Abstract
Purpose
To assess the association between minimal ablative margin (MAM) and local tumor progression (LTP) following CT-guided thermal ablation of colorectal liver metastases (CRLM) in a multicenter cohort and across two confirmation software.
Materials and methods
This multicenter retrospective study included patients who underwent CT-guided radiofrequency or microwave ablation for CRLM between 2009 and 2021 in three institutions. Three-dimensional (3D) MAM was retrospectively assessed using dedicated ablation confirmation software by automatic non-rigid (Ablation-fit) or semi-automatic rigid co-registration (SAFIR) of intraprocedural pre- and post-ablation contrast-enhanced CT scans by two independent reader teams blinded to patient outcomes. LTP was assessed on a per-tumor basis. Factors associated with LTP-free survival were assessed using multivariable Cox regression analysis.
Results
Overall, 113 patients (mean age: 67 ± 10 years; 78 men) who underwent thermal ablation for 189 CRLM (mean diameter: 1.9 ± 1.1 cm) met the inclusion criteria. 173/189 (92%) CRLM could be successfully analyzed using both software. Over a median follow-up of 31 months (IQR: 22–47), 21 of 173 CRLM (12.1%) developed LTP. On multivariable analysis, 3D MAM was independently associated with LTP in both software (Ablation-fit: HR 0.47, 95% CI: 0.36–0.61, p < 0.001; SAFIR: HR 0.42, 95% CI: 0.32–0.55, p < 0.001). No LTP was observed in CRLM ablated with MAM ≥ 4 mm (Ablation-fit) and ≥ 5 mm (SAFIR). The per-tumor median absolute difference in MAM quantification between both software was 2 mm (IQR: 1–3).
Conclusion
MAM was independently associated with LTP after thermal ablation of CRLM across multicenter data and two confirmation software. Ablations achieving a MAM ≥ 5 mm were associated with local control in both software.
Clinical relevance statement
MAMs from intraprocedural contrast-enhanced CT were independently associated with LTP after thermal ablation of CRLM across multicenter data and two confirmation software, with a margin ≥ 5 mm associated with local control in both software.
Key Points
-
Sufficient ablative margins are critical for local control following thermal ablation of CRLM.
-
Intraprocedural CT-derived MAM was the only independent factor associated with LTP across two confirmation software.
-
No LTP was observed in CRLM ablated with a MAM ≥ 5 mm.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Percutaneous thermal ablation is a well-established minimally invasive local treatment option for colorectal liver metastases (CRLM) with widely expanded indications over the past decade [1, 2]. Radiofrequency (RFA) and microwave ablation (MWA) are the most commonly used thermal ablative modalities and have been demonstrated as parenchyma-sparing treatment techniques with low rates of major complications and treatment-related mortality [3,4,5].
Despite these advantages, thermal ablation has historically been reserved for patients who are not eligible for surgery due to its relatively higher rates of local tumor progression (LTP) (2–60%) [6, 7]. Among several tumor- or patient-related characteristics, numerous studies have recently identified the minimal ablative margin (MAM) as the most critical predictor for LTP [8,9,10,11]. In a recent meta-analysis of retrospective studies, complete coverage of the target lesion with sufficient ablative margin (i.e., > 5–10 mm) was shown associated with reduced risk of LTP [12].
In current practice however, many physicians rely on assessing the MAM by a side-by-side comparison of pre- and post-interventional CT scans—a method shown to be poorly reproducible and unreliable for predicting treatment success in various studies [13, 14]. Image fusion by (semi-)automatic confirmation software with three-dimensional (3D) volumetric assessment has proven to overcome these difficulties [8, 15,16,17,18]. Currently available confirmation software solutions however have been reported mainly in retrospective, single-center studies with rather small sample sizes, providing an overall low level of evidence [19]. An extensive multi-institutional evaluation is presently lacking. Adding to this, the current literature is characterized by wide variation in margin quantification approaches [20] and the consistency of the optimal ablative margin across different software is still unknown.
