Abstract
A recently reported deletion of about 40 Mb in length between 6.12/6.57 and 46.73/47.31 Mb on the Y chromosome long arm of the C57BL/6JBomTac inbred strain made us closely examine the strain’s breeding history and reproductive characteristics. We verified that the two copies of Rbm31y that are present inside the putative deletion were indeed deleted. This inbred strain presents an expected litter size for a C57BL/6 substrain. In vitro fertilization (IVF) efficiency and breeding efficiencies are comparable to those of the C57BL/6NTac substrain; however, the male/female sex ratio in the C57BL/6JBomTac is mildly skewed towards females. There is an increase in the percentage of sperm shape abnormalities found in C57BL/6JBomTac (35%) versus C57BL/6NTac (11%). The most frequent type of sperm abnormality observed is bent heads (19%). Additionally, there is deregulation of several transcripts expressed in the testes. We determined that this mutation arose in the C57BL/6JBomTac Foundation Colony in 2008, and it was completely fixed in the colony by 2009.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Y chromosome in mammals is quite unique; it is the smallest of the chromosomes and is found only in males. In the mouse, the Pseudo Autosomal Region (PAR) is found at the tip of the Yq long arm and it spans about 700 Kb. This is the site of obligatory pairing and recombination between the X and Y chromosomes during male meiosis (Perry et al. 2001). The rest of the Yq chromosome is the non-pairing Yq region (NPYq) or male-specific Y chromosome (MSY) region that is thought to be involved in spermatogenesis and fertility (Burgoyne et al. 1992; Burgoyne 1998). The mouse MSY is quite large (89.6 Mb) when compared to human (22.8 Mb), chimpanzee (25.8 Mb), and rhesus (11 Mb) (Soh et al. 2014). The sequencing of the mouse MSY was an endeavor that took 12 years. It corroborated previous studies that showed several MSY genes present in multiple copies in the order of hundreds (Conway et al. 1994) and other studies that showed many of these genes to be amplified en bloc and transcribed bidirectionally (Ellis et al. 2007). Additionally, these studies showed that these genes are mostly expressed in the testes (Toure et al. 2005; Cocquet et al. 2009; Ellis et al. 2011). The mouse sequencing project provided the detailed architecture of the ampliconic 86.4 Mb of MSY sequence which consists of a 0.5 Mb unit amplified 200 times (Soh et al. 2014).
GVG Genetic Monitoring informed us of a 40 Mb deletion in the C57BL/6JBomTac inbred strain detected using short tandem repeat (STR) analysis (Fischer et al. 2016). This deletion represents 46% of the MSY. In this study, we verify a deletion in the Y chromosome of C57BL/6JBomTac mice, and we evaluate the effects of this deletion on the reproductive characteristics of this inbred strain. Another C57BL/6 substrain, C57BL/6NTac, was used as a comparator for many of the experiments described.
Materials and methods
Mouse strains
C57BL/6JBomTac (B6JBom) and C57BL/6NTac (B6NTac) male mice at 11–24 weeks old, and B6JBom and B6NTac female mice at 3–4 weeks old, all from Taconic Biosciences were used in all experiments. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
DNA and RNA isolation, cDNA synthesis, and qPCR
Mice were euthanized according to the protocols approved by the IACUC. The testes were removed and total RNA was extracted using the Qiagen RNeasy-Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Genomic DNA was extracted from tail biopsies using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol.
For real-time reverse transcriptase polymerase chain reaction (RT PCR), total testis RNA was extracted and amplification of cDNA was performed using 1 µg of purified RNA with anchored oligo-dT primers (NEB, Ipswich, MA) using M-MuLV reverse transcriptase (NEB, Ipswich, MA), following the manufacturer’s recommendations.
List of primers used for genomic PCR amplification and gene expression is provided as Table S1 (Online Resource). Gene expression was analyzed using the 2ΔΔCt method. All genes were normalized to β-actin and the expression level for each gene is presented as the ratio of B6JBom divided by B6NTac. The threshold for statistical significance was set at p-value of 0.05 and was generated using Microsoft Excel 2-tailed Student’s t-test.
Mouse breeding performance and female/male sex ratio
Mice were bred in Taconic Isolated Barrier Units and were fed NIH #31M Rodent Diet. Breeding performance indicators such as production efficiency index (PEI), which is the measure of the total number of pups weaned per breeding female, and litter size were collected on a weekly basis. Female and male sex ratios were calculated by the proportion of males and females produced by each strain.
