Abstract
Objective
In nonalcoholic fatty liver disease (NAFLD), liver fibrosis is the strongest predictor of adverse outcomes. We sought to investigate the relationship between liver fibrosis and cardiac remodeling in participants from the general population using magnetic resonance imaging (MRI), as well as explore potential mechanistic pathways by analyzing circulating cardiovascular biomarkers.
Methods
In this cross-sectional study, we prospectively included participants with type 2 diabetes and individually matched controls from the SCAPIS (Swedish CArdioPulmonary bioImage Study) cohort in Linköping, Sweden. Between November 2017 and July 2018, participants underwent MRI at 1.5 Tesla for quantification of liver proton density fat fraction (spectroscopy), liver fibrosis (stiffness from elastography), left ventricular (LV) structure and function, as well as myocardial native T1 mapping. We analyzed 278 circulating cardiovascular biomarkers using a Bayesian statistical approach.
Results
In total, 92 participants were enrolled (mean age 59.5 ± 4.6 years, 32 women). The mean liver stiffness was 2.1 ± 0.4 kPa. 53 participants displayed hepatic steatosis. LV concentricity increased across quartiles of liver stiffness. Neither liver fat nor liver stiffness displayed any relationships to myocardial tissue characteristics (native T1). In a regression analysis, liver stiffness was related to increased LV concentricity. This association was independent of diabetes and liver fat (Beta = 0.26, p = 0.0053), but was attenuated (Beta = 0.17, p = 0.077) when also adjusting for circulating levels of interleukin-1 receptor type 2.
Conclusion
MRI reveals that liver fibrosis is associated to structural LV remodeling, in terms of increased concentricity, in participants from the general population. This relationship could involve the interleukin-1 signaling.
Clinical relevance statement
Liver fibrosis may be considered a cardiovascular risk factor in patients without cirrhosis. Further research on the mechanisms that link liver fibrosis to left ventricular concentricity may reveal potential therapeutic targets in patients with non-alcoholic fatty liver disease (NAFLD).
Key Points
-
Previously, studies on liver fibrosis and cardiac remodeling have focused on advanced stages of liver fibrosis.
-
Liver fibrosis is associated with left ventricular (LV) concentricity and may relate to interleukin-1 receptor type 2.
-
Interleukin-1 signaling is a potential mechanistic interlink between early liver fibrosis and LV remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD, also termed metabolic dysfunction-associated steatotic liver disease—MASLD), estimated to affect more than half of the global type 2 diabetes population [1], has emerged as a risk factor for cardiac remodeling and development of heart failure [2,3,4]. Although the mechanisms are not yet fully understood, the underlying degree of hepatic fibrosis in NAFLD has been linked to both higher mortality in acute heart failure [5] and left ventricular (LV) diastolic dysfunction [6, 7].
To date, however, research on liver fibrosis and cardiac remodeling has focused on patients from tertiary centers with advanced liver scarring (i.e., cirrhosis) [8,9,10]. This is despite the fact that NAFLD is typically a long-term progressive disease, where cirrhosis is preceded by several years of steatosis, inflammation, and subclinical fibrosis in the liver [11]. Few studies have reported on the relationship between liver fibrosis and cardiac remodeling in patients free of cirrhosis. Moreover, none of these studies has used magnetic resonance imaging (MRI), a method that provides accurate and non-invasive measurements of both hepatic fat content and fibrosis [12], as well as enabling detailed assessment of cardiac function, structure, and tissue characteristics [13, 14].
In order to investigate the relationships between liver fibrosis and cardiac remodeling in the early stages of fatty liver disease, we applied advanced cardiac and hepatic magnetic resonance techniques in participants sampled from the general population. The aim was to assess the relationships between liver fibrosis and cardiac structure, function, and tissue characteristics. We also sought to explore potential underlying mechanistic pathways by analyzing circulating biomarkers of cardiovascular inflammation and fibrosis. The hypothesis was that liver fibrosis had a significant association to LV structure, function, and tissue characteristics.
Methods
Study design and population
The study was approved by the Regional Ethics Committee Linköping and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent to participate.