The primary aim of this study was to assess the association between 3D MAM and LTP following CT-guided thermal ablation of CRLM in a multicenter cohort and across two confirmation software. The secondary objectives were to evaluate the diagnostic performance and inter-software consistency of MAM quantification using each software.
Materials and methods
Patients
This multicenter retrospective study included data from three academic institutions (Radboud University Medical Center [RUMC]; Medical University Innsbruck [MUI]; Amsterdam University Medical Center, location VUMC [VUMC]). The study was approved by the institutional review board at each institution and informed consent was waived. Institutional databases were queried to identify all patients that had undergone RFA or MWA for CRLM and met the following inclusion criteria: (i) treated for local treatment-naïve lesion(s); (ii) intraprocedural pre- and immediate post-ablation contrast-enhanced CT (CE-CT) imaging available; (iii) > 12 months imaging follow-up available. For RUMC, all consecutive patients treated between March 2015 and May 2021 were included. For MUI, all consecutive patients treated between January 2009 and October 2018 were included. For VUMC, a randomly selected subset of 55 patients treated between October 2014 and April 2020 was included. Part of the data (45 of 113 patients) used in this study overlaps with a previous study [16]. The prior study was a single-center study utilizing one confirmation software whereas in this study we report results in a multicenter cohort and all data was analyzed by a second confirmation software.
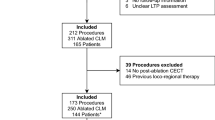
In all patients, the decision to perform thermal ablation was made by the multidisciplinary tumor board. A detailed flowchart is shown in Fig. 1.
Ablation procedures
All thermal ablation procedures were performed in the CT room by a board-certified interventional radiologist. Patients were treated under general anesthesia in two centers and under general anesthesia or propofol sedation in one center. Depending on local treatment protocol, intraprocedural pre- and immediate post-ablation CE-CT imaging was performed using either intravenous contrast injection or transcatheter hepatic arteriography (CTHA) [21]. In one center, a stereotactic navigation device was used in all cases for probe placement of which the methodology has been described in detail before [22, 23]. In the other two centers, probe placement was performed under ultrasound or CT fluoroscopy guidance. Thermal ablation was performed using RFA (Cool-tip, Covidien; LeVeen, Boston Scientific; Starburst, Angiodynamics) or MWA (Emprint, Medtronic; NeuWave, Ethicon; Solero, Angiodynamics) depending on site and historic availability. Technical success was defined as complete ablation with an ablative margin of ≥ 5 mm as evaluated at the end of each procedure by the treating physician on two-dimensional side-by-side comparison or rigid superposition of pre- and post-ablation CE-CT images.
Outcome assessment
Ablation outcomes were assessed following standardized reporting guidelines for tumor ablation [3, 24]. All patients underwent routine imaging follow-up consisting of CE-CT, CE-MRI, or PET-CT imaging every 3 months for the first year followed by 3–6 month intervals thereafter. All follow-up imaging was reviewed by a board-certified abdominal radiologist. LTP was defined as tumor focus within or at the border of the ablation zone on follow-up imaging after the immediate post-ablation CE-CT documented complete ablation. Time to LTP was defined as the time between thermal ablation and imaging evidence of LTP.
MAM quantification
All imaging data was independently reviewed at two institutions (RUMC and MUI). At each institution, a reader team consisting of a senior radiology resident and an interventional radiologist in consensus performed MAM quantification blinded to patient outcome using a dedicated ablation confirmation software: (i) Ablation-fit (R.A.W. SRL), commercially available software for liver thermal ablation, at MUI or (ii) Software Assistant for Interventional Radiology (SAFIR) (Fraunhofer MEVIS), research software for liver thermal ablation, at RUMC.