Assessment of sperm morphology
The cauda epididymis from each male was dissected and placed into a dish containing a 120 µl drop of sperm freezing media (R183S) under oil at room temperature. The epididymis was then cut with fine scissors and gently pressed to release the sperm. Using fine forceps, the sperm clot was pulled into the freezing medium drop and allowed to disperse for a few minutes. An aliquot of sperm was mixed with Eosin-Nigrosin stain (Newcomer supply, Middleton, WI, USA) following the manufacturer’s recommendations, and smears were made on clean glass slides. The morphology of the sperm was assessed using light microscopy at 1000× magnification. Sperm morphology analysis was performed for at least three males from each strain, and a minimum 150 spermatozoa were analyzed per male. Sperm with any deviation from normal morphology was counted as abnormal. Sperm head abnormalities were classified as slight or gross abnormalities (Yamauchi et al. 2009). Microsoft Excel was used to calculate standard deviations and statistical significance set at p-value of 0.05 using 2-tailed Student’s t-test.
In vitro fertilization
In vitro fertilization was performed following the protocol described by Takeo and Nakagata (2010). Briefly, pre-pubertal female mice were superovulated by intraperitoneal injections of 5 IU of equine chorionic gonadotropin (eCG), followed by human chorionic gonadotropin (hCGn) at 48 h interval. Oviducts were collected at 14–15 h post hCG injection, and transferred into a dish containing a 90 µl drop of FERTIUP fertilization medium (Cosmo Bio USA, Carlsbad, CA) under oil. Using a fine forceps, cumulus-oocyte complex (COC) was pulled from ampulla of oviducts into the fertilization drop and incubated at 37 °C for 30 min. Cauda epididymal sperm was placed into a 100 µl drop of FERTIUP sperm pre-incubation medium (Cosmo Bio USA, Carlsbad, CA), and incubated at 37 °C for 60 min. After 60 min, actively motile sperm from the periphery of drop was collected and added to the oocytes in the fertilization drop and co-incubated for 3 h. Then the oocytes were washed of excess sperm and cultured overnight for development to 2-cell embryos.
Single-nucleotide polymorphism (SNP) analysis
Tail biopsies were sent to the University of North Carolina, Systems Genetics Core for GigaMuga SNP analysis (Morgan et al. 2016). 143,259 SNP probes were tested along the mouse genome, with 83 SNPs in the Y chromosome. SNP genotyping was done at Taconic’s Molecular Biology Laboratory using various SNP marker panels developed by Taconic, such as 96 SNP marker GenMon panel or 1449 SNP marker panel using Illumina’s platform (Illumina, Inc., San Diego, CA), following the manufacturer’s protocols. SNP analysis was done using Genome Studio Software (Illumina, Inc, San Diego, CA).
Short tandem repeat (STR) analysis
Tail biopsies were sent to GVG Genetic Monitoring (Leipzig, Germany) to be tested by a panel of 246 STR markers along all mouse chromosomes, 18 of them on the Y chromosome. List of STR markers and their Y chromosome position used by GVG are provided on Table S2 (Online Resource).
Multiparametric flow cytometry analysis on spleen and bone marrow
Mice were sent to Maine Medical Research Institute for analysis. Mice were euthanized and spleens were dissected onto a petri dish. Each spleen was minced using a scalpel and small scissors. Five milliliters of RPMI media were added to the minced tissue and then transferred into a syringe with a 70 µm filter. Cells that passed through the filter were collected into a 50 ml conical tube. The filter was washed with additional RPMI and cells were pooled with first pass-through. The cell suspension was spun at 500 g for 5 min. The supernatant was discarded and cells were resuspended in 10 ml of FACS buffer (PBS/0.5%BSA/2 mM EDTA). Cells were passed once more through a 70 µm filter. Cells were counted and diluted into a single cell suspension of 1 × 107 cells/ml. Splenocytes were placed on ice until flow cytometry commenced.
For bone marrow isolation, hind legs of euthanized mice were removed and femurs and tibia were dissected from surrounding tissue using sterile scissors exposing the interior marrow shaft. The contents were flushed from the marrow with RPMI using a 5 ml syringe and a 27G × 1/2 needle and collected into a 50 ml conical tube and filtered through a 70 µm filter. Contents were spun at 500 g for 5 min, the supernatant was discarded and cells were resuspended in 10 ml of FACS buffer (PBS/0.5%BSA/2 mM EDTA). Cells were counted and diluted into a single cell suspension of 1 × 107 cells/ml. Bone marrow cells were placed on ice until flow cytometry commenced. P values were calculated using unpaired t test.
