Abstract
Objectives
To compare the quantitative background parenchymal enhancement (BPE) in women with different lifetime risks and BRCA mutation status of breast cancer using screening MRI.
Materials and methods
This study included screening MRI of 535 women divided into three groups based on lifetime risk: nonhigh-risk women, high-risk women without BRCA mutation, and BRCA1/2 mutation carriers. Six quantitative BPE measurements, including percent enhancement (PE) and signal enhancement ratio (SER), were calculated on DCE-MRI after segmentation of the whole breast and fibroglandular tissue (FGT). The associations between lifetime risk factors and BPE were analyzed via linear regression analysis. We adjusted for risk factors influencing BPE using propensity score matching (PSM) and compared the BPE between different groups. A two-sided Mann–Whitney U-test was used to compare the BPE with a threshold of 0.1 for multiple testing issue-adjusted p values.
Results
Age, BMI, menopausal status, and FGT level were significantly correlated with quantitative BPE based on the univariate and multivariable linear regression analyses. After adjusting for age, BMI, menopausal status, hormonal treatment history, and FGT level using PSM, significant differences were observed between high-risk non-BRCA and BRCA groups in PEFGT (11.5 vs. 8.0%, adjusted p = 0.018) and SERFGT (7.2 vs. 9.3%, adjusted p = 0.066).
Conclusion
Quantitative BPE varies in women with different lifetime breast cancer risks and BRCA mutation status. These differences may be due to the influence of multiple lifetime risk factors. Quantitative BPE differences remained between groups with and without BRCA mutations after adjusting for known risk factors associated with BPE.
Clinical relevance statement
BRCA germline mutations may be associated with quantitative background parenchymal enhancement, excluding the effects of known confounding factors. This finding can provide potential insights into the cancer pathophysiological mechanisms behind lifetime risk models.
Key Points
-
Expanding understanding of breast cancer pathophysiology allows for improved risk stratification and optimized screening protocols.
-
Quantitative BPE is significantly associated with lifetime risk factors and differs between BRCA mutation carriers and noncarriers.
-
This research offers a possible understanding of the physiological mechanisms underlying quantitative BPE and BRCA germline mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent noncutaneous malignancy among women and is ranked as the second leading cause of cancer-related deaths [1]. The standard imaging tool for breast cancer screening is digital mammography. However, mammography sensitivity may be compromised in women with dense-breast tissue, as the masking effect of such tissue can obscure potential malignancies [2]. As a result, the American Cancer Society recommends that women with a lifetime risk exceeding 20% of developing breast cancer undergo breast magnetic resonance imaging (MRI) screening in addition to mammography [3]. MRI and mammography combined screening improves breast cancer survival in these individuals [4].
Predicting breast cancer risk would enhance patient stratification and lead to tailored screening tactics. Established breast cancer risk assessment models such as the Gail, Claus, and Tyrer-Cuzick models are widely utilized for stratifying patients into different risk groups but offer moderate predictive accuracy at the individual level [5,6,7,8,9,10]. There is an ongoing interest in the physiological mechanisms underlying these risk models. One potential factor is fibroglandular tissue (FGT) enhancement after contrast injection, i.e., background parenchymal enhancement (BPE). Previous studies have revealed a possible correlation between BPE and breast cancer risk [11,12,13,14,15], although this relationship remains controversial [16, 17]. Potential variations in BPE’s vascular and molecular characteristics may account for differences in breast cancer risk [18]. It also remains uncertain whether quantitative BPE characteristics significantly differ between women with and without a high lifetime risk. Such studies may uncover the role of BPE as an underlying physiological factor behind the classical risk models.
