Abstract
Objectives
To investigate associations between tissue diffusion, stiffness, and different tumor microenvironment features in resected hepatocellular carcinoma (HCC).
Methods
Seventy-two patients were prospectively included for preoperative magnetic resonance (MR) diffusion-weighted imaging and MR elastography examination. The mean apparent diffusion coefficient (ADC) and stiffness value were measured on the central three slices of the tumor and peri-tumor area. Cell density, tumor-stroma ratio (TSR), lymphocyte-rich HCC (LR-HCC), and CD8 + T cell infiltration were estimated in resected tumors. The interobserver agreement of MRI measurements and subjective pathological evaluation was assessed. Variables influencing ADC and stiffness were screened with univariate analyses, and then identified with multivariable linear regression. The potential relationship between explored imaging biomarkers and histopathological features was assessed with linear regression after adjustment for other influencing factors.
Results
Seventy-two patients (male/female: 59/13, mean age: 56 ± 10.2 years) were included for analysis. Inter-reader agreement was good or excellent regarding MRI measurements and histopathological evaluation. No correlation between tumor ADC and tumor stiffness was found. Multivariable linear regression confirmed that cell density was the only factor associated with tumor ADC (Estimate = −0.03, p = 0.006), and tumor-stroma ratio was the only factor associated with tumor stiffness (Estimate = −0.18, p = 0.03). After adjustment for fibrosis stage (Estimate = 0.43, p < 0.001) and age (Estimate = 0.04, p < 0.001) in the multivariate linear regression, intra-tumoral CD8 + T cell infiltration remained a significant factor associated with peri-tumor stiffness (Estimate = 0.63, p = 0.02).
Conclusions
Tumor ADC surpasses tumor stiffness as a biomarker of cellularity. Tumor stiffness is associated with tumor-stroma ratio and peri-tumor stiffness might be an imaging biomarker of intra-tumoral immune microenvironment.
Clinical relevance statement
Tissue stiffness could potentially serve as an imaging biomarker of the intra-tumoral immune microenvironment of hepatocellular carcinoma and aid in patient selection for immunotherapy.
Key Points
-
Apparent diffusion coefficient reflects cellularity of hepatocellular carcinoma.
-
Tumor stiffness reflects tumor-stroma ratio of hepatocellular carcinoma and is associated with tumor-infiltrating lymphocytes.
-
Tumor and peri-tumor stiffness might serve as imaging biomarkers of intra-tumoral immune microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the second leading cause of cancer-related deaths worldwide [1]. Magnetic resonance imaging (MRI) plays an essential role in the diagnosis and local staging of HCC. However, beyond its great diagnostic capacity, MRI also provides valuable insights into the histopathology of HCC, which holds significant clinical implications.
One of the key metrics derived from MRI is the apparent diffusion coefficient (ADC), which is quantified from diffusion-weighted imaging (DWI), a commonly used non-contrast quantitative MRI technique. Accumulating evidence suggests a correlation between ADC and several critical histopathological features of HCC, including tumor differentiation, proliferation, and microvascular invasion [2,3,4,5]. A recent study further highlighted a weak association between ADC and tumor-stroma ratio (STR) in HCC [6], which is considered a crucial microenvironment feature of HCC and might be associated with treatment response and patient prognosis [7, 8]. Additionally, ADC values have also been linked to the density of tumor-infiltrating lymphocytes in several malignancies such as head and neck squamous cell cancer, breast cancer, and intrahepatic cholangiocarcinoma [9,10,11]. However, the exploration of such a relationship in the context of HCC remains unclear.
Another MRI technique gaining prominence in HCC assessment is magnetic resonance elastography (MRE). MRE provides a non-invasive non-contrast method for quantifying tissue stiffness, a biomechanical property associated with extracellular matrix deposition and a key parameter in the evaluation of liver fibrosis [12]. Prior studies have shown that tumor stiffness was associated with the presence of microvascular invasion (MVI), response to transarterial chemoembolization treatment and immunotherapy, and tumor recurrence [13,14,15]. Understanding the relationship between tumor stiffness and tumor microenvironments, such as cellularity, TSR, and immune microenvironment, could potentially enhance patient stratification and guide personalized management strategies using MRI-derived information. However, little has been known about the relationship between tumor stiffness and histopathology in HCC.