Bone marrow and splenic cell suspensions were used to analyze subpopulations of myeloid cells and lymphocytes. The total number of viable cells was also determined. Cells were treated with TrueStain fcX (Biolegend, San Diego, CA) to prevent non-specific binding. Cells are then stained with antibodies against specific cell surface markers. Multiparametric flow cytometry analysis was performed using a combination of antibodies (see Table S3 Online Resource). DAPI was used to exclude dead cells. At least 10,000 events were collected using MACSQuant 10 analyzer (Miltenyi Biotec). Flow cytometric analysis was done using WinList 5.0. P values were calculated using unpaired t test.
Results
STR analysis shows absence of specific STR markers
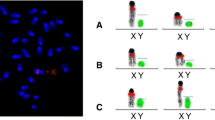
Personal communication from GVG Genetic Monitoring had alerted us of a Y chromosome deletion of approximately 40 Mb on the B6JBom strain. In order to verify that B6JBom indeed lacked various STR markers in the Y chromosome, we sent samples from B6JBom and B6NTac females and males from our colony to GVG Genetic Monitoring. The data showed that, indeed, some markers localized between 6.57 and 46.73 Mb (personal communication by GVG, Fischer et al. 2016) were absent in the electropherograms from B6JBom shown on Fig. 1. B6NTac presented with the 18 expected microsatellite markers present (Fig. 1a), while only 9 of the 18 markers were detected in B6JBom (Fig. 1b). Markers absent are between position 12.87 and 46.6 Mb (Table S2). The size and location of the mutation between 6.12/6.57 and 46.73/47.31 Mb have been defined by GVG (Fischer et al. 2016).
Electropherograms of multiplex STR profile of 11 Y chromosome STR markers in C57BL/6NTac and C57BL/6JBomTac. Y-axis represents the fluorescence intensity of the amplified band and the X-axis represents approximate size of the amplified band in base pairs. Since some primer pairs target more than one specific region, a total of 18 target regions are analyzed simultaneously. a C57BL/6NTac male Y chromosome STR profile shows all 18 STR markers. b C57BL/6JBomTac male Y chromosome STR profile shows only nine STR markers
GigaMuga SNP analysis does not provide informative SNPs in the Y chromosome in the region of the putative deletion
In order to verify the reported Y chromosome deletion with another method, tail biopsies of B6JBom and B6NTac mice were sent to the University of North Carolina Systems Genetics Core to be tested with the GigaMuga SNP Panel (Morgan et al. 2016), which has 83 SNP markers in the Y chromosome. No unique SNP markers were found in the region of the putative 40 Mb deletion, and we were not able to verify the presence of the deletion with this methodology (data not shown).
Rbm31y is deleted in B6JBom
We extracted from the NCBI database all reported genes and open reading frames within the putative deleted region (Online Resource Table S4) and found that gene Rbm31y has two copies in the putative deleted area and none outside of this region. We designed primers based on sequence from the NCBI database. PCR genotyping results show that Rbm31y is indeed deleted (Fig. 2a).
Genomic DNA PCR amplification of Rbm31y and transcript level of Rbm31y and other Y chromosome genes. a PCR amplification of genomic DNA from B6NTac and B6JBom males using primers for a housekeeping gene Cd79b and Y chromosome encoded Rbm31y. b Expression level of selected Yq transcripts: Rbm31y, Ssty1, Ssty2, Asty, Sly global (Sly1 and Sly2), and Sly long (only Sly1), a Yp gene: Zfy2, and selected genes encoded on other chromosomes: Prm1 (Chromosome 16); Acrv1 (Chromosome 9), and housekeeping gene β-actin (Chromosome 5) from male B6NTac and B6JBom. P values are represented by asterisks; *p < 0.05, **p < 0.01, ***< p < 0.001
Genes on both the Y chromosome and the autosomes are deregulated
Mice with Y chromosome deletions were previously reported to have deregulation of Y chromosome genes expressed in the testes (Reynard and Turner 2009; Ellis et al. 2005). We therefore quantified levels of selected transcripts in testes from B6JBom and B6NTac. As expected, Rbm31y transcripts were missing in B6JBom since the 2 copies of this gene are deleted (Fig. 2a, b). The levels of Acrv1 and Zfy2 transcripts were increased while the levels of Ssty1, Ssty2, and Sly global (Sly1 and Sly2) and Sly (Sly1 only) transcripts were significantly decreased in B6JBom compared to B6NTac.
B6JBom males have normal breeding performance but distorted male/female sex ratio
The PEI was collected from Taconic Murine Pathogen-Free™ (MPF™) colonies containing 1:1 breeding pairs. There was no difference between the PEIs of B6JBom and B6NTac (Table 1).