For both software, intraprocedural pre- and post-ablation arterial and portal-venous phase CE-CT scans (in-plane resolution 0.6–1.0 mm; slice thickness 0.5–3.0 mm) were imported. First, the liver parenchyma was automatically segmented and manually adjusted if needed. Second, in each software, each colorectal liver metastasis (CRLM) and corresponding coagulation zone were manually identified by the reader and semi-automatically segmented in the pre- and post-ablation CE-CT scans, respectively. Manual corrections were performed until segmentation results were deemed satisfactory. Third, the pre- and post-ablation CE-CT scans were co-registered. For Ablation-fit, an automatic non-rigid co-registration was performed by the software. For SAFIR, a semi-automatic rigid co-registration was performed and manually adjusted if needed. Co-registration was optimized based on local landmarks, in particular liver vessels, to obtain optimal local registration in the segment of interest. In both software, co-registration accuracy was assessed using a blending slider allowing to switch between pre- and post-ablation CE-CT scans. Insufficient co-registration accuracy was defined as a mismatch in more than one local landmark (e.g., vessels) of > 3 mm, and such cases were excluded from further MAM quantification.
Hereafter, 3D MAM quantification was automatically obtained in each software. For Ablation-fit, incomplete tumor coverage was labeled by the software as residual tumor, and was classified as MAM < 0 mm. For remaining ablations, unablated volumes for all 3D safety margins from 1 mm to 10 mm were calculated. The MAM was defined as the maximum 3D safety margin that was completely ablated. For SAFIR, the MAM was calculated as the smallest 3D distance between the tumor and ablation zone rounded to the nearest millimeter, where negative values indicate incomplete coverage. For both software, the side(s) of the tumor where suspected insufficient margins (< 5 mm) occurred were documented by dividing the tumor in octants along the left-right, cranio-caudal, and anterior-posterior axes. A schematic overview of MAM quantification is summarized in Fig. 2. Additional details are provided in S1.
Schematic overview of MAM quantification steps for each software. Semi-automatic segmentation of the liver, tumor, and ablation zone is performed on the pre- and post-ablation CE-CT. Automatic non-rigid (Ablation-fit) or semi-automatic rigid (SAFIR) co-registration is performed to align the image volumes. Finally, MAM calculation is performed and visualized in two-dimensional and 3D views
Statistical analyses
The primary endpoint was to determine whether the MAM was independently associated with LTP per software. Secondary endpoints were the diagnostic performance of MAM generated by each software to discriminate between cases with and without LTP and per-tumor inter-software agreement in MAM quantification. Uni- and multivariable analyses for LTP-free survival (LTPFS) were performed using the Cox proportional hazard regression model. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Survival curves were estimated using the Kaplan–Meier method and differences were assessed using the log-rank test. ROC analysis was used to determine diagnostic performance and area under the curve (AUC) of MAM generated by each software. For cases with MAM < 0 mm or > 10 mm, Ablation-fit does not provide absolute MAM values precluding direct comparison between both software. For cases with MAM between 0 mm and 10 mm, inter-software agreement was assessed using single-measurement, absolute agreement intraclass correlation coefficient (ICC), and absolute difference. Continuous data are expressed as means ± standard deviation with range or medians with interquartile range (IQR) or range. The Shapiro-Wilk method was used for normality testing. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (v23.0; IBM) and R (v3.6.3; R Foundation).
Results
Patient cohort and baseline demographics
Overall, 113 patients who underwent thermal ablation for 189 CRLM met the inclusion criteria. A total of 173/189 (92%) CRLM could be successfully analyzed using both software and could be included for final analysis. Sixteen tumors were excluded based on suboptimal tumor visibility to allow segmentation (n = 4) or failed image co-registration (n = 12) (Fig. 1).
The final cohort thus consisted of 103 patients (mean age: 67 years ± 10 [SD]; 78 men) treated in 120 sessions (median number of treated CRLM per session: 1 [IQR: 1–2]) for 173 CRLM (mean diameter: 1.9 cm ± 1.1 [SD]). The distribution of included CRLM per institution was 85 (VUMC), 73 (MUI), and 15 (RUMC). Baseline patient, procedure, and tumor characteristics are summarized in Table 1.