Therefore, the purpose of this study was to investigate possible associations between ADC, MRE-derived stiffness, and different histopathological features including cell density, TSR, tumor-infiltrating lymphocytes, and CD8 + T cell infiltration in resected HCC.
Materials and methods
Study population
This prospective study was approved by our institutional review board and written informed consent was obtained from each participant. From January 2021 to March 2023, patients suspected with HCC were recruited for preoperative MRI/MRE examination. The inclusion criteria for initial patient recruitment included patients with liver lesions suspected of HCC detected by other imaging modalities (ultrasonography or computed tomography) and/or elevated serum tumor markers (Alpha-fetoprotein and/or Protein Induced by Vitamin K Absence or Antagonist-II). The final exclusion criteria were: (a) patients with pathology-proven non-HCC lesions, (b) pathology from surgically resected tumor samples within one month after the MRI examination was not available, (c) patients received local regional or systemic antitumoral therapy before MRI examination, (d) tumor diameter was less than 1 centimeter, (e) tumor located at the hepatic dome, (f) mechanical coupling failure due to a loose driver, and (g) technical failure due to severe respiratory motion artifacts. Demographic information and laboratory results were collected from the electronic medical system.
MRI examination
All MRI examinations were performed prospectively on a 3.0 T MR scanner (Discovery MR750, GE Healthcare) using a 16-channel phased-array body coil. Participants were fasted at least 4 h before the MRI scan. Routine axial DWI was scanned under free breath using the following parameters: TR = 6315 msec, TE = 70.7 msec, FOV = 400 × 320 mm, matrix = 80 × 128, slice thickness = 6.0 mm, b values = 0, 50, and 1000 sec/mm2, and acceleration factor = 2. Apparent diffusion coefficient (ADC) was calculated from b values of 0 and 1000 sec/mm2 by using the mono-exponential model.
MRE examination was conducted after a routine pre-scan and before the application of the contrast agent. For liver MRE, a customized flexible acoustic passive driver (30 × 10 cm, Mayo Clinic) was placed over the right upper abdomen and was secured using an elastic strap. 3D-MRE data was acquired during breath hold at the end of expiration by a single-shot echo-planar imaging sequence using the following parameters: TR = 1600 msec, TE = 57.8 msec, FOV = 440 × 440 mm, matrix = 96 × 96, slice thickness = 3.6 mm, mechanical vibration frequency = 60 Hz, and acceleration factor = 2. Post-processing of 3D-MRE was conducted by using a 3D multimode direct inversion algorithm to generate corresponding wave images, phase images, magnitude images, and shear stiffness maps.
Image analysis
Image analysis was conducted using the ITK-SNAP software (Version 3.6.0) by two independent investigators (C.J. and Z.Z., with 10- and 6-years’ experience in liver MRI, respectively) who were blinded to histopathological findings. By using T2-weighted and contrast-enhanced images as references, the freehand region of interest was manually drawn on the central three slices of the tumor on the stiffness and ADC map to encircle the tumor area. Tumor edges and areas with necrosis, intra-tumoral hemorrhage, artifacts, and poor wave penetration, as determined by the investigator, were excluded from the analysis. Peri-tumor stiffness and peri-tumor ADC were quantified by placing freehand regions of interest on the same three slices on peri-tumor liver parenchyma (within 1 cm from the tumor edge), while excluding artifacts and major vasculatures. When multiple lesions were detected, the lesion with the largest diameter was selected for imaging and pathological analysis. The volumetric mean value of each parameter was extracted, and the final measurement was calculated as the average value of two measurements.
Histopathological analysis
Tumor specimen was obtained from the tumor center section following surgical resection, as per the prior recommendation [16]. For each specimen, a standard hematoxylin and eosin (HE) staining and a multiplex immunohistochemical (mIHC) staining were performed. Histopathological findings were evaluated in the scanned whole slide image using the open-source software of QuPath (Version 0.4.3). For quantitative evaluation of cell density and TSR, a pathologist (W.Z., 10 years’ experience in liver pathology) first reviewed the 5-channal mIHC slide and a free hands annotation was defined to include all available tumor area for quantitative analysis. The watershed cell detection method was then applied to detect and segment all cells based on the nucleus stain. Following a similar approach to previous studies, TSR was quantified on a cellular level using machine learning-based methods in Qupath [17,18,19] (detailed information is provided in supplementary methods). Cell density was calculated as the total number of cells divided by the annotation area at a low power field of 4X on mIHC slides, and TSR was calculated as the total number of tumor cells divided by the total number of stroma cells based on cell detection and classification on mIHC slides, as described in supplementary methods.