The BRCA1 and BRCA2 genes, known as tumor suppressor genes, encode proteins essential for DNA repair. Germline mutations in the BRCA1/2 increase the risk of several cancers, most notably breast and ovarian cancers [19]. Comparison of BPE kinetic properties between BRCA mutation carriers and noncarriers could help explain the impact of BRCA on breast physiology, thereby further improving the diagnostics accuracy of MRI in populations at high risk of breast cancer. While previous studies have reported a lower BPE level in BRCA1/2 mutation carriers in high-risk women using qualitative and quantitative methods [18, 20, 21], Goodburn et al. [22] has presented contrasting findings by showing no differences in BPE between carriers and noncarriers. BPE level is known to be hormone-sensitive and is associated with menstrual cycle, age, menopausal status, corresponding to hormonal changes, and breast density [23, 24], which may contribute to the conflicting results.
The variability in radiologist-assigned BPE categories within and between readers highlights the necessity for the quantitative study of BPE. Therefore, our study aimed to compare quantitative BPE measurements based on screening MRI between radiologically normal women with and without high lifetime risk and between BRCA mutation carriers and noncarriers. We determined which clinical factors in classical risk models are associated with quantitative BPE measurements. In addition, we investigated whether differences in BPE remained after adjusting for clinical factors that might influence BPE.
Materials and methods
Patient population
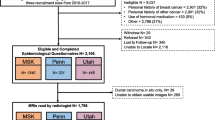
Our retrospective study was approved by the Institutional Review Board (IRB) and was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA). IRB waived the requirement to obtain informed consent. We reviewed 4859 contrast-enhanced bilateral breast MRI exams for women between January 2017 and December 2019. Exclusions were made for patients with prior breast cancer, mastectomy history, unknown lifetime risk scores, and tamoxifen use within the last 6 months. The MRI exams that were not for screening purposes or not eligible for BPE quantification were further excluded. Further exclusion details are in Fig. 1. Premenopausal women underwent MRI screenings during the second week of their menstrual cycle to minimize the amount of estrogen-induced BPE [25].
The following characteristics were collected for eligible women based on the records at the MRI exam: age, body mass index (BMI), menopausal status, personal history of hormonal therapy within six months before MRI, and genetic test results. Breast density was derived from the MRI report as four FGT levels (almost entirely fat, scattered FGT, heterogeneous FGT, and extreme FGT). Menopausal status was determined based on patient-submitted questionnaires. Two breast fellowship-trained radiologists evaluated BPE levels (W.M. and S.M.). The Tyrer-Cuzick model [7] was used to calculate the lifetime risk score. Based on these scores and BRCA1/2 mutation status, the eligible women were stratified into three groups: (1) nonhigh-risk group including women with lifetime risk < 20%; (2) high-risk non-BRCA group including women with 20% or higher lifetime risk without BRCA1/2 mutation; (3) BRCA group including women with BRCA1/2 mutation. Including nonhigh-risk women who underwent MRI scans could be attributed to the primary care providers’ limited familiarity with the latest screening guidelines or the patient’s personal choice for more comprehensive testing. Women with high-risk lesions and those with mantle field irradiation due to Hodgkin’s lymphoma were later eliminated from the nonhigh-risk group. The complete flowchart of the study is presented in Fig. 1.
MRI protocols
MRI scans were performed in the prone position in the axial plane on a 3-T scanner (Siemens Verio, Erlangen, Germany). Image sequences included a T1-weighted non-fat-suppressed (T1-NFS) image and a T1-weighted fat-suppressed dynamic contrast-enhanced (DCE) MRI series with one precontrast and four postcontrast images. Gadolinium-based contrast (Magnevist, Bayer, Leverkusen, Germany) was administered at 0.1 mmol/kg, 2 mL/s, followed by a 20 mL saline flush. The first postcontrast sequence was acquired 120 s after the precontrast sequence, with other postcontrast sequences acquired every 90 s. The breast MRI protocol details are in Supplementary Material S1.
FGT and BPE quantification
We used a fully automated method modified from a previous publication [26], to segment the whole breast and FGT. As shown in Fig. 2, after the N4 bias field correction [27], the entire breast and FGT three-dimensional volumes were segmented using a 3D U-net deep learning model on T1-NFS images. After applying image rigid registration, the segmented masks were transferred to the DCE-MRI series. The anterior border of the pectoralis muscle was defined as the edge between the breast and chest. The nipple and skin were excluded from the whole breast segmented masks. Vessels were excluded from the FGT segmentation masks if they were visible.