A bias towards females in the male to female sex ratio has been previously reported for Y chromosome deletion mutants (Styrna et al. 1991; Conway et al. 1994; Ward and Burgoyne 2006). We reviewed historical information on both Taconic B6 substrains to see if there was such a bias in the B6JBom substrain. We found that in 2009, there was a shift in the B6JBom male/female sex ratio, and the shift has been maintained since then in our breeding colonies (Table 2). The B6NTac substrain male/female sex ratio remained ~1.00 during the same period.
Fertilization rate of B6JBom is comparable to B6NTac
In order to assess whether the Y chromosome deletion had any impact on IVF rates, we did a comparative study using sperm from B6JBom and B6NTac. Two sets of IVF experiments were performed, the first with sperm from four individual males and the second with three individual males, each male tested with two sets of pooled oocytes from five females for B6JBom. For B6NTac, five individuals males were used each tested with two sets of pooled oocytes from five females. No differences in the IVF fertilization rates, measured as a proportion of inseminated oocytes that developed to the two-cell stage embryos, were observed (Table 3).
Sperm morphology analysis shows a range of sperm abnormalities in B6JBom
Abnormal sperm morphology has been previously observed in various Y chromosome deletion mutants and the severity of the deletion correlates with the percentage of abnormal sperm (Styrna et al 1991, Toure et al 2004, Ward et al 2006). We quantified the number of sperm with normal and abnormal shape, and among abnormal sperm differentiated between those with bent heads and those with abnormal head shape. B6JBom males had significantly more morphologically abnormal sperm (35 vs. 11%, P < 0.05) than B6NTac (Table 4). The predominant defect observed in B6JBom was ‘bent heads’ (Table 4; Fig. 3b–c).
Some statistically significant differences in immune cell frequency or count, but no immunodeficiency
A recent publication (Sun et al. 2013) presented evidence of a potential link between Y chromosome deletion and B and NK cell deficiencies. To investigate potential differences in the immune characteristics between B6JBom and B6NTac, a multiparametric flow cytometry analysis of spleen and bone marrow cells was performed. B6JBom presented with larger spleens when compared to B6NTac, evidenced by a higher organ weight and a higher number of spleen cells (Fig. 4). The distribution of all cells (Fig. 5a), viable cells (Fig. 5b) and immune (CD45+) and non-immune (CD45−) cells (Fig. 5c) was similar in spleens from B6JBom and B6NTac. However, in-depth characterization of CD45− and CD45+ spleen cell subpopulations revealed some differences between B6JBom and B6NTac (Fig. 5d–f, S2, Table S5). B6JBom had more mature erythrocytes but fewer T lymphocytes, T helper cells and cytotoxic T cells, natural killer cells, myeloid phagocytic cells, and macrophages, when compared to B6NTac.
Flow cytometry characterization of mouse spleen cells from B6NTac and B6JBom males. a SSC/FSC characteristics of spleen cell suspension; b viable cells from spleen cell suspension were stained with DAPI. A total of 50,000 events were collected. c Viable CD45−, non-immune cells (left gate) versus CD45+, immune cells (right gate). d Viable erythrocytes (CD45−, CD71−, Ter119+) versus mature erythrocytes (CD45−, CD71+, Ter119+). e Viable endothelial cells (CD45−, CD71−, Ter119−, CD31+) versus fibroblasts (CD45−,CD71−, Ter119−, Sca-1+). f Viable myeloid cells (CD45+, CD11b+) versus dendritic cells (CD11c+, CD11b−). Top row B6NTac, bottom row B6JBom
The analysis of immune and non-immune bone marrow cell populations did not show any significant differences between the B6 substrains (Table S6). There were also no differences observed in immune and non-immune cell populations from spleen or bone marrow of female B6JBom and B6NTac mice (data not shown).
Discussion
All the evidence acquired in this study, including absence of both copies of the Rbm31y gene, altered levels of examined transcripts in testes, biased male/female sex ratio, and sperm shape abnormalities, supports the presence of a fixed Y chromosome deletion in Taconic’s B6JBom inbred strain, reported recently by Fischer et al. 2016. With respect to reproductive abilities, this deletion thus far seems to mostly affect sperm morphology, with a mild impact on the male/female sex ratio, but no alterations on the natural mating breeding performance or IVF efficiency. The data indicate that for the most part, the phenotype observed mainly affects the male germline, which is in agreement with several publications that describe other mouse and human Y chromosome deletions (Conway et al. 1994; Reynard and Turner 2009; Toure et al. 2004; Ma et al. 2000).