Additionally, a qualitative assessment of tumor-infiltrating lymphocytes and CD8 + T cells was conducted by two independent investigators (W.Z. and C.J.), and a consensus-reached categorization was used for analysis. Lymphocytes-rich HCC (LR-HCC) was defined as HCC with a diffuse dense intra-tumoral lymphocytes infiltration and involving more than 50% of the tumor on HE slides [20]. CD8 + T cell infiltration was categorized as negative (none or mild) and positive (moderate or dense) based on mIHC slides. Other histopathological features such as the Edmondson-Steiner grade, MVI, the fibrosis stage, and inflammation grade of peri-tumor liver parenchyma were also assessed by the pathologist.
Statistical analysis
The interobserver agreement was assessed using the intraclass correlation coefficient (ICC) for quantitative MRI measurements and Cohen’s Kappa for subjective pathological evaluation. The correlation between imaging biomarkers and histopathological features was calculated by Spearman’s rank correlation coefficient. Variables influencing ADC and stiffness were screened with univariable analyses, and then identified with multivariable linear regression. The potential relationship between explored imaging biomarkers and histopathological features was assessed with linear regression after adjustment for other influencing factors. Differences in imaging and histopathological biomarkers were compared between HCCs with and without MVI using the Mann-Whitney test or independent-sample T-test when appropriate. Statistical analysis was performed using the SPSS software (IBM SPSS Statistics for Windows, version 28: IBM corporation, 2021) and R software (R version 4.3.1). A significance level of p < 0.05 was used to determine statistical significance.
Results
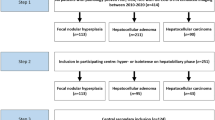
The final study cohort included 72 patients (male/female: 59/13, mean age: 56 ± 10.2 years), sixty-three of them had hepatitis B virus infection. Among them, 57 patients had solitary lesion, 9 patients had 2 lesions, and 6 patients had 3 lesions. The distribution of the Barcelona Clinic Liver Cancer (BCLC) stage was 54 in stage A, 12 in stage B, and 6 in stage C. All patients received liver resection in accordance with the guideline recommendation for BCLC stage A or the expanded criteria for selected patients with BCLC stage B or C (preserved liver function, no more than 3 nodules, or portal invasion to the first-, second- and third-order branch). Macrovascular invasion was present in 6 patients, while 30 patients had MVI. The median tumor diameter was 3.70 cm. Figure 1 represents the flowchart of this study. Demographic, clinical, and pathological characteristics of included patients were summarized in Table 1.
Association between ADC value and stiffness
Inter-reader agreement was good or excellent regarding ADC and stiffness measurement. The ICC was 0.82 (0.69, 0.90) for tumor ADC, 0.94 (0.87, 0.98) for tumor stiffness, 0.75 (0.57, 0.91) for peri-tumor ADC, and 0.95 (0.84, 0.99) for peri-tumor stiffness. The mean tumor ADC was 1.12 ± 1.34 mm2/s, mean tumor stiffness was 4.52 ± 1.48 kPa, mean peri-tumor ADC was 1.34 ± 0.26 mm2/s, and mean peri-tumor stiffness was 3.34 ± 1.08 kPa. Figure 2 demonstrates the measurement of imaging biomarkers, more cases with MRI images are provided in Supplementary Figs. 1, 2, and 3. The influence of intra-tumoral necrosis on the agreement and measurement of each variable was summarized in Supplementary Table 1 and Table 2.
The measurement of apparent diffusion coefficient and stiffness in the tumor (white) and peri-tumor area (red). a T2 weighted image; b diffusion-weighted image; c the T2-weighted magnitude image of magnetic resonance elastography; d late arterial phase of dynamic contrast enhanced imaging; e the apparent diffusion coefficient map; f the color-coded stiffness map
There were significant correlations between tumor ADC and peri-tumor ADC (rho = 0.49, p < 0.001), between tumor stiffness and peri-tumor stiffness (rho = 0.50, p < 0.001), and between peri-tumor ADC and peri-tumor stiffness (rho = −0.35, p = 0.003). However, no significant correlation was found between tumor ADC and tumor stiffness (rho = 0.08, p = 0.54) (Table 2). Figure 3 demonstrates the association between tumor and peri-tumor measurements.