Overview of BPE quantification process. The three-dimensional volumes of breast and FGT were segmented on T1-NFS images. The segmented masks of the breast (red outlines) and FGT (green) were then transferred to the DCE-MRI. Enhancement maps, including the PE map and SER map, were generated based on the masks from which PEFGT, PEBreast, SERFGT, and SERBreast were derived. The primary eigenvector of DCE-MRI was used to calculate another two BPE measurements, i.e., IER and DER. DCE dynamic contrast-enhanced, PE percent enhancement, SER signal enhancement ratio, IER initial enhancement ratio, DER delayed enhancement ratio
We measured PE, representing wash-in enhancement characteristics, and SER, representing delayed enhancement characteristics, over the FGT volume (FGT-wise BPE) and the whole breast volume (breast-wise BPE). The four quantitative BPE measurements were denoted as PEFGT, PEBreast, SERFGT, and SERBreast. The enhancement ratio threshold for each voxel in the PE map and SER map were set as 30% and 90%, respectively, referring to previous studies [28, 29]. The details of these four quantitative BPE calculations are explained in Supplementary Material S2. We also measured the IER and DER of the BPE based on the three-dimensional volume of the FGT. We applied the principal component analysis (PCA) method [18, 25] to the DCE-MRI image series. The principal eigenvector with the highest eigenvalue captures the maximum signal fluctuation from enhancing tissue in the FGT volume, which is supposed to reflect BPE kinetics. Therefore, based on the principal eigenvector, IER was defined as the percent increase of postcontrast 120-s early phase compared to precontrast, and DER was the percent increase of postcontrast 390-s delayed-phase compared to precontrast, as shown in Fig. 2. BPE quantification was performed using MATLAB (MathWorks, Natick, MA).
Statistical analyses
We reported descriptive statistics for study group characteristics. We conducted two comparisons: high-risk non-BRCA vs. nonhigh-risk (among women without BRCA1/2 mutations) and high-risk non-BRCA vs. BRCA (among high-risk women). Ages and BMI were compared using the Mann–Whitney U-test, while menopausal status, hormonal treatment history, FGT level, and BPE level were compared using the chi-squared test with a significance level of 0.05.
Furthermore, corresponding to the data distribution, two-sided Mann–Whitney U-tests were used to compare the BPE measurements. To account for multiple testing issues, Bonferroni procedure was applied to correct the significance level for the number of hypothesis testing, m, with the family-wise error rate (FWER) at threshold of 0.1. The patients were further divided into subcohorts based on breast density and menopausal status. “Dense-breast women” includes women with heterogeneous and extreme FGT. Quantitative BPE was compared within the subcohorts, and adjusted p values, as unadjusted p value/m, were reported.
We used univariate and multivariable linear regression analysis to evaluate the association between BPE measurements and clinical factors, including age, BMI, menopausal status, hormonal treatment, FGT level, and BRCA gene mutation status. We reported correlation coefficients with 95% confidence intervals and corresponding p values with a significance level of 0.05.
Since BPE is sensitive to endogenous hormonal changes and other factors [23, 24], we used propensity score matching (PSM) to control for confounders [30], including age, BMI, menopausal status, hormonal treatment history, and FGT level. PSM was performed twice using nearest-neighbor matching at a 1:1 ratio, first matching nonhigh-risk (N = 71) to high-risk non-BRCA and then BRCA (N = 165) to high-risk non-BRCA. We reported patient characteristics after PSM and compared BPE measurements in matched groups.
We tested the reliability of our results by examining the effect of BPE thresholds and early phase selection on BPE differences. PE and SER were estimated using thresholds of 10–90% in 10% increments and different postcontrast phases as the early phase. We compared BPE between risk groups using the Mann–Whitney U-test for each threshold and phase. We reported unadjusted p values with a significance level of 0.05. Statistical analyses were performed using Python’s library SciPy (version 1.9.3) and R (version 4.1.2).