The current study was a prospective cross-sectional sub-study within SCAPIS (Swedish CArdioPulmonary bioImage Study). SCAPIS is a nation-wide cohort study including over 30,000 men and women in the ages 50–65, all randomly sampled from the general Swedish population [15]. As described previously [16], we consecutively recruited participants with type 2 diabetes (T2D) as well as controls free of diabetes from the SCAPIS cohort in Linköping (n = 5 000). Participants were identified as having diabetes through SCAPIS health forms, having fasting P-glucose ≥ 7.0 mmol/L on two occasions or HbA1C ≥ 48 mmol/mol by sampling at SCAPIS. Controls were individually matched to the diabetes participants with respect to age, sex, and smoking habits. Participants with type 1 diabetes, general contraindications to MRI, or significantly irregular ventricular rhythm were excluded.
SCAPIS data
All subjects in this study underwent the core SCAPIS data acquisition. Accordingly, we had access to the following data for our study population: demographics (age, sex), alcohol consumption and smoking, self-reported cardiac disease (coronary heart disease, atrial fibrillation, heart failure or valvular heart disease), drug use, anthropometrics (height, weight, and body mass index [BMI]), clinical biochemistry and echocardiography (diastolic functional parameters).
See Supplementary material for additional information on the acquisition and analysis of echocardiographic data.
Magnetic resonance
Between November 2017 and July 2018, and within four weeks after the core SCAPIS data collection, all participants underwent a magnetic resonance scan at Linköping University Hospital. Data were acquired in a single scan at 1.5 T using Philips Achieva dStream (Philips Healthcare). The scan covered several sequences, e.g., for quantification of liver fat, liver stiffness, LV structure, and function, as well as LV tissue characteristics.
Liver
Elastography
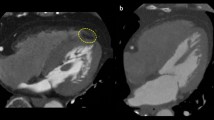
As a metric of fibrosis in the liver, liver stiffness was measured using 3D magnetic resonance elastography (MRE). The procedure was performed as previously described [17]. In short, an active electrodynamic transducer at 56 Hz was used, positioned to the right of the xiphoid process on the anterior chest wall, and held firmly in place using a broad elastic band. From four breath holds, a 3D image was acquired with nine slices, each with a slice thickness of 4 mm. Mean shear stiffness was determined using the KIR-software (for 3D processing), originally described in [18], and the results were reported in kPa. Regions of interest for the ROI measurements were defined away from large vessels and organ edges and drawn in the right liver lobe. See Fig. 1 for an example liver MR (magnetic resonance) elastogram.
A Liver elastogram from magnetic resonance elastography (3D MRE) in a 53-year-old male in the diabetes group, with a liver shear stiffness of 2.2 kPa. The position of the external transducer on the right anterior chest wall and the axial waves are schematically illustrated. The region of interest (ROI-2) for measurement of shear stiffness, located in the right liver lobe, is shown in blue (dashed). The larger region of interest (ROI-1) for defining the input region to the KIR-software is shown in orange (dotted), and the coloured overlay shows the result of the calculation within the region representing liver, on a background anatomical image. The image is presented using radiological convention. B Liver proton spectra from magnetic resonance spectroscopy, used for proton density fat fraction (PDFF) calculation, with water (H2O) resonance at 4.76 ppm and major fatty-acyl chain (-CH2-) resonance at 1.21 ppm
Spectroscopy
Liver proton density fat fraction (PDFF) was measured using magnetic resonance spectroscopy (MRS), as described in previous publications [16, 19, 20]. In short, data was acquired with a 1H-MRS PRESS sequence and subsequently post-processed to quantify the integrals of water and fat resonance, using the jMRUI and AMARES algorithms, respectively [21, 22]. Regions of interest of 30 × 30 × 30 mm3 were placed in the right liver lobe, in an area free from major blood vessels. All resonances from different lipid moieties were included in the spectral analysis. See Fig. 1 for an example liver spectrum from the MRS sequence.
Cardiac
Cine imaging
A 3D cine balanced steady state-free precession sequence was used to assess LV structure (mass, end-diastolic volume, concentricity) and function (ejection fraction, stroke volume). Images were acquired during breath hold and subsequently post-processed by a reader (CE) with five years of cardiac imaging experience using Segment CMR version 3.2 R8452 (Medviso AB) [23]. LV mass was acquired in end-diastole, and the papillary muscles were regarded as part of the blood pool. LV concentricity was calculated as the ratio between LV mass and LV end-diastolic volume. Cardiac MR has previously shown excellent reproducibility for LV mass and volumes [24]. Where applicable, the Mosteller algorithm [25] was used to index the LV parameters and left atrial volume to body surface area (BSA): BSA (m2) = (height (cm) × weight (kg)/3600)0.5. See Fig. 2 for example images from the cardiac cine sequence.