Association between different histopathological features
Inter-reader agreement was good regarding qualitative histopathological evaluation. The Kappa was 0.71 (0.49, 0.88) for the identification of LR-HCC and 0.88 (0.70, 0.99) for the identification of positive intra-tumoral CD8 + T cell infiltration. Figure 4 demonstrates the expression of each biomarker and their combined expression on the mIHC stain, along with the results of cell classification. Figure 5 presents examples of HCC cases with different histopathological features.
Demonstration of each biomarker and their combined expression on the multiplex immunohistochemical stain, along with the results of cell classification. a The nucleus stain by 4',6-diamidino-2-phenylindole (DAPI). b The expression of α-smooth muscle actin (α-SMA). c The expression of CD163. d The expression of CD86. e Combined expression of multiple biomarkers. f Results of cell classification. Red indicates tumor cells and green indicates stroma cells
Cases of hepatocellular carcinoma (HCC) with different histopathological features. a, b HCC with high cell density (a) and low cell density (b) as assessed by the DAPI channel of mIHC. Blue dots are stained nuclei. c, d HCC with low tumor-stroma ratio (c) and high tumor-stroma ratio (d) according to cell classification on mIHC. Red represents tumor cells and green represents stroma cells. e, f Lymphocytes-rich HCC (e) and non-lymphocytes-rich HCC (f) as assessed by HE slides. g, h HCC with (g) and without (h) intra-tumoral CD8 + T cell infiltration
There were significant correlations between cell density and TSR (rho = 0.33, p = 0.005) and between TSR and LR-HCC (rho = −0.53, p < 0.001). No significant correlation was found between other histopathological features (Table 3).
Association between ADC value and histopathological features
At univariable analysis, cell density was the only histopathological feature associated with tumor ADC (rho = −0.39, p = 0.001). No significant correlation between tumor ADC and TSR (rho = −0.13, p = 0.29), LR-HCC (rho = 0.11, p = 0.37), intra-tumoral CD8 + T cell infiltration (rho = 0.04, p = 0.71), peri-tumor inflammation (rho = 0.12, p = 0.31), and peri-tumor fibrosis (rho = 0.05, p = 0.68) was found. After adjustment for cell density in the multivariable linear regression, the relationship between TSR and tumor ADC was still non-significant (Estimate = 0.001, p = 0.93, Table 4).
The peri-tumor ADC showed a weak negative correlation with the grade of peri-tumor inflammation (rho = −0.25, p = 0.04). However, it did not show a significant correlation with the stage of peri-tumor fibrosis (rho = −0.10, p = 0.42) or with any of the intra-tumoral histopathological features investigated, including cell density (rho = −0.04, p = 0.76), TSR (rho = 0.03, p = 0.84), LR-HCC (rho = 0.05, p = 0.70), and CD8 + T cell infiltration (rho = 0.04, p = 0.76).
Association between stiffness and histopathological features
At univariable analysis, TSR (rho = −0.33, p = 0.005), LR-HCC (rho = 0.28, p = 0.02), and tumor size (rho = 0.40, p = 0.001) were significant factors associated with tumor stiffness. The tumor stiffness in LR-HCC was significantly higher than that in non LR-HCC (5.40 ± 1.65 Kpa vs. 4.33 ± 1.40 Kpa, p = 0.02). Since tumor-infiltrating lymphocytes were also counted as stroma cells when calculating the TSR, an intrinsic collinearity was expected between TSR and LR-HCC (rho = −0.53, p < 0.001). Therefore, only TSR and tumor size were included in the multivariable linear regression, where TSR was a significant factor influencing tumor stiffness (Estimate = −0.18, p = 0.03, Table 4). After adjustment for TSR and tumor size in the multivariable linear regression, the relationship between cell density and tumor stiffness remained non-significant (Estimate = −0.03, p = 0.61).
Intra-tumoral CD8 + T cell infiltration (rho = 0.27, p = 0.02), fibrosis stage (rho = 0.39, p = 0.001), and age (rho = 0.41, p < 0.001) were significant variables associated with peri-tumor stiffness at univariable analysis. After adjustment for fibrosis stage (Estimate = 0.43, p < 0.001) and age (Estimate = 0.04, p < 0.001) in the multivariable linear regression, intra-tumoral CD8 + T cell infiltration remained a significant factor associated with peri-tumor stiffness (Estimate = 0.63, p = 0.02). The peri-tumor stiffness in HCC with CD8 + T cell infiltration was significantly higher than that in HCC without CD8 + T cell infiltration (4.09 ± 1.44 Kpa vs. 3.16 ± 0.91 Kpa, p = 0.02). The associations between MRI measurements and histopathological features are summarized in Table 4 and Fig. 6.