Clinical outcomes
The median follow-up was 31 months (IQR: 22–47). Technical success was achieved in all (173/173) lesions. The overall incidence of LTP was 21/173 (12.1%) after a mean time to progression of 14.7 months. The per-center incidences of LTP were 4/85 (5%), 10/73 (14%), and 7/15 (47%).
MAM quantification
Of 173 ablated CRLM, MAM ≥ 5 mm; > 0 mm and < 5 mm; and ≤ 0 mm were measured in 80 (46.2%), 80 (46.2%), and 13 (7.6%) using Ablation-fit and 79 (45.7%), 79 (45.7%), and 15 (8.6%) using SAFIR. Of tumors with MAM ≥ 5 mm; > 0 mm and < 5 mm; and ≤ 0 mm measured using Ablation-fit, LTP was observed in 0/80 (0%), 13/80 (16.3%) and 8/13 (61.5%), respectively (Fig. 3a). Of tumors with MAM ≥ 5 mm; > 0 mm and < 5 mm; and ≤ 0 mm measured using SAFIR, LTP was observed in 0/79 (0%), 6/79 (7.6%) and 15/15 (100%), respectively (Fig. 3b). Smallest MAM where no LTP was observed was ≥ 4 mm for Ablation-fit and ≥ 5 mm for SAFIR. The side of LTP corresponded to the side(s) of the tumor treated with insufficient margins in 20/21 (95%) and 20/21 (95%) cases for Ablation-fit and SAFIR, respectively.
LTPFS
Univariable Cox regression analysis showed that age (HR: 0.95, 95% CI: 0.92–0.99, p = 0.02), tumor size (HR: 1.04, 95% CI: 1.00–1.07, p = 0.045), and MAM of each software (Ablation-fit: HR: 0.51, 95% CI: 0.41–0.64, p < 0.001; SAFIR: HR: 0.49, 95% CI: 0.41–0.59, p < 0.001) were significantly associated with LTPFS (Table 2). On multivariable Cox regression analysis, the MAM of each software (Ablation-fit: HR: 0.47, 95% CI: 0.36–0.61, p < 0.001; SAFIR: HR: 0.42, 95% CI: 0.32–0.55, p < 0.001) were the only independent factors significantly associated with LTPFS (Table 3).
Diagnostic performance
ROC analysis showed excellent diagnostic performance for discriminating between cases with and without LTP of MAM measured by Ablation-fit (AUC: 0.90, 95% CI: 0.86–0.95) and SAFIR (AUC: 0.96, 95% CI: 0.91–1.00). The detection rate of LTP (sensitivity) and false positive rate (1-specificity) as a function of MAM thresholds are shown for each software (Fig. 4). Corresponding sensitivity and false positive rate values for MAM thresholds between 0 mm and 5 mm are shown in Table 4.
Inter-software agreement
Inter-software agreement could be calculated for 147/173 tumors with a MAM between 0 mm and 10 mm. The per-tumor ICC showed moderate agreement for MAM quantification between both software and was 0.53 (95% CI: 0.41–0.64). The per-tumor median difference in MAM quantification between both software was 1 mm (IQR: −2 mm to 2 mm) and the median absolute difference was 2 mm (IQR: 1–3 mm).
Discussion
Achieving sufficient ablative margins has been shown critical to improve local control following thermal ablation of CRLM in multiple single-center studies evaluating single software approaches. In this study, we found the 3D MAM derived from intraprocedural contrast-enhanced pre- and post-ablation CT imaging to be the only independent predictor of LTP following thermal ablation of CRLM across multicenter data and two dedicated ablation confirmation software. Ablations achieving a MAM ≥ 4 mm for Ablation-fit and ≥ 5 mm for SAFIR were associated with local control. Consistency of these findings across two different software, different users, and intraprocedural CT data from three institutions represents an important step toward clinical integration.