T1 and T2 mapping
Quantitative mapping of native T1 and T2 of the heart was performed using 3D-QALAS (3D-quantification using an interleaved Look-Locker sequence with T2 preparation pulse), enabling three-dimensional mapping in the whole heart during a single breath hold [26, 27]. Quantitative maps were generated and post-processed using software SyMRI version 0.45.38 (SyntheticMRI AB). All datasets were assessed by C.E. Ten datasets were randomly selected for inter- and intrareader variability analysis and assessed by E.K. (two years of cardiac imaging experience), as well as a second time by C.E. 8 weeks after the first assessment. As proposed by Puntmann et al [28], regions of interest were drawn manually in a short axis view, in the septal wall of all 3 to 5 mid-ventricular slices where the papillary muscles of the LV were visible. In order to avoid border artefacts towards the blood pool, only the innermost ~50% of the thickness of the septal myocardium was included. Average T1 and T2 values were calculated from all slices. See Fig. 3 for an example image from the 3D-QALAS sequence.
Proteomics
Blood samples were collected during the core SCAPIS data collection and accessible through the Linköping bio-bank facility. Three commercially available biomarker panels were analyzed externally using proximity extension assay technology: Cardiovascular II, Cardiovascular III, and Cardiometabolic (Olink Proteomics AB), totaling 276 unique biomarkers for cardiovascular inflammation, metabolism, and remodeling. In addition, PRO-C6, a marker of fibroblast activity, was also analyzed externally (Nordic Bioscience). A linear scale was used for all biomarkers.
Statistical analyses
Group comparisons and linear regression analyses
Statistical comparison of groups and quartiles, as well as multiple linear regression analyses, were performed in IBM SPSS version 29. Independent samples T-tests were used to compare continuous data between the diabetes and the control group. Chi-square tests were used to compare categorical data between the two groups. To compare cardiac parameters between quartiles of liver stiffness and liver PDFF, we used the Kruskal-Wallis test for all parameters due to n < 30 in each quartile. Intra- and interreader variability was assessed by single measures intraclass correlation coefficient (ICC), using the absolute-agreement two-way mixed-effect model. Pearson correlation coefficients were used to assess the relationships of cardiac remodeling parameters to clinical liver biochemistry. Multiple linear regression was used to assess linear relationships between cardiac remodeling and hepatic MRI parameters as well as to cardiovascular proteomics. Due to left-skewness, the liver fat percentage was logarithmically transformed for the linear regression analyses. For all regression models, the normal distribution of residuals was affirmed using Shapiro-Wilks test. For each model, we calculated the p value and standardized beta coefficient for each independent variable, as well as the overall R2 value. p values < 0.05 were considered statistically significant.
Stochastic search variable selection
To explore the relationships between the circulating cardiovascular proteomics and measures of LV remodeling, we used the Stochastic Search Variable Selection (SSVS) [29, 30]. SSVS is a Bayesian statistical method that, for a given dependent variable, efficiently samples a smaller subset of predictors from a larger set of predictors. This is done by stochastically iterating over thousand several subsets of predictors to simultaneously model the strength of predictors and uncertainty in the regression parameters. The proportion of times each predictor is included in the model, the ‘posterior inclusion probability’, can be used to assess the relative strength for each predictor. The closer the posterior inclusion probability is to 1, the stronger a predictor is to explain the dependent variable.
SSVS was performed by BW using the R package SSVS. Four models were estimated, each with a different dependent variable: LV concentricity, LV end-diastolic volume, LV mass and LV E/é. All biomarkers were used in all four models. We selected a standard choice of non-informative prior distributions, with a prior inclusion probability of 0.5 for each predictor. As an analogous interpretation of Bayes factors [31], a posterior inclusion probability above ~0.76 was considered substantial and above ~0.91 was considered strong. Less-than-substantial predictors were not considered. Beta coefficients were standardized, to enable comparison between predictors.