Results
The final cohort includes 535 eligible patients (high-risk non-BRCA, 299 patients; nonhigh-risk, 71 patients; BRCA, 165 patients). The clinical and radiographic characteristics of the study cohort are summarized in Table 1. There were significant differences in age, menopause status, FGT level, and BPE level distribution in the pairwise comparison of the three groups. The BMI significantly differed between high-risk non-BRCA and BRCA groups (unadjusted p = 0.018).
Comparisons of quantitative BPE for all women
As shown in Table 2, after adjusting for multiple testing issues, for high-risk non-BRCA vs. nonhigh risk, we found significant differences in PEbreast (1.6 vs. 0.8%, adjusted p < 0.001), SERFGT (7.4 vs. 10.2%, adjusted p = 0.006), IER (33.5 vs. 26.4%, adjusted p = 0.018), and DER (85.6 vs. 68.4%, adjusted p = 0.005). For high-risk non-BRCA vs. BRCA, we found significant differences in PEFGT (10.1 vs. 8.0%, adjusted p = 0.036), PEbreast (1.6 vs. 1.0%, adjusted p = 0.005), SERFGT (7.4 vs. 9.3%, adjusted p = 0.047), IER (33.5 vs. 28.2%, adjusted p = 0.047), and DER (85.6 vs. 73.9%, adjusted p = 0.035). Figure 3 provides a detailed illustration of BPE data distributions with adjusted p values.
Comparisons of quantitative BPE for subcohorts
The comparison of women stratified by breast density is shown in Table 3. In dense-breast women, significant differences in PEFGT, PEBreast, IER, and DER exist between the high-risk non-BRCA and nonhigh-risk groups. Figure 4 displays example images of two dense-breast women. The high-risk non-BRCA woman (Fig. 4a) has higher PEFGT, PEbreast, IER, and DER than the nonhigh-risk woman (Fig. 4b). In nondense-breast women, the difference in PEBreast between high-risk non-BRCA and BRCA groups is significant (0.6 vs. 0.4%, adjusted p = 0.096). Figure 5 presents example images of two nondense-breast women. The high-risk non-BRCA woman (Fig. 5a) has higher PEBreast than the BRCA1-positive woman (Fig. 5b).
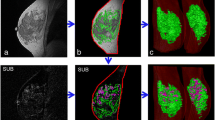
MRIs and BPE of two representative dense-breast normal-weight postmenopausal women with (a) high-risk non-BRCA mutation and (b) nonhigh-risk. MRI images include precontrast DCE-MRI, MIP of first postcontrast subtraction DCE-MRI, MIP of ten slices of PE map, and the primary eigenvector used to measure IER and DER. a A 49-year-old woman with a lifetime risk of 45.0% and a BMI of 22.45. FGT level is extreme fibroglandular tissue, and BPE level is marked. b A 53-year-old woman with a lifetime risk of 7.7% and a BMI of 20.08. FGT level is heterogeneously fibroglandular tissue, and BPE level is minimal
MRIs and BPE of two representative nondense-breast overweight/obesity postmenopausal women with (a) high-risk non-BRCA mutation and (b) BRCA mutation. MRI images include precontrast DCE-MRI, MIP of first postcontrast subtraction DCE-MRI, MIP of ten slices of PE map, and the primary eigenvector used to measure IER and DER. a A 53-year-old woman with a lifetime risk of 22.2% and a BMI of 25.12. FGT level is scattered fibroglandular tissue, and BPE level is marked. b A 48-year-old BRCA1-positive woman with a BMI of 31.46. FGT level is scattered fibroglandular tissue, and BPE level is mild
The comparison of women stratified by menopausal status is demonstrated in Supplementary Material S3. We found no significant differences in both the premenopausal subcohort and postmenopausal subcohort.