In mice, the Y chromosome long arm (Yq) consists almost entirely of massively amplified sequence, with three primary gene families, Sly, Sry, and Ssty. These genes are present in 132, 197, and 317 copies, respectively (Soh et al. 2014). This highly repetitive sequence has made interrogation of the mouse Y chromosome difficult.
Our goal was to confirm the reported mutation and understand the phenotypic impact of this deletion to the widely used B6JBom inbred strain. Our study provided additional supportive evidence to a recently reported 40 Mb deletion that has been mapped close to 6.12/6.57 and 46.73/47.31 Mb in the Y chromosome of B6JBom (Fischer et al. 2016) and that has become fixed in the Taconic B6JBom foundation colony. The STR marker analysis showed the absence of markers in the Y chromosome of B6JBom, and we verified that both copies of Rbm31y were deleted from the B6JBom genome. However, the high density SNP panels, the current gold standard for mouse genetic monitoring, were uninformative due to a lack of unique SNP markers in the mouse Yq arm.
We observed a specific testicular phenotype in B6JBom males as compared to B6NTac controls. Specifically, we observed alterations in levels of testis transcripts of several genes. Similar transcriptional alterations were observed before in mice with known NPYq deletions, and were shown to correlate with the deletion size (Toure et al. 2005; Ellis et al. 2005; Reynard and Turner 2009; Coquet et al. 2009). The reduced transcript levels of genes encoded within NPYq of B6JBom (Sly, Ssty, and Asty) are reflective of the loss of several of their copies encoded within the deleted region. Our reported B6JBom deletion is the smallest of all reported thus far, approximately 40 Mb, equivalent to a 46% deletion of the Y chromosome, representing a 40% reduction on the levels of Sly transcription. The smallest previously reported Yq deletion mutant, Del(Y)B10.BR-Ydel/Ms, which had 66% of Y chromosome deleted, presented with a 46% reduction on Sly transcription (Toure et al. 2005) which is a higher reduction than in B6JBom.
A similar pattern was observed when the expression of Ssty1 and Ssty2 in B6JBom was related to that reported for Del(Y)B10.BR-Ydel/Ms. For all previously reported models with NPYq deletions, the exact deletion coordinates have not been determined. It is therefore possible that the differences in transcript expression between our study and those of others are due not only to differences in deletion size but also its location. It would be interesting to determine more precisely the gene breakpoints of these other NPYq deletion mutants.
NPYq encoded Sly has been shown to be a key regulator of sex chromosome gene expression by Coquet et al. (2009). Its deficiency or absence leads to a global upregulation of sex chromatin and occasional alterations of genes encoded on the autosomes. In agreement with the previous data by Cocquet et al. (2009), we demonstrated a significant upregulation of NPYp encoded Zfy2 and autosomally encoded Acrv1, but lack of deregulation of autosomally encoded Prm1. Zfy2 has been proposed to be essential in sperm head remodeling and sperm tail development (Vernet et al. 2016; Yamauchi et al. 2015), therefore upregulation of Zfy2 might explain the sperm abnormalities we see in B6JBom.
Other characteristics of MSYq deletion mutants are that they present sperm abnormalities, decreased rates of fertilization, and distortion of the male/female sex ratio (Xian et al. 1992). We did not observe differences in the reproductive performance or IVF rates between the B6JBom substrain compared to B6NTac. The PEI for B6JBom in Taconic’s MPF barriers is comparatively similar to B6NTac. Litter sizes for B6JBom are also as expected for a B6 substrain (6–8 pups/litter, data not shown). This lack of breeding deficiency is possible considering the size of deletion, the smallest of reported thus far.
In respect to sperm morphology anomalies, we observed 35% of sperm from B6JBom were abnormal, compared to 11% in B6NTac. We defined the severity of head deformities based on criteria described before (Yamauchi et al. 2009) and noted that most of head shape defects fell into the 1 and 2 S category (slight) with a few G (gross deformities). The observed sperm shape abnormalities did not negatively influence breeding or production efficiencies in breeding colonies. So while the mutation does cause alterations in sperm morphology, there is no impact on natural or assisted reproduction in B6JBom.