Results
Clinical and biochemical data
92 participants were enrolled (mean age 59.5 ± 4.6 years, 32 women); 46 with type 2 diabetes and 46 controls without diabetes. 11 participants (12.0%) reported previous cardiac disease. The average alcohol consumption was 45 g/week. One participant reported excessive alcohol consumption (> 210 g/week). Women had higher high-density lipoprotein (HDL) cholesterol than men (1.8 vs. 1.3 mmol/L, p < 0.001), otherwise there were no sex differences in clinical and biochemical data.
The diabetes group had higher BMI (29.6 vs. 26.2 kg/m2, p < 0.001), higher HbA1c (54 vs. 34 mmol/mol, p < 0.001), and higher alanine aminotransferase (ALT) (0.70 vs 0.50 µkat/L, p = 0.017) compared to the control group. 10 participants in the diabetes group (21.7%) had insulin treatment (Table 1).
Magnetic resonance and echocardiography data
The mean liver PDFF for all 92 subjects was 8.7%, where the diabetes group displayed higher liver PDFF than the control group (11.7 vs. 5.7%, p < 0.001). A total of 53 participants (57.6%) displayed hepatic steatosis defined as liver PDFF ≥ 5%, 33 (71.7%) in the diabetes group and 20 (43.5%) in the control group (p = 0.011 for group difference). The mean liver stiffness value was 2.1 kPa in the diabetes group and 2.0 kPa in the control group (p = 0.062 for group difference). The highest observed liver stiffness value was 2.9 kPa.
Compared to the control group, the diabetes group had lower LV end-diastolic volume (69.6 vs. 76.0 mL/m2, p = 0.047), higher LV concentricity (0.91 vs. 0.79 g/mL, p < 0.001), and higher LV E/é ratio (13.0 vs. 9.9, p < 0.001). There were no statistically significant differences in LV ejection fraction, LV stroke volume, LV mass, septal T1, septal T2, left atrial volume, or LV E/A ratio (Table 2).
In 15 cases, septal T1 and T2 could not be adequately quantified due to image quality issues and were therefore excluded in the statistical analyses. For native T1, ICC for intra- and interreader agreement was 0.998 and 0.996, respectively.
Circulating cardiovascular biomarkers
For all four Bayesian models, predictors that were at least substantial (posterior inclusion probability > 0.76) were as follows:
-
IL-1RT2 (interleukin-1 receptor type 2) was a strong positive predictor for LV concentricity.
-
Leptin was a strong negative predictor for both LV mass and LV end-diastolic volume.
-
BNP (brain natriuretic peptide) was a strong positive predictor for LV mass, and a substantial positive predictor for LV end-diastolic volume.
For LV E/é, no predictors passed the substantial cut-off in posterior inclusion probability.
Relationship between liver characteristics and cardiac remodeling
ALT was significantly correlated to LV concentricity (R = 0.23, p = 0.029). GGT was significantly correlated to LV end-diastolic volume (R = −0.25, p = 0.017), LV concentricity (R = 0.31, p = 0.0030), and LV E/é ratio (R = 0.435, p < 0.001).
Over quartiles of liver stiffness, there was an increase in LV concentricity (p = 0.0032) (Table 3, Fig. 4). Over quartiles of liver PDFF, there was a decrease in LV end-diastolic volume (p = 0.0025), an increase in LV concentricity (p = 0.0017), and an increase in LV E/é ratio (p = 0.015) (Table 4).
In a multiple linear regression analysis, liver stiffness was positively associated with LV concentricity (Beta = 0.26, p = 0.0053), independently of liver PDFF and diabetes. This association was attenuated (Beta = 0.17, p = 0.077), when also adjusting the model for levels of IL-1 RT2 (Table 5).
Discussion
In this study, using advanced magnetic resonance techniques, we found that a majority of the participants displayed hepatic steatosis. NAFLD is likely the predominating etiology for these cases, as only one participant reported excessive alcohol consumption, and due the fact that NAFLD is the dominant cause of chronic liver disease in the Swedish population.