Correlation analysis of quantitative BPE
In the univariate analysis, age and menopausal status significantly correlate with all BPE measurements (Supplementary Material S4). However, only menopausal status significantly correlates with all BPE measurements in the multivariable analysis (Supplementary Material S5). With the identified difference for PEbreast comparison based on the smallest p value among the comparison for six measures in Table 2, linear regression results of PEbreast was further assessed in Table 4. Both univariate and multivariable analyses of PEbreast found significant correlations with age, BMI, menopausal status, and FGT level. Specifically, higher PEbreast was correlated with younger age, lower BMI, premenopausal status, and higher FGT levels.
Comparisons of quantitative BPE after PSM
The clinical and radiographic characteristics of the patient cohort after PSM are summarized in Supplementary Material S6. There were no significant differences in age, BMI, menopause status, hormone treatment history, and FGT level among matched groups. Table 5 shows a quantitative BPE comparison after PSM. We found no significant differences between the high-risk non-BRCA and nonhigh-risk groups in all BPE measurements. However, significant differences were observed between high-risk non-BRCA and BRCA groups in PEFGT (11.5 vs. 8.0%, adjusted p = 0.018) and SERFGT (7.2% vs. 9.3%, adjusted p = 0.066).
Analyses of enhancement thresholds and phase selection for BPE quantification
The Manhattan plots in Fig. 6 present the unadjusted p values for quantitative BPE comparisons using varying PE, SER enhancement thresholds, and postcontrast phases. We observed significant differences in both comparisons for PEFGT and PEbreast across all thresholds (10–90%) and all three postcontrast phases. SERFGT was significantly different using thresholds between 60 and 90% and using the second phase, while SERbreast was significantly different using thresholds between 10 and 40% and using the fourth phase in both comparisons.
Manhattan plot of the unadjusted value profiles of Mann–Whitney U-test comparing four quantitative BPE measurements, PEFGT, PEBreast, SERFGT, and SERBreast, computed by using (a, b) a wide range of BPE enhancement threshold and (c, d) different postcontrast phases as the early phase. A reference line with a p of 0.05 is shown as the gray dashed line. The data point above the reference line indicated a significant difference
Discussion
Our study demonstrated a difference in quantitative BPE among different groups stratified by lifetime breast cancer risk and BRCA germline mutation status. Specifically, BPE is higher for the high-risk non-BRCA group than for the nonhigh-risk group, especially in the dense-breast subcohort. More importantly, BPE is higher for the high-risk non-BRCA group than for the BRCA group, especially in the nondense-breast subcohort. Linear regression analysis shows that factors significantly affecting BPE include age, BMI, menopausal status, and FGT level. After adjusting for these confounding factors, the difference in BPE between BRCA carriers and noncarriers can still be observed.
There is ongoing controversy regarding the relationship between BPE and various lifetime risk factors. In our study, PEbreast is significantly associated with age, BMI, menopausal status, and FGT level. BPE is known to be hormone-sensitive, which could explain the potential reason for the impact of age and menopausal status on BPE [23, 24, 31]. Previous studies reported an association between higher BMI and higher qualitative BPE [31, 32], while the underlying mechanism is not fully understood. A possible explanation is that adipose tissue can serve as a significant source of estrogen [33]. In our analysis, however, BMI is inversely correlated with quantitative BPE, possibly due to differences in patient selection and BPE quantification methods. Besides, the positive correlation between FGT and BPE in our study is consistent with previous literature [34, 35].
Prior studies of qualitative and quantitative BPE have shown that high-risk women with higher BPE had a higher chance of breast cancer [15, 36]. Compared with previous studies that correlated BPE with cancer development and mainly focused on high-risk patients, our study included women without a high lifetime breast cancer risk. According to our findings, individuals with high lifetime risk at baseline tended to have higher BPE than those without before adjusting for confounding factors. This finding may be attributed to the difference in known factors, like age, menopausal status, and FGT levels, since the differences in BPE disappeared after adjusting for these confounding factors.
The results of our study in the BPE comparison of BRCA1/2 mutation carriers and noncarriers in high-risk patients are consistent with previous studies [18, 21], which found that BRCA mutation carriers had lower BPE than noncarriers. BRCA patients (assumed to be at the highest risk for developing breast cancer compared to everyone else) do not necessarily have the highest levels of BPE. After accounting for other potential influencing variables using PSM, these two groups have residual BPE differences. These findings may suggest that the BRCA germline mutation may affect quantitative BPE. Further investigation into the biological underpinnings of these effects is essential for leveraging quantitative BPE in breast cancer risk stratification.