The male:female sex ratio distortion towards females observed in B6JBom due to the Y deletion is in agreement with skewed sex ratios reported for other mouse models with NPYq deletions (Styrna et al. 1991; Conway et al. 1994; Ward and Burgoyne 2006). This sex ratio bias has been hypothesized to reflect an intragenomic conflict between the X and Y chromosome (Coquet et al. 2009; Ellis et al. 2011; Coquet at al. 2012). Spermatid-specific multicopy Y chromosome transcripts, i.e., Sly, Asty, Ssty1, Ssty2, have a high degree of homology to multicopy spermatid-specific transcripts in the X chromosome, i.e., Slx and Slxl1, Astx, Ssxb. In mice with NPYq deletions, when the Y chromosome transcripts are downregulated, their X homologous counterparts are upregulated (Toure et al. 2005; Ellis et al. 2005; Raynard and; Turner 2009; Coquet et al. 2009).
The Y-encoded Sly and X-encoded Slx/Slxl1 have been proposed to be the key players in intragenomic conflict in the mouse (Cocquet et al. 2009, 2012). Sly represses sex chromatin while Slx/Slxl1 plays the opposite role and stimulates XY gene expression in spermatids. Sly deficiency leads to XY gene upregulation, sperm differentiation defects, subfertility/sterility, and sex ratio bias towards females (Cocquet et al. 2009). Slx/Slxl1 deficiency rescues sperm differentiation defects and subfertility/sterility caused by Sly deficiency. Importantly, while Sly deficiency leads to sex ratio bias towards females, Slx/Slxl1 deficiency causes a sex ratio distortion towards males (Coquet et al. 2012). These data support that Sly and Slx/Slx1l have antagonistic effects during sperm differentiation.
To investigate the origin of the deletion in our colonies, we looked into the breeding history of the B6JBom. The origin of Taconic’s C57BL/6JBomTac is as follows. The Jackson Laboratory received C57BL/6 in 1948 from Hall. In 1971, the Zentralinstitut fur Versuchsteirzucht in Hannover, Germany, received stock from the Jackson Laboratory. In 1988, M&B (now Taconic Biosciences) received stock from the Zentralinstitut fur Versuchsteirzucht. The mice were derived by Taconic in 2000 by embryo transfer. Taconic maintains the B6JBom as an inbred strain with a single Foundation Colony, which feeds all production colonies globally. Taconic’s Foundation Colony was refreshed in 2015 using cryopreserved embryos from 2010. We went back to 2004 records when reviewing the breeding history of this inbred strain. When we analyzed the collected breeding information from B6JBom and compared it to B6NTac, there was a clear breakpoint in the male/female sex ratio from 2008 to 2009. The male/female sex ratio shifted in the production colonies from a 1 to a 0.76 in 2009, and since then it has fluctuated between 0.76 and 0.82 (Table 2). This indicates that the mutation arose in the Foundation Colony, propagated to all production colonies and was fixed by 2009.
It is important to point out that the history of C57BL/6J (B6J), C57BL/6NTac, and C57BL/6JBomTac are of three different substrains that have independently drifted from each other. Jackson cryopreserved its B6J stock in 2003 at generation F226 to maintain their Genetic Stability program. The C57BL/6N (B6N) substrain diverged from B6J when stock was transferred from Jax to NIH in 1951. B6N was then transferred to Taconic from NIH at generation F151 in 1991. At this point, Taconic cryopreserved its B6NTac. Meanwhile, Jackson transferred B6J to Hannover in 1971, and in 1988 M&B (now Taconic Biosciences) received B6JBom from Hannover at F141, Taconic cryopreserved the B6JBom in 2010 at F207. Therefore, all 3 substrains are quite different and have drifted apart for at least 39 years (B6JBom from B6J), 40 years B6NTac from B6J (became N when it was received by NIH). We thus chose the B6NTac as a control for these experiments as a readily available strain that was separated from B6JBom a similar length of time as other possible control substrains.
Sun et al. (2013) reported a B and NK cell deficiency in the bone marrow of mice which was linked to a Y chromosome deletion of 1/3 of its length but were not able to determine how the Y chromosome deletion mediated B and NK cell defects. Here, we have shown differences between B6JBom and B6NTac in respect to spleen size and characteristics of immune and non-immune spleen cells. The biological relevance of these differences is not clear, but they do not seem to represent any sort of obvious immunodeficiency. These differences could be due to genetic modifiers characteristic of the B6JBom relative to the B6NTac substrain.