In spite of the high proportion of subjects with hepatic steatosis, liver stiffness values were not suggestive of advanced liver fibrosis in any participant. To the best of our knowledge, there are no published reference values on liver fibrosis stages, for the specific 3D MRE method with a transducer frequency of 56 Hz used in the current study. However, previous studies using 60–65 Hz find cut-off values for cirrhosis ranging from 4.1 to 4.4 kPa [32, 33], which is more than 1 kPa above the highest value of 2.9 kPa observed in our cohort. We believe this gap is too large to be explained by different MRE settings, thus still suggesting the absence of advanced liver fibrosis among our subjects. Nonetheless, stiffness in the liver was related to increased LV concentricity, independently of liver fat and diabetes. This association was attenuated when also adjusting for circulating levels of IL-1 receptor type 2, the biomarker with the strongest association to LV concentricity in the Bayesian statistical analysis.
Pre-cirrhotic liver fibrosis and cardiac remodeling
The term ‘liver-heart axis’ [8], has been used to describe the phenomenon where the diseased liver adversely affects the heart. In liver cirrhosis, patients are prone to developing ‘cirrhotic cardiomyopathy’, a distinct cardiac phenotype characterized by impaired systolic response to stress, resting diastolic impairment and QT interval prolongation [34]. With the emerging ability to study cardiac and hepatic tissues through magnetic resonance techniques, several recent MRI studies have linked cirrhotic cardiomyopathy to a concurrent increase in diffuse myocardial fibrosis [8, 9], a process that appears to reverse when cirrhosis is treated with liver transplantation [10].
However, only two previous studies have specifically investigated the relationship between LV remodeling and liver stiffness in non-cirrhotic individuals. Using ultrasound-based liver elastography, Lee et al [6] studied patients with and without NAFLD and showed that both liver fat as well as liver stiffness was associated with higher resting LV E/é. Canada et al [7] found in 36 subjects with NAFLD that increasing histological severity of liver fibrosis was associated with lower peak oxygen uptake during exercise, higher LV E/é during exercise, and slower heart rate recovery after exercise, but no relationship was found to resting LV E/é. Notably, both of these studies used echocardiography to determine cardiac structure and function, and neither study reported any clear relationship between cardiac structure and liver fibrosis.
Compared to earlier works, our current study provides several significant contributions to the so far limited literature on early liver fibrosis and LV remodeling, as MRI studies on participants from the general population are lacking [35]. As Canada et al and Lee et al could only demonstrate relationships to diastolic function, our more accurate assessment of cardiac morphology from cardiac MRI, add new insights into the specific structural aspects of LV remodeling in relation to liver fibrosis. Moreover, we used magnetic resonance techniques also for the assessment of liver characteristics. This method enables high-precision and noninvasive measurement of both liver fat and liver stiffness and outperforms ultrasound techniques, at least in terms of liver fat quantification [12]. Although liver biopsy is regarded as the best standard method to assess liver fibrosis [36], the limited number of subjects in the study by Canada et al may reflect the high cost and invasiveness associated with this method. Last, our extensive analysis of circulating cardiovascular biomarkers, as discussed below, also has the potential to provide new mechanistic understandings of the relationship between liver fibrosis and LV remodeling.
Although concentric LV remodeling was independently related to both liver fat and fibrosis, we did not find any evidence of concurrent alterations in native septal T1 or T2. One possible explanation is that LV remodeling in this setting is not followed by any simultaneous change in myocardial tissue characteristics, such as diffuse myocardial fibrosis. Another perhaps more likely explanation is that potential myocardial tissue alterations are too subtle to be detected by native T1 mapping. When Kim et al found that a significant decrease in myocardial extracellular volume (ECV) fraction occurred after liver transplantation, they observed no changes in native T1 [10]. This supports the fact that alterations in myocardial tissue characteristics could be detected through, for example, ECV quantification but not by native T1 mapping.