Current literature investigating the relation between ablative margins and LTP is characterized by a wide variety of margin quantitation methods, utilizing various combinations of preceding diagnostic, intraprocedural or follow-up imaging as the pre- and post-ablation references, as well as different imaging modalities [20]. Recent work has shown ablative margins quantified on intraprocedural CT to significantly outperform initial follow-up CT in predicting LTP following thermal ablation of CRLM [25]. This work utilized solely intraprocedurally acquired pre- and post-ablation CE-CT imaging, allowing the methodology to be applicable during the actual intervention itself. In addition to superior accuracy in predicting LTP, a fully intraprocedural method for ablation endpoint assessment is particularly desirable as to enable the ability to immediately re-ablate site(s) of inadequate coverage and potentially improve local control rates without the need for a re-do procedure. Importantly, identified site(s) at risk for LTP were co-localized with the site of actual LTP in > 95% of cases and were consistent between both software.
Several studies have investigated optimal cut-off points for margin adequacy in thermal ablation of CRLM. Initial studies using landmark-based visual comparison of diagnostic and initial follow-up CT imaging recommended a MAM greater than 5 mm, and preferably greater than 10 mm all around the tumor to achieve optimal local control [9, 11, 26, 27]. Recent studies that used 3D approaches to quantify the MAM utilizing rigid or non-rigid image co-registration techniques have indicated an ablative margin greater than 2–5 mm to be associated with no LTP [8, 16, 18]. In a large single-institutional cohort Lin et al recently demonstrated a MAM threshold greater than 5 mm as the optimal endpoint following CT-guided CRLM ablation [8]. Our study confirms these findings, where no LTP was observed for respective MAMs of ≥ 4 mm and ≥ 5 mm using both elastic deformation and rigid image fusion software. Nevertheless, with Ablation-fit and SAFIR we observed a MAM of ≥ 5 mm in only 46.2% and 45.7% of ablated tumors, respectively. This is particularly interesting given that in all cases the achievement of technical success including a ≥ 5 mm ablative margin was perceived by the treating physician. On the other hand, as shown in Table 4 this finding also implies that applying a ≥ 5 mm threshold as the ablation endpoint using the software evaluated in this work would yield a substantial false positive rate (~ 48%), i.e., lesions treated with margins < 5 mm which would not develop LTP. We additionally provided diagnostic performance as a function of different margin thresholds, which should be important data for determining the clinical implications of each margin endpoint. Given the different performances per software found in this study, the definition of optimal ablative margin endpoints should require careful validation for each ablation confirmation approach in order to optimize the identification of tumors at high risk of developing LTP while minimizing the number of false positives.
Accurate image co-registration of pre- and post-ablation imaging is a prerequisite for robust MAM quantification [28]. In this study, only a small subset (12/189; 6%) of initially included CRLM had to be excluded due to insufficient image registration quality in one or both software. Interestingly, the use of two different approaches (i.e., semi-automatic rigid vs. automatic non-rigid) for image registration between both software did not result in large differences in diagnostic performance. Possibly, this is due to the use of solely intraprocedural pre- and post-ablation imaging inherently reducing the between-scan variability as compared to preceding or follow-up imaging acquired at different time points. Nevertheless, our study found an average per-tumor margin discrepancy of 2 mm between both software, which indicates the confidence with which margins can be quantified in individual cases.
Finally, comparative trials and prospective evidence are needed to steer and confirm the impact of margin confirmation software on improving local control following CRLM ablation. The generation of an open, standardized multicenter dataset may be beneficial to serve as a benchmark for confirmation software performance evaluations and facilitate the determination of optimal margin endpoints before clinical integration. Furthermore, prospective trials, such as the ongoing ACCLAIM trial (NCT05265169), CIEMAR registry [29] and recently published COVER-ALL study protocol [30] are urgently needed to support clinical adoption of margin confirmation to confirm treatment adequacy. Particularly considering the evolving role of thermal ablation in the management of CRLM, currently studied as an equivalent to surgical resection in phase III randomized controlled COLLISION trial [31], optimization of treatment outcomes and standardization of outcome evaluation appear desirable.