The finding of a Y chromosome deletion in the B6JBom strain was a surprise. From the perspective of animal production breeding, monitoring the Y chromosome has indeed been a challenge due to the nature of its many repeats and therefore lack of accessibility of its sequence. In the specific case of B6JBom, the genetic monitoring program was not able to detect the Y chromosome mutation due to the lack of informative SNP markers as previously discussed. While the B6JBom was cryopreserved per the requirements of the genetic quality program, the frozen stock already had the fixed Y chromosome deletion. We are working on developing additional markers in the repetitive region of the Y chromosome to include in our routine genetic monitoring program for all our inbred strains and outbred stocks. Taconic has a robust genetic quality program that aligns with industry standards. Inbred strains are cryopreserved and Foundation Colonies are refreshed every 5 years from cryopreserved stock to lessen the risk of genetic drift. All strains are tested using well characterized SNP panels performed on the Illumina platform. Breeding paradigms for inbred strains include unidirectional flow of mice, brother by sister mating as well as careful record-keeping. Breeding is harmonized globally for all production sites and distributors. In addition, we are in the process of performing whole genome sequencing of all our inbred strains.
Our study emphasizes on the importance of substrain differences. The Y chromosome deletion identified in B6JBom is not the first mutation that has been described on a C57BL/6 substrain; there are many known, and each of them present different effects in various organs/systems. The Nnt C57BL/6J mutation, a 17 Kb mutation that was discovered in B6J and affects several organ systems under stress conditions, has well-characterized effects on diabetes (Freeman et al. 2006). The Snca and Mmrn1 deletions, a 300 Kb deletion discovered on the C57BL/6JOlaHsd (B6JOlaHsd), affect metabolism of brain chemical compounds (Specht and Schoepfer 2001; Gajovic et al. 2006). The Crb1 rd8 mutation, a one base-pair deletion in all commercially available B6N substrains, causes mild retinal degeneration which produces visual impairment but does not render the mouse completely blind (Mattapallil et al. 2012). The Cyfip2 Min mutation, a G to A base change on all commercially available B6N substrains, causes acute and sensitized cocaine-response phenotypes (Kumar et al. 2013). As can be appreciated, findings of additional mutations in inbred strains will become more common as more substrains are sequenced. Research scientists should carefully choose a substrain when designing experiments and be cautious to be consistent in substrain usage for ongoing experiments.
References
Burgoyne P (1998) The role of Y-encoded genes in mammalian spermatogenesis. Semin Cell Dev Biol 9:423–432
Burgoyne PS, Mahadevaiah SK, Sutcliffe MJ, Palmer SJ (1992) Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell 71:391–398
Cocquet J, Ellis PJ, Yamauchi Y, Mahadewaiah SK, Affara NA, Ward MA, Burgoyne PS (2009) The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biology 7:1–19
Cocquet J, Ellis PJ, Mahadevaiah SK, Affara NA, Vaiman D, Burgoyne PS (2012) A genetic basis for a postmeiotic X versus Y chromosome intragenomic conflict in the mouse. PLoS Genet 8(9):e1002900
Conway SJ, Mahadevaiah SK, Darling SM, Capel B, Rattigan AM, Burgoyne PS (1994) Y353/B: a candidate multiple-copy spermiogenesis gene on the mouse Y chromosome. Mamm Genome 5:203–210
Ellis PJI, Clemente EJ, Ball P, Toure A, Ferguson L, Turner JMA, Loveland, KL, Affara NA, Burgoyne PS (2005) Deletions on mouse Yq lead to upregulation of multiple X- and Y-linked transcripts in spermatids. Hum Mol Genet 14:2705–2715
Ellis PJI, Ferguson L, Clemente EJ, Affara NA (2007) Bidirectional transcription of a novel chimeric gene mapping to mouse chromosome Yq. BMC Evol Biol 7:171
Ellis PJI, Bacon J, Affara NA (2011) Association of Sly with sex-linked gene amplification during mouse evolution: a side effect of genomic conflict in spermatids? Hum Mol Genet 20:3010–3021
Fischer M, Kosyakova N, Liehr T, Dobrowolski P. (2016) Large deletion on the Y-chromosome long arm (Yq) of C57BL/6JBomTac inbred mice. Mamm Genome. 31 Oct 2016 [Epub ahead of print]
Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD (2006) Deletion of nicotinamide nucleotide transhydrogenase. Diabetes 55:2153–2156