Mechanistic interlinks and circulating cardiovascular biomarkers
To explore possible mechanistic interlinks between cardiac remodeling and the liver, we analyzed cardiovascular biomarkers of inflammation and fibrosis and found that the association between liver stiffness and LV concentricity was attenuated when adjusting for circulating levels of interleukin-1 receptor type 2 (IL-1RT2). This could indicate a mechanistic role of IL-1RT2 in concentric LV remodeling. IL-1RT2 is a soluble decoy receptor that negatively regulates the inflammatory activity of IL-1β [37]. Circulating levels of IL-1RT2 have previously been associated with long-term adverse cardiac remodeling. Orrem et al [38] found that levels of IL-1RT2 were elevated in ST-elevation myocardial infarction and correlated with higher myocardial infarct size and long-term adverse LV remodeling, including lower LV ejection fraction four months after infarction. The authors proposed that, as IL-1RT2 negatively regulates IL-1β stimuli, it might counteract the beneficial inflammatory response necessary for infarct healing and adaptive LV remodeling. Similar to Orrem et al, we did not find any associations to LV remodeling for IL-1 receptor antagonist protein or IL-1 receptor type 1.
Though the structural and functional cardiac alterations are likely to have a different underlying nature in liver disease compared to in myocardial infarction, it appears at least plausible that IL-1RT2 is related to cardiac remodeling. Moreover, as IL-1 family cytokines have been shown to play an important role for hepatic inflammation and fibrosis progression in NAFLD [39, 40], the role of IL-1 cytokines as a mechanistic interlink between liver fibrosis and LV remodeling is suggestive and may warrant further research.
Limitations
The current study poses considerable strengths in the study of LV remodeling and liver disease, such as the use of magnetic resonance techniques for the assessment of both liver and heart, as well as the extensive number of analyzed cardiovascular biomarkers. However, some limitations need to be mentioned. First, as no liver biopsies were performed on the participants in this study, we are unable to determine histopathological inflammation in the liver. Second, no external contrast agent was administered during the magnetic resonance examinations, and we are therefore unable to assess late gadolinium enhancement and ECV in the myocardium. Last, further research on larger study populations could improve the generalizability of the findings.
Conclusions
In the current study, advanced magnetic resonance imaging was used to bring novel insights into the liver-heart axis in participants from the general population. The results show that liver fibrosis is independently associated with concentric LV remodeling, a relationship that may involve IL-1 signaling. Future studies could establish causal relationships between liver fibrosis and LV remodeling, as well as provide further understanding of the role of IL-1 signaling in this clinical context.
Abbreviations
- A:
-
Late diastolic velocity of mitral inflow
- ALT:
-
Alanine aminotransferase
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- E:
-
Early diastolic velocity of mitral inflow
- é:
-
Early diastolic mitral annular velocity
- GGT:
-
Gamma glutamyl transferase
- HDL:
-
High density lipoprotein
- IL-1RT2:
-
Interleukin 1 receptor type 2
- LV:
-
Left ventricular
- MRE:
-
Magnetic resonance elastography
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- NAFLD:
-
Non-alcoholic fatty liver disease
- PDFF:
-
Proton density fat fraction
- SCAPIS:
-
Swedish CArdioPulmonary bioImage Study
- SSVS:
-
Stochastic search variable selection
- T2D:
-
Type 2 diabetes
References
Younossi ZM, Golabi P, de Avila L et al (2019) The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 71:793–801. https://doi.org/10.1016/j.jhep.2019.06.021
Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G (2022) Risk of heart failure in patients with nonalcoholic fatty liver disease: JACC review topic of the week. J Am Coll Cardiol 79:180–191. https://doi.org/10.1016/j.jacc.2021.11.007
Fudim M, Zhong L, Patel KV et al (2021) Nonalcoholic fatty liver disease and risk of heart failure among medicare beneficiaries. J Am Heart Assoc 10:e021654
Styczynski G, Kalinowski P, Michałowski Ł et al (2021) Cardiac morphology, function, and hemodynamics in patients with morbid obesity and nonalcoholic steatohepatitis. J Am Heart Assoc 10:e017371