In our study, we found that PEFGT and PEbreast showed consistent differences between different groups across a wide range of intensity enhancement ratio thresholds and postcontrast phases. Currently, there is no standardized approach for determining this threshold value and selecting this postcontrast phase in the BPE quantification process. The consistent results that we observed suggest the potential robustness of BPE measurement as a biomarker correlated with breast cancer lifetime risk.
Our study has some limitations. One limitation is that vessels are challenging to visualize in dense breasts with MRI. Some vessels were likely counted as FGT during the segmentation procedure, resulting in a little overestimation of FGT and BPE. Additionally, due to the limited number of patients, the inclusion of patients with germline mutations other than BRCA1/2, such as TP53, STK11, and ATM, as a separate group was not feasible. However, these gene mutations are less common, and there is still much to learn about them. The absence of short-term MRI follow-up might be a limitation in confirming the complete absence of breast cancer due to the retrospective nature of the study. However, our stringent screening process reduced the likelihood of including patients with breast cancer. Finally, to assess if this study may have wide clinical use, it is necessary to undertake more research in a prospective environment for extended validation.
In conclusion, our study reveals that quantitative BPE measures are associated with lifetime breast cancer risk in non-BRCA mutation carriers and BRCA germline mutation status in high-risk women. These associations have been attributed to the presence of several lifetime risk factors. Differences in quantitative BPE between BRCA mutation carriers and high-risk noncarriers persisted after adjusting for known factors. Our work provides a potential explanation for the cancer pathophysiological mechanisms underlying the lifetime risk model from the perspective of BPE. In the future, additional research is necessary to determine if quantitative BPE can function as an independent risk factor enhancing breast cancer risk stratification.
Abbreviations
- BPE:
-
Background parenchymal enhancement
- DCE:
-
Dynamic contrast-enhanced
- DER:
-
Delayed enhancement ratio
- FGT:
-
Fibroglandular tissue
- FWER:
-
Family-wise error rate
- IER:
-
Initial enhancement ratio
- IQR:
-
Interquartile range
- MIP:
-
Maximum-intensity projection
- PE:
-
Percent enhancement
- PSM:
-
Propensity score matching
- SER:
-
Signal enhancement ratio
- T1-NFS:
-
T1-weighted non-fat-suppressed
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Hunter A (2007) Mammographic density: the risk and detection of breast caner. J BioLaw Bus 10:60–61
Saslow D, Boetes C, Burke W et al (2007) American Cancer Society Guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75–89
Bae MS, Sung JS, Han W et al (2017) Survival outcomes of screening with breast MRI in high-risk women. J Clin Oncol 35:1508–1508
Gail MH, Brinton LA, Byar DP et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879–1886
Claus EB, Risch N, Thompson WD (1991) Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet 48:232–242
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130
Amir E, Freedman OC, Seruga B, Evans DG (2010) Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst 102:680–691
Cummings SR, Tice JA, Bauer S et al (2009) Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst 101:384–398
Amir E, Evans DG, Shenton A et al (2003) Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet 40:807–814
King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA (2011) Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260:50–60
Dontchos BN, Rahbar H, Partridge SC et al (2015) Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 276:371–380
Sippo DA, Rutledge GM, Burk KS et al (2019) Effect of background parenchymal enhancement on cancer risk across different high-risk patient populations undergoing screening breast MRI. AJR Am J Roentgenol 212:1412–1418
Saha A, Grimm LJ, Ghate SV et al (2019) Machine learning-based prediction of future breast cancer using algorithmically measured background parenchymal enhancement on high-risk screening MRI. J Magn Reson Imaging 50:456–464
Niell BL, Abdalah M, Stringfield O et al (2021) Quantitative measures of background parenchymal enhancement predict breast cancer risk. AJR Am J Roentgenol 217:64–75
