developmental defects in mouse embryos lacking N-cadherin. Dev Biol 181(1):64–78
Ramsbottom SA, Sharma V, Rhee HJ, Eley L, Phillips HM, Rigby HF, Dean C, Chaudhry B, Henderson DJ (2014) Vangl2-regulated polarisation of second heart field-derived cells is required for outflow tract lengthening during cardiac development. PLoS Genet 10(12):e1004871
Rana MS, Théveniau-Ruissy M, De Bono C, Mesbah K, Francou A, Rammah M, Domínguez JN, Roux M, Laforest B, Anderson RH et al (2014) Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart. Circ Res 115(9):790–799
Rawles ME (1943) The heart-forming areas of the early chick blastoderm. Physiol Zool 16:22–43
Reuter MS, Chaturvedi RR, Liston E, Manshaei R, Aul RB, Bowdin S, Cohn I, Curtis M, Dhir P, Hayeems RZ et al (2020) The cardiac genome clinic: implementing genome sequencing in pediatric heart disease. Genet Med 22(6):1015–1024
Roberts C, Ivins SM, James CT, Scambler PJ (2005) Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev Dyn 232(4):928–938
Rochais F, Mesbah K, Kelly RG (2009) Signaling pathways controlling second heart field development. Circ Res 104(8):933–942
Rones MS, McLaughlin KA, Raffin M, Mercola M (2000) Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127(17):3865–3876
Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K (2008) Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci USA 105(8):2913–2918
Ryckebüsch L, Bertrand N, Mesbah K, Bajolle F, Niederreither K, Kelly RG, Zaffran S (2010) Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of DiGeorge syndrome. Circ Res 106(4):686–694
Rydeen AB, Waxman JS (2016) Cyp26 enzymes facilitate second heart field progenitor addition and maintenance of ventricular integrity. PLoS Biol 14(11):e2000504
Seo S, Kume T (2006) Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol 296(2):421–436
Shenje LT, Andersen P, Uosaki H, Fernandez L, Rainer PP, Cho GS, Lee DI, Zhong W, Harvey RP, Kass DA et al (2014) Precardiac deletion of Numb and Numblike reveals renewal of cardiac progenitors. Elife 3:e02164
Shinohara K, Hamada H (2017) Cilia in left-right symmetry breaking. Cold Spring Harb Perspect Biol 9(10):a028282
Sirbu IO, Zhao X, Duester G (2008) Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn 237(6):1627–1635
Snarr BS, O’Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A (2007a) Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res 101(10):971–974
Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A (2007b) A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn 236(5):1287–1294
Soh BS, Buac K, Xu H, Li E, Ng SY, Wu H, Chmielowiec J, Jiang X, Bu L, Li RA et al (2014) N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res 24(12):1420–1432
Song YC, Dohn TE, Rydeen AB, Nechiporuk AV, Waxman JS (2019) HDAC1-mediated repression of the retinoic acid-responsive gene ripply3 promotes second heart field development. PLoS Genet 15(5):e1008165
Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP (2002) Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3’UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int J Dev Biol 46(4):431–439
Stolfi A, Gainous TB, Young JJ, Mori A, Levine M, Christiaen L (2010) Early chordate origins of the vertebrate second heart field. Science 329(5991):565–568
Takeichi M (2014) Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15(6):397–410
Takeuchi JK, Bruneau BG (2009) Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459(7247):708–711
Théveniau-Ruissy M, Dandonneau M, Mesbah K, Ghez O, Mattei MG, Miquerol L, Kelly RG (2008) The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circ Res 103(2):142–148
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED et al (2010) Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell 18(2):275–287
Tirosh-Finkel L, Zeisel A, Brodt-Ivenshitz M, Shamai A, Yao Z, Seger R, Domany E, Tzahor E (2010) BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development 137(18):2989–3000
Toomer KA, Fulmer D, Guo L, Drohan A, Peterson N, Swanson P, Brooks B, Mukherjee R, Body S, Lipschutz JH et al (2017) A role for primary cilia in aortic valve development and disease. Dev Dyn 246(8):625–634
van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF (2009) A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res 104(2):179–188
Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE (2006) Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn 235(1):19–28
Van Praagh R (2009) The first Stella van Praagh memorial lecture: the history and anatomy of tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 12(1):19–38
Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL (2005) The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 287(1):134–145
Vincent SD, Buckingham ME (2010) How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol 90:1–41
von Both I, Silvestri C, Erdemir T, Lickert H, Walls JR, Henkelman RM, Rossant J, Harvey RP, Attisano L, Wrana JL (2004) Foxh1 is essential for development of the anterior heart field. Dev Cell 7(3):331–345
Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML (2001) Conotruncal myocardium arises from a secondary heart field. Development 128(16):3179–3188
Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML (2005a) Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol 281(1):66–77
Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML (2005b) Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol 281(1):78–90
Wang W, Razy-Krajka F, Siu E, Ketcham A, Christiaen L (2013) NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol 11(12):e1001725
Watanabe Y, Miyagawa-Tomita S, Vincent SD, Kelly RG, Moon AM, Buckingham ME (2010) Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries. Circ Res 106(3):495–503
Watanabe Y, Zaffran S, Kuroiwa A, Higuchi H, Ogura T, Harvey RP, Kelly RG, Buckingham M (2012) Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc Natl Acad Sci USA 109(45):18273–18280
Witzel HR, Jungblut B, Choe CP, Crump JG, Braun T, Dobreva G (2012) The LIM protein Ajuba restricts the second heart field progenitor pool by regulating Isl1 activity. Dev Cell 23(1):58–70
Wyatt TP, Harris AR, Lam M, Cheng Q, Bellis J, Dimitracopoulos A, Kabla AJ, Charras GT, Baum B (2015) Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc Natl Acad Sci USA 112(18):5726–5731
Xia M, Luo W, Jin H, Yang Z (2019) HAND2-mediated epithelial maintenance and integrity in cardiac outflow tract morphogenesis. Development 146(13):dev177477
Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP (2012) Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell 23(2):280–291
Xue Z, Hennelly S, Doyle B, Gulati AA, Novikova IV, Sanbonmatsu KY, Boyer LA (2016) A G-Rich Motif in the lncRNA braveheart interacts with a Zinc-Finger transcription factor to specify the cardiovascular lineage. Mol Cell 64(1):37–50
Yuan X, Qi H, Li X, Wu F, Fang J, Bober E, Dobreva G, Zhou Y, Braun T (2017) Disruption of spatiotemporal hypoxic signaling causes congenital heart disease in mice. J Clin Invest 127(6):2235–2248
Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA (2004) Right ventricular myocardium derives from the anterior heart field. Circ Res 95(3):261–268
Zeng XX, Yelon D (2014) Cadm4 restricts the production of cardiac outflow tract progenitor cells. Cell Rep 7(4):951–960
Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, Schmuck EG, Raval AN, da Rocha AM, Herron TJ et al (2019) Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat Commun 10(1):2238
Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE et al (2011) Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474(7353):645–648
Zhou L, Liu J, Olson P, Zhang K, Wynne J, Xie L (2015) Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol 85:1–12
Zhou L, Liu J, Xiang M, Olson P, Guzzetta A, Zhang K, Moskowitz IP, Xie L (2017a) Gata4 potentiates second heart field proliferation and Hedgehog signaling for cardiac septation. Proc Natl Acad Sci USA 114(8):E1422-e1431
Zhou Z, Wang J, Guo C, Chang W, Zhuang J, Zhu P, Li X (2017b) Temporally distinct six2-positive second heart field progenitors regulate mammalian heart development and disease. Cell Rep 18(4):1019–1032
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31930029 and 91854111) and from the National Key Research and Development Program of China (2019YFA0801601) to Zhongzhou Yang
Author information
Authors and Affiliations
Contributions
KZ. wrote the draft and ZY. corrected and edited.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, K., Yang, Z. The second heart field: the first 20 years. Mamm Genome 34, 216–228 (2023). https://doi.org/10.1007/s00335-022-09975-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-022-09975-8