Gajovic S, Mitrecic DA, Iaconcig A, Muro AF (2006) Unexpected rescue of alpha-synuclein and multimerin 1 deletion in C57BL/6JOlaHsd mice by beta-adducin knockout. Transgenic Res 15:255–259
Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, Vitaterna MH, Pardo-Manuel de Villena F, Churchill G, Bonci A, Takahashi JS (2013) C57BL/6 N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342:1508–1512
Ma K, Mallidis C, Bhasin S (2000) The role of Y chromosome deletions in male fertility. Eur J Endocrinol 142:418–430
Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6 N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci 53(6):2921–2927
Morgan AP, Fu C, Kao C, Welsh CE, Didion JP, Yadgary L, Hyacinth L, Ferris MT, Bell TA, Miller DR, Giusti-Rodriguez P, Nonneman RJ, Cook KD, Whitmire JK, Gralinski LE, Keller M, Attie AD, Churchill GA, Petkov P, Sullivan PF, Brennan JR, McMillan L, Pardo-Manuel de Villena F (2016) The mouse universal genotyping array: from substrains to subspecies. G3 6(2):263–279
Perry J, Palmer S, Gabriel A, Ashworth A (2001) A short pseudoautosomal region in laboratory mice. Genome Res 11:1826–1832
Reynard LN, Turner, JMA (2009) Increased sex chromosome expression and epigenetic abnormalities in spermatids from male mice with Y chromosome deletions. J Cell Sci 122:4239–4248
Soh YQS, Alfoldi J, Pyntikova T, Brown LG, Graves T, Minx PJ, Fulton RS, Kremitzki C, Koutseva N, Mueller JL, Rozen S, Hughes JF, Owens E, Womack JE, Murphy WJ, Cao Q, De Jong P, Warren WC, Wilson RK, Skaletsky H, Page DC (2014) Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 159:800–813
Specht CG, Schoepfer R (2001) Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6 J inbred mice. BMC Neurosci 2:11
Styrna J, Imai HT, Moriwaki K (1991) An increased level of sperm abnormalities in mice with a partial deletion of the Y chromosome. Genet Res 57:195–199
Sun S, Horino S, Itoh-Nakadai A, Kawabe T, Asao A, Takahashi T, So T, Funayama R, Kondo M, Saitsu H, Matsumoto N, Nakayama K, Ishii N (2013) Y chromosome-linked B and NK cell deficiency in mice. J Immunol 190:6209–6220
Takeo T, Nakagata N (2010) Combination medium of cryoprotective agents containing L-glutamine and methyl-{beta}-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 44:132–137
Toure A, Szot M, Mahadevaiah SK, Rattigan A, Ojarikre OA, Burgoyne PS (2004) A new deletion of the mouse Y chromosome long arm associated with loss of Ssty expression, abnormal sperm development and sterility. Genetics 166:901–912
Toure A, Clemente EJ, Ellis P, Mahadevaiah SK, Ojarikre OA, Ball PAF, Reynard L, Loveland KL, Burgoyne PS, Affara NA (2005) Identification of novel Y chromosome encoded transcripts by testis transcriptome analysis of mice with deletions of the Y chromosome long arm. Genome Biol 6:R102
Vernet N, Mahadevaiah SK, Decarpentrie F, Longepied G, de Rooij DG, Burgoyne PS, Mitchell MJ (2016) Mouse Y-encoded transcription factor Zfy2 is essential for sperm head remodeling and sperm tail development. PLoS ONE 11 (1):e0145398
Ward MA, Burgoyne PS (2006) The effects of deletions of the mouse Y chromosome long arm on sperm function intracytoplasmic sperm injection (ICSI)-based analysis. Biol Reprod 74:652–658
Xian M, Azuma S, Naito K, Kuneida, T, Moriwaki K, Toyoda Y (1992) Effect of a partial deletion of Y chromosome on in vitro fertilizing ability of mouse spermatozoa. Biol Reprod 47:549–553
Yamauchi Y, Riel JM, Wong SJ, Ojarikre OA, Burgoyne PS, Ward MA (2009) Live offspring from mice lacking the Y chromosome long arm gene complement. Biol Reprod 81:353–361
Yamauchi Y, Riel JM, Ruthig V, Ward MA (2015) Mouse Y-encoded transcription factor Zfy2 is essential for sperm formation and function in assisted fertilization. PLoS Genetics 11(12):e1005476
Acknowledgements
We thank the Taconic embryology laboratory, in particular Rosemary Leonard, for their contribution to the IVF experiments, Jochen Welcker for genome data mining, Michael Seiler for discussion regarding the flow cytometry analysis, Alane Hogan for assistance with figures, and Monika Ward for helpful discussions regarding Y chromosome mutations, sperm abnormalities, and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employed by Taconic Biosciences, Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
MacBride, M.M., Navis, A., Dasari, A. et al. Mild reproductive impact of a Y chromosome deletion on a C57BL/6J substrain. Mamm Genome 28, 155–165 (2017). https://doi.org/10.1007/s00335-017-9680-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-017-9680-0