Thompson CM, Mallawaarachchi I, Dwivedi DK et al (2019) The association of background parenchymal enhancement at breast mri with breast cancer: a systematic review and meta-analysis. Radiology 292:552–561
Liao GJ, Henze Bancroft LC, Strigel RM et al (2020) Background parenchymal enhancement on breast MRI: a comprehensive review. J Magn Reson Imaging 51:43–61
Lewin AA, Gene Kim S, Babb JS et al (2016) Assessment of background parenchymal enhancement and lesion kinetics in breast MRI of BRCA 1/2 mutation carriers compared to matched controls using quantitative kinetic analysis. Acad Radiol 23:358–367
Kuchenbaecker KB, Hopper JL, Barnes DR et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317:2402–2416
Murakami W, Mortazavi S, Yu T et al (2023) Clinical significance of background parenchymal enhancement in breast cancer risk stratification. J Magn Reson Imaging 59:1742–1757
Grubstein A, Rapson Y, Benzaquen O et al (2018) Comparison of background parenchymal enhancement and fibroglandular density at breast magnetic resonance imaging between BRCA gene mutation carriers and non-carriers. Clin Imaging 51:347–351
Goodburn R, Kousi E, Sanders C et al (2023) Quantitative background parenchymal enhancement and fibro-glandular density at breast MRI: association with BRCA status. Eur Radiol 1:1–9
Brooks JD, Sung JS, Pike MC et al (2018) MRI background parenchymal enhancement, breast density and serum hormones in postmenopausal women. Int J Cancer 143:823–830
King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA (2012) Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 22:2641–2647
Amarosa AR, McKellop J, Leite APK et al (2013) Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology 268:356–365
Dalmiş MU, Litjens G, Holland K et al (2017) Using deep learning to segment breast and fibroglandular tissue in MRI volumes. Med Phys 44:533–546
Tustison NJ, Avants BB, Cook PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Wu S, Weinstein SP, DeLeo MJ et al (2015) Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res 19:67
Wu S, Berg WA, Zuley ML et al (2016) Breast MRI contrast enhancement kinetics of normal parenchyma correlate with presence of breast cancer. Breast Cancer Res 18:76
Deebajah M, Keeley J, Park H et al (2019) A propensity score matched analysis of the effects of African American race on the characteristics of regions of interests detected by magnetic resonance imaging of the prostate. Urol Oncol 37:531.e1–531.e5
Hellgren R, Saracco A, Strand F et al (2020) The association between breast cancer risk factors and background parenchymal enhancement at dynamic contrast-enhanced breast MRI. Acta Radiol 61:1600–1607
Gillman J, Chun J, Schwartz S, Schnabel F, Moy L (2016) The relationship of obesity, mammographic breast density, and magnetic resonance imaging in patients with breast cancer. Clin Imaging 40:1167–1172
Mair KM, Gaw R, MacLean MR (2020) Obesity, estrogens and adipose tissue dysfunction – implications for pulmonary arterial hypertension. Pulm Circ 10:1–21
Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA (2011) Background parenchymal enhancement on baseline screening breast MRI: Impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 196:218–224
Uematsu T, Kasami M, Watanabe J (2012) Should breast MRI be performed with adjustment for the phase in patients’ menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients’ menstrual cycle. Eur J Radiol 81:1539–1542
Watt GP, Sung J, Morris EA et al (2020) Association of breast cancer with MRI background parenchymal enhancement: the IMAGINE case-control study. Breast Cancer Res 22:138
Funding
This study was in part supported by the Tanner Project Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is one of the authors, Kyunghyun Sung.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Fang-I Chu, has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
The Institutional Review Board of the University of California (Los Angeles) approval was obtained.
Study subjects or cohorts overlap
None study subjects or cohorts have been previously reported.
Methodology
-
Retrospective
-
Experimental
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, R., Murakami, W., Mortazavi, S. et al. Quantitative assessment of background parenchymal enhancement is associated with lifetime breast cancer risk in screening MRI. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10758-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10758-9