Valbusa F, Agnoletti D, Scala L et al (2018) Non-alcoholic fatty liver disease and increased risk of all-cause mortality in elderly patients admitted for acute heart failure. Int J Cardiol 265:162–168. https://doi.org/10.1016/j.ijcard.2018.04.129
Lee YH, Kim KJ, Yoo ME et al (2018) Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol 68:764–772. https://doi.org/10.1016/j.jhep.2017.11.023
Canada JM, Abbate A, Collen R et al (2019) Relation of hepatic fibrosis in nonalcoholic fatty liver disease to left ventricular diastolic function and exercise tolerance. Am J Cardiol 123:466–473. https://doi.org/10.1016/j.amjcard.2018.10.027
Isaak A, Praktiknjo M, Jansen C et al (2020) Myocardial fibrosis and inflammation in liver cirrhosis: MRI study of the liver-heart axis. Radiology 297:51–61. https://doi.org/10.1148/radiol.2020201057
Wiese S, Hove JD, Mo S et al (2019) Cardiac dysfunction in cirrhosis: a 2-yr longitudinal follow-up study using advanced cardiac imaging. Am J Physiol Gastrointest Liver Physiol 317:G253–G263. https://doi.org/10.1152/ajpgi.00402.2018
Kim HM, Kim H-K, Lee J-H et al (2020) Myocardial structural and functional changes in patients with liver cirrhosis awaiting liver transplantation: a comprehensive cardiovascular magnetic resonance and echocardiographic study. J Cardiovasc Magn Reson 22:25
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R (2015) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatology 13:643–654.e649. https://doi.org/10.1016/j.cgh.2014.04.014
Castera L, Friedrich-Rust M, Loomba R (2019) Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156:1264–1281.e1264. https://doi.org/10.1053/j.gastro.2018.12.036
Zhang X, Yang S, Hao S et al (2023) Myocardial fibrosis and prognosis in heart failure with preserved ejection fraction: a pooled analysis of 12 cohort studies. Eur Radiol. https://doi.org/10.1007/s00330-023-10218-w
Liu X, Gao Y, Guo Y-K et al (2022) Cardiac magnetic resonance T1 mapping for evaluating myocardial fibrosis in patients with type 2 diabetes mellitus: correlation with left ventricular longitudinal diastolic dysfunction. Eur Radiol 32:7647–7656. https://doi.org/10.1007/s00330-022-08800-9
Bergström G, Berglund G, Blomberg A et al (2015) The Swedish CArdioPulmonary BioImage Study: objectives and design. J Inter Med 278:645–659. https://doi.org/10.1111/joim.12384
Edin C, Ekstedt M, Scheffel T et al (2022) Ectopic fat is associated with cardiac remodeling-A comprehensive assessment of regional fat depots in type 2 diabetes using multi-parametric MRI. Front Cardiovasc Med 9:813427. https://doi.org/10.3389/fcvm.2022.813427
Forsgren MF, Nasr P, Karlsson M et al (2020) Biomarkers of liver fibrosis: prospective comparison of multimodal magnetic resonance, serum algorithms and transient elastography. Scand J Gastroenterol 55:848–859. https://doi.org/10.1080/00365521.2020.1786599
Sinkus R, Tanter M, Xydeas T, Catheline S, Bercoff J, Fink M (2005) Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn Reson Imaging 23:159–165. https://doi.org/10.1016/j.mri.2004.11.060
Karlsson M, Ekstedt M, Dahlström N et al (2019) Liver R2* is affected by both iron and fat: a dual biopsy‐validated study of chronic liver disease. J Magn Reson Imaging 50:325–333. https://doi.org/10.1002/jmri.26601
Nasr P, Forsgren M, Ignatova S et al (2017) Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology 153:53–55 e57. https://doi.org/10.1053/j.gastro.2017.03.005
Vanhamme L, Van Den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior nowledge. J Magn Reson Imaging 129:35–43. https://doi.org/10.1006/jmre.1997.1244
Naressi A, Couturier C, Devos JM et al (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12:141–152. https://doi.org/10.1007/bf02668096
Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H (2010) Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med Imaging 10:1. https://doi.org/10.1186/1471-2342-10-1
Grothues F, Smith GC, Moon JC et al (2002) Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 90:29–34. https://doi.org/10.1016/s0002-9149(02)02381-0
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098. https://doi.org/10.1056/nejm198710223171717
Kvernby S, Rönnerfalk M, Warntjes MJB et al (2018) Longitudinal changes in myocardial T1 and T2 relaxation times related to diffuse myocardial fibrosis in aortic stenosis; before and after aortic valve replacement. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25980
Kvernby S, Warntjes MJB, Haraldsson H, Carlhäll C-J, Engvall J, Ebbers T (2014) Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson 16:102