Comparison of imaging and histopathological biomarkers between HCC with and without MVI
Tumor stiffness and peri-tumor stiffness were significantly higher in HCC with MVI. The mean tumor stiffness was 5.04 ± 1.79 kPa in HCC with MVI, and 4.09 ± 1.17 kPa in HCC without MVI. The mean peri-tumor stiffness was 3.70 ± 1.25 kPa in HCC with MVI, and 3.09 ± 0.89 in HCC without MVI. No significant difference was found in ADC measurements and histopathological biomarkers between groups. Table 5 summarizes the comparison of explored imaging and histopathological biomarkers between HCC with and without MVI.
Discussion
This study investigated associations between ADC, stiffness, and several histopathological features of HCC. Results showed that ADC and stiffness were robust imaging biomarkers with excellent inter-reader agreement and were associated with different histopathologies of HCC. This analysis could provide further insights into the histopathological underpinnings of HCC and the role of MRI-derived imaging biomarkers in capturing these features.
The negative correlation between ADC and cell density in this study is consistent with prior conclusions [6, 21, 22], while the correlation between tumor stiffness and cell density was weak and non-significant. One potential explanation for the observed differences could be the higher spatial resolution of DWI compared to MRE imaging. Consequently, DWI may provide more detailed and accurate information regarding cellularity than MRE despite variations in physiological mechanisms. Previous studies have shown that both tumor ADC and stiffness can document the effects of cytoreductive treatment. For example, according to Kostek et al, advanced HCC patients with a favorable response to sorafenib had a significant increase in ADC value at the first radiological evaluation [23]. Qayyum et al found that changes in HCC stiffness at 6 weeks of immunotherapy correlated significantly with overall survival and time to disease progression [15]. Those findings suggested that changes in tumor ADC and stiffness might also serve as imaging biomarkers of treatment responders. Accordingly, for patients without these changes or with the opposite changes in corresponding imaging biomarkers, the treatment approach may need to be adjusted. Together with prior studies, the results of this study indicate that although tumor ADC and stiffness can both reflect tumor microstructure, ADC might be a better image biomarker for cellularity.
Tumor stroma is an important component of the tumor microenvironment, which provides favorable conditions for tumor growth and invasion [24, 25]. Recent studies have highlighted the role of stromal cells in modulating tumor immune status and its response to immunotherapy [25]. So far, only one study investigated the association between ADC and TSR as well as intra-tumoral lymphocytes in 53 biopsied HCCs [6]. Theoretically, increased stroma cells are associated with increased extracellular matrix deposition, which eventually leads to increased diffusion restriction (i.e., decreased ADC) [26]. Inconsistent with the weak correlation between ADC and TSR by prior study, our study found no significant correlation between tumor ADC and TSR. We speculate that this might be affected by the calculation of TSR on the cellular level, the lack of precise image-pathology match, and the use of different b values when calculating the ADC value (1000 s/mm2 in our study versus 600 s/mm2 in prior study). Nevertheless, tumor ADC continues to show a lack of association with intra-tumoral lymphocyte infiltration. On the other hand, it is well-known that the ADC value decreases with the increase of liver fibrosis stage [27]. However, the peri-tumor ADC did not correlate significantly with peri-tumor fibrosis in this study. This can be explained by the fact that most patients already had advanced fibrosis or cirrhosis at diagnosis. Under this situation, the peri-tumor ADC was more affected by other pathological conditions such as the grade of inflammation in this study.