Our study had several limitations. First, our study pertains to a retrospective non-consecutive multicenter cohort which was selected based on the inclusion criteria. Although these were mostly related to the exclusion of cases with prior local treatment of the target tumor and insufficient follow-up, this may have introduced a bias to our results. Yet, this study presents the first large multicenter cohort evaluating MAM quantitation after CRLM ablation utilizing solely intraprocedural CT imaging. Furthermore, due to inherent differences between both ablation confirmation software, the MAM could not be calculated in the ranges < 0 mm and > 10 mm in one software, precluding the direct comparison of MAM results for these cases. Also, this study did not evaluate whether immediate re-ablation within the same session (or second-hit) could have been performed in each case of LTP, therefore restricting its clinical implication. Finally, even though a two-reader consensus approach was applied the semi-automatic assessments used in the study are subject to reader variability, which requires further study.
In conclusion, MAM was independently associated with LTP after thermal ablation of CRLM across multicenter data and two confirmation software. Ablations achieving a MAM ≥ 5 mm were associated with local control in both software. Ultimately, optimal cut-off points for margin adequacy and its implications for clinical use require validation per confirmation software.
Abbreviations
- AUC:
-
Area under the curve
- CE-CT:
-
Contrast-enhanced CT
- CRLM:
-
Colorectal liver metastasis
- 3D:
-
Three-dimensional
- HR:
-
Hazard ratio
- ICC:
-
Intraclass correlation coefficient
- LTP:
-
Local tumor progression
- MAM:
-
Minimal ablative margin
References
Crocetti L, de Baére T, Pereira PL, Tarantino FP (2020) CIRSE standards of practice on thermal ablation of liver tumours. Cardiovasc Intervent Radiol 43:951–962
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386–1422
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–260
Livraghi T, Meloni F, Solbiati L, Zanus G, Collaborative Italian Group using AMICA system (2012) Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 35:868–874
Schullian P, Johnston E, Laimer G et al (2021) Frequency and risk factors for major complications after stereotactic radiofrequency ablation of liver tumors in 1235 ablation sessions: a 15-year experience. Eur Radiol 31:3042–3052
Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L (2005) Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–171
Takahashi H, Berber E (2020) Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 9:49–58
Lin YM, Paolucci I, O’Connor CS et al (2023) Ablative margins of colorectal liver metastases using deformable CT image registration and autosegmentation. Radiology 307:e221373
Han K, Kim JH, Yang SG et al (2021) Single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology 298:212–218
Calandri M, Yamashita S, Gazzera C et al (2018) Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol 28:2727–2734
Shady W, Petre EN, Gonen M et al (2016) Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 278:601–611
Chlorogiannis DD, Sotirchos VS, Georgiades C et al (2023) The importance of optimal thermal ablation margins in colorectal liver metastases: a systematic review and meta-analysis of 21 studies. Cancers 15:5806
Laimer G, Schullian P, Putzer D, Eberle G, Goldberg SN, Bale R (2020) Can accurate evaluation of the treatment success after radiofrequency ablation of liver tumors be achieved by visual inspection alone? Results of a blinded assessment with 38 interventional oncologists. Int J Hyperthermia 37:1362–1367
Schaible J, Pregler B, Bäumler W et al (2020) Safety margin assessment after microwave ablation of liver tumors: inter- and intrareader variability. Radiol Oncol 54:57–61
Kaye EA, Cornelis FH, Petre EN et al (2019) Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol 29:2698–2705
Laimer G, Jaschke N, Schullian P et al (2021) Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur Radiol 31:6489–6499
Faber RA, Burghout KST, Bijlstra OD et al (2022) Three-dimensional quantitative margin assessment in patients with colorectal liver metastases treated with percutaneous thermal ablation using semi-automatic rigid MRI/CECT-CECT co-registration. Eur J Radiol 156:110552
Ruiter SJS, Tinguely P, Paolucci I et al (2021) 3D quantitative ablation margins for prediction of ablation site recurrence after stereotactic image-guided microwave ablation of colorectal liver metastases: a multicenter study. Front Oncol 11:757167