Puntmann VO, Peker E, Chandrashekhar Y, Nagel E (2016) T1 Mapping in characterizing myocardial disease: a comprehensive review. Circul Res 119:277–299. https://doi.org/10.1161/CIRCRESAHA.116.307974
George EI, McCulloch RE (1993) Variable Selection Via Gibbs Sampling. J Am Stat Assoc 88:881–889
Bainter SA, McCaulley TG, Wager T, Losin ER (2020) Improving practices for selecting a subset of important predictors in psychology: an application to predicting pain. Adv Methods Pract Psychol Sci 3:66–80. https://doi.org/10.1177/2515245919885617
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90:773–795
Han MAT, Vipani A, Noureddin N et al (2020) MR elastography‐based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver Int 40:2242–2251. https://doi.org/10.1111/liv.14593
Venkatesh SK, Yin M, Ehman RL (2013) Magnetic resonance elastography of liver. J Comput Assist Tomogr 37:887–896. https://doi.org/10.1097/rct.0000000000000032
Møller S, Bernardi M (2013) Interactions of the heart and the liver. Eur Heart J 34:2804–2811. https://doi.org/10.1093/eurheartj/eht246
Edin C, Ekstedt M, Karlsson M et al (2023) Liver fibrosis is associated with left ventricular remodeling: insights into the liver-heart axis. Eur Heart J 44:ehad655.2415
Bedossa P, Carrat F (2009) Liver biopsy: The best, not the gold standard. J Hepatol 50:1–3. https://doi.org/10.1016/j.jhep.2008.10.014
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–241. https://doi.org/10.1038/nrrheum.2010.4
Orrem HL, Shetelig C, Ueland T et al (2018) Soluble IL-1 receptor 2 is associated with left ventricular remodelling in patients with ST-elevation myocardial infarction. Int J Cardiol 268:187–192. https://doi.org/10.1016/j.ijcard.2018.05.032
Barbier L, Ferhat M, Salame E et al (2019) Interleukin-1 family cytokines: keystones in liver inflammatory diseases. Front Immunol 10:2014. https://doi.org/10.3389/fimmu.2019.02014
Tilg H, Moschen AR, Szabo G (2016) Interleukin‐1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64:955–965. https://doi.org/10.1002/hep.28456
Acknowledgements
We thank Charlotte Brage, Shan Cai, Markus Henningsson, Emilia Karlborg, Christian Simonsson, Jens Tellman and the Bio-bank facilities in Linköping, for their help with data management and technical assistance. We also thank the study participants in SCAPIS for enabling this research.
Funding
This study has received funding by the Swedish Research Council, the Swedish Heart and Lung Foundation, and from the ALF Grants Region Östergötland. Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Carl-Johan Carlhäll.
Conflict of interest
MW is a consultant for SyntheticMR AB and holds stock in SyntheticMR AB. The remaining authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all participants in this study.
Ethical approval
Institutional Review Board (Regional Ethics Committee Linköping) approval was obtained.
Study subjects or cohorts overlap
The current study population (n = 92) has previously undergone MR-based measurement of regional fat depots and LV structure and function. These results were reported in: Edin C, Ekstedt M, Scheffel T et al Ectopic fat is associated with cardiac remodeling—A comprehensive assessment of regional fat depots in type 2 diabetes using multiparametric MRI. Front Cardiovasc Med. 2022 Jul 28;9:813427. https://doi.org/10.3389/fcvm.2022.813427. In the current paper, several additional MR sequences were utilized, yielding data on liver fibrosis (MR elastography), myocardial tissue characteristics (native T1 and T2). We have also quantified circulating cardiovascular biomarkers of inflammation and fibrosis, as well as applied for and received access to additional core data from SCAPIS (e.g., liver biochemistry). The current results have been presented as a conference abstract at ESC Congress 2023 and published in an abstract supplement: Edin C, Ekstedt M, Karlsson M et al Liver fibrosis is associated with left ventricular remodeling: insights into the liver-heart axis. Eur Heart J, Volume 44, Issue Supplement_2, November 2023, ehad655.191, https://doi.org/10.1093/eurheartj/ehad655.191.
Methodology
-
Prospective
-
Cross-sectional study
-
Observational
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Edin, C., Ekstedt, M., Karlsson, M. et al. Liver fibrosis is associated with left ventricular remodeling: insight into the liver-heart axis. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10798-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10798-1