To the best of our knowledge, this is the first study regarding associations between tumor stiffness and TSR in HCC. Gordic et al previously reported positive correlations between tumor stiffness and enhancement ratios [28], which might be partly explained by its correlation with TSR. Stroma-rich HCCs tend to have high microvascular density at pathology. The association between tumor stiffness and LR-HCC might also be explained by its association with TSR, given that tumor-infiltrating lymphocytes mainly present in tumor stroma. This result echoes with previous report about the strong positive correlation between HCC stiffness and intra-tumoral T lymphocytes in nine HCC biopsies [15]. However, there was only a weak and non-significant association between tumor stiffness and intra-tumoral CD8 + T cell infiltration, probably due to limited sample size. Interestingly, peri-tumor stiffness also showed a weak, yet significant, correlation with the intra-tumoral CD8 + T cell infiltration even after adjustment with other factors, indicating that the microenvironment remodeling of peri-tumor parenchyma is also involved in modulating the intra-tumoral immune status. This study suggested that tissue stiffness might be a promising image biomarker of intra-tumoral immune status of HCC. With meticulous evaluation in ongoing clinical trials, MRE might offer insights into identifying patients who could benefit from immunotherapy, potentially enabling the avoidance of unnecessary immunotherapy in non-responders.
Our study also showed that tumor ADC and stiffness correlated positively with peri-tumor ADC and stiffness, respectively. This observation supported the current conclusion in basic research regarding the interaction between tumor and peri-tumor microenvironments in the progression and treatment of HCC [29, 30]. According to Chen et al, both tumor stiffness and its stiffness difference between tumor and non-tumoral liver were significantly associated with tumor response to transarterial chemoembolization treatment [14]. Notably, no significant correlation was found between tumor ADC and tumor stiffness, suggesting that tumor ADC and stiffness were independent MRI biomarkers.
Our study does possess certain limitations. First, despite its prospective design, there was an absence of precise matching between the sites of histopathology and imaging analysis, which may have potentially weakened the association. second, the sample size of our study was limited, particularly in relation to the number of HCC cases with positive intra-tumoral CD8 + T cell infiltration. Third, due to limited sample size and follow-up time, we could not further validate the prognostic value of those imaging and histopathological biomarkers. Further studies are needed to verify the prognostic value of those biomarkers, especially the role of imaging biomarkers in patient stratification and monitoring treatment response.
In conclusion, ADC and stiffness are independent and robust image biomarkers that are associated with different microenvironment features of HCC. Tumor ADC surpasses tumor stiffness as a biomarker of cellularity. Tumor stiffness is associated with tumor-stroma ratio and peri-tumor stiffness might be an imaging biomarker of intra-tumoral immune microenvironment.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BCLC stage:
-
The Barcelona Clinic Liver Cancer stage
- DWI:
-
Diffusion-weighted imaging
- HCC:
-
Hepatocellular carcinoma
- HE:
-
Hematoxylin and eosin staining
- ICC:
-
Intraclass correlation coefficient
- LR-HCC:
-
Lymphocytes-rich hepatocellular carcinoma
- mIHC:
-
Multiplex immunohistochemical staining
- MRE:
-
Magnetic resonance elastography
- MRI:
-
Magnetic resonance imaging
- MVI:
-
Microvascular invasion
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Chen J, Wu M, Liu R et al (2015) Preoperative evaluation of the histological grade of hepatocellular carcinoma with diffusion-weighted imaging: a meta-analysis. PLoS One 10:e0117661. https://doi.org/10.1371/journal.pone.0117661
Yan C, Han Z, Chen X et al (2023) Diffusion-weighted imaging as a quantitative imaging biomarker for predicting proliferation rate in hepatocellular carcinoma: developing a radiomics nomogram. J Comput Assist Tomogr 47:539–547. https://doi.org/10.1097/RCT.0000000000001448
Zhang L, Li M, Zhu J et al (2023) The value of quantitative MR elastography-based stiffness for assessing the microvascular invasion grade in hepatocellular carcinoma. Eur Radiol 33:4103–4114. https://doi.org/10.1007/s00330-022-09290-5