Minier C, Hermida M, Allimant C et al (2022) Software-based assessment of tumor margins after percutaneous thermal ablation of liver tumors: a systematic review. Diagn Interv Imaging 103:240–250
Verdonschot KHM, Arts S, Van den Boezem PB et al (2023) Ablative margins in percutaneous thermal ablation of hepatic tumors: a systematic review. Expert Rev Anticancer Ther 23:977–993
Puijk RS, Nieuwenhuizen S, van den Bemd BAT et al (2020) Transcatheter CT hepatic arteriography compared with conventional CT fluoroscopy guidance in percutaneous thermal ablation to treat colorectal liver metastases: a single-center comparative analysis of 2 historical cohorts. J Vasc Interv Radiol 31:1772–1783
Bale R, Schullian P, Eberle G et al (2019) Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology 70:840–850
Bale R, Widmann G, Haidu M (2011) Stereotactic radiofrequency ablation. Cardiovasc Intervent Radiol 34:852–856
Puijk RS, Ahmed M, Adam A et al (2021) Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology 301:533–540
Lin YM, Paolucci I, Albuquerque Marques Silva J et al (2023) Intraprocedural versus initial follow-up minimal ablative margin assessment after colorectal liver metastasis thermal ablation: Which one better predicts local outcomes? Invest Radiol 9:314–319
Wang X, Sofocleous CT, Erinjeri JP et al (2013) Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 36:166–175
Kurilova I, Bendet A, Petre EN et al (2021) Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer 20:e82–e95
Vasiniotis Kamarinos N, Gonen M, Sotirchos V et al (2022) 3D margin assessment predicts local tumor progression after ablation of colorectal cancer liver metastases. Int J Hyperthermia 39:880–887
Pereira PL, Bale R, Fretland AA (2023) Local tumour control following microwave ablation: protocol for the prospective observational CIEMAR study. Cardiovasc Intervent Radiol. https://doi.org/10.1007/s00270-023-03573-0
Lin YM, Paolucci I, Anderson BM et al (2022) Study protocol COVER-ALL: clinical impact of a volumetric image method for confirming tumour coverage with ablation on patients with malignant liver lesions. Cardiovasc Intervent Radiol 45:1860–1867
Puijk RS, Ruarus AH, Vroomen LGPH et al (2018) Colorectal liver metastases: surgery versus thermal ablation (COLLISION)—a phase III single-blind prospective randomized controlled trial. BMC Cancer 18:821
Funding
This study was partially funded by Stiftung Propter Homines, Vaduz, Liechtenstein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Christiaan G. Overduin.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: M.R.M. Medtronic-Covidien, Johnson & Johnson, Angiodynamics, Philips, Immunophotonics, and Guerbet. R.B. with Interventional Systems. The remaining authors declare no conflicts of interest.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Only if the study is on human subjects: written informed consent was waived by the Institutional Review Board. For this retrospective study, informed consent to use and exchange pseudonymized data was waived by the local institutional review board at each institution (2018–2053; 2020.077; 1316/2021) for all study participants. All data was shared under data sharing agreements (A20-0423; A21-0442) in compliance with local regulations.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
In a prior study (Laimer 2021—PMID: 33447860), we reported on 45 of 113 patients included in the current study. The previous study was a single-center study utilizing one confirmation software (Ablation-fit). The current study expands on this by having a multicenter cohort and all data analyzed by a second confirmation software, providing a significantly increased study population and additional and new analyses per software, including inter-software agreement. The extent of overlap has been explicitly mentioned in the Materials and Methods section of the manuscript (Page 4, 1st paragraph), including reference to the prior study.
Methodology
-
Retrospective
-
Observational
-
Multicenter study
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laimer, G., Verdonschot, K.H.M., Kopf, L. et al. Multicenter and inter-software evaluation of ablative margins after thermal ablation of colorectal liver metastases. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10956-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10956-5