Zhang L, Chen J, Jiang H et al (2022) MR elastography as a biomarker for prediction of early and late recurrence in HBV-related hepatocellular carcinoma patients before hepatectomy. Eur J Radiol 152:110340. https://doi.org/10.1016/j.ejrad.2022.110340
Surov A, Eger KI, Potratz J et al (2023) Apparent diffusion coefficient correlates with different histopathological features in several intrahepatic tumors. Eur Radiol 33:5955–5964. https://doi.org/10.1007/s00330-023-09788-6
Lv Z, Cai X, Weng X et al (2015) Tumor–stroma ratio is a prognostic factor for survival in hepatocellular carcinoma patients after liver resection or transplantation. Surgery 158:142–150. https://doi.org/10.1016/j.surg.2015.02.013
Wang D, Luo J, Tao Y (2023) Tumor–stroma ratio predicts prognosis and PD-L1 expression in hepatocellular carcinoma. BMC Cancer 23:434. https://doi.org/10.1186/s12885-023-10859-6
Meyer H-J, Höhn AK, Surov A (2022) Associations between ADC and tumor infiltrating lymphocytes, tumor-stroma ratio and vimentin expression in head and neck squamous cell cancer. Acad Radiol 29:S107–S113. https://doi.org/10.1016/j.acra.2021.05.007
Tang W-J, Jin Z, Zhang Y-L et al (2020) Whole-lesion histogram analysis of the apparent diffusion coefficient as a quantitative imaging biomarker for assessing the level of tumor-infiltrating lymphocytes: value in molecular subtypes of breast cancer. Front Oncol 10:611571. https://doi.org/10.3389/fonc.2020.611571
Miyazaki K, Morine Y, Yamada S et al (2021) Stromal tumor-infiltrating lymphocytes level as a prognostic factor for resected intrahepatic cholangiocarcinoma and its prediction by apparent diffusion coefficient. Int J Clin Oncol 26:2265–2274. https://doi.org/10.1007/s10147-021-02026-3
Venkatesh SK, Wells ML, Miller FH et al (2018) Magnetic resonance elastography: beyond liver fibrosis-a case-based pictorial review. Abdom Radiol (NY) 43:1590–1611. https://doi.org/10.1007/s00261-017-1383-1
Abe H, Shibutani K, Yamazaki S et al (2023) Tumor stiffness measurement using magnetic resonance elastography can predict recurrence and survival after curative resection of hepatocellular carcinoma. Surgery 173:450–456. https://doi.org/10.1016/j.surg.2022.11.001
Chen R, Kong W, Gan Y et al (2019) Tumour stiffness associated with tumour response to conventional transarterial chemoembolisation for hepatocellular carcinoma: preliminary findings. Clin Radiol 74:814.e1–814.e7. https://doi.org/10.1016/j.crad.2019.07.008
Qayyum A, Hwang K-P, Stafford J et al (2019) Immunotherapy response evaluation with magnetic resonance elastography (MRE) in advanced HCC. J Immunother Cancer 7:329. https://doi.org/10.1186/s40425-019-0766-y
Cong W-M, Bu H, Chen J et al (2016) Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 22:9279–9287. https://doi.org/10.3748/wjg.v22.i42.9279
Zheng Q, Jiang Z, Ni X et al (2023) Machine learning quantified tumor-stroma ratio is an independent prognosticator in muscle-invasive bladder cancer. Int J Mol Sci 24:2746. https://doi.org/10.3390/ijms24032746
Millar EK, Browne LH, Beretov J et al (2020) Tumour stroma ratio assessment using digital image analysis predicts survival in triple negative and luminal breast cancer. Cancers 12:3749. https://doi.org/10.3390/cancers12123749
Xu Q, Chen Y-Y, Luo Y-H et al (2023) Proposal of an automated tumor-stromal ratio assessment algorithm and a nomogram for prognosis in early-stage invasive breast cancer. Cancer Med 12:131–145. https://doi.org/10.1002/cam4.4928
Kim H, Leow W-Q, Lo R et al (2022) Lymphocyte-Rich Hepatocellular Carcinoma. In: Atlas of Hepatocellular Carcinoma Pathology. Springer Nature Singapore, Singapore, pp 69–75
Santucci D, Faiella E, Calabrese A et al (2021) On the additional information provided by 3T-MRI ADC in predicting tumor cellularity and microscopic behavior. Cancers 13:5167. https://doi.org/10.3390/cancers13205167
Surov A, Meyer HJ, Wienke A (2017) Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget 8:59492–59499. https://doi.org/10.18632/oncotarget.17752
Kostek O, Yilmaz E, Bekir Hacıoglu M et al (2018) Value of MRI apparent diffusion coefficient for assessment of response to sorafenib in hepatocellular carcinoma. J BUON 23:979–984
Dzobo K, Senthebane DA, Dandara C (2023) The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers 15:376. https://doi.org/10.3390/cancers15020376
Liu Y, Xun Z, Ma K et al (2023) Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol S0168-8278:00023–00025. https://doi.org/10.1016/j.jhep.2023.01.011
Lewis S, Dyvorne H, Cui Y, Taouli B (2014) Diffusion-weighted imaging of the liver: techniques and applications. Magn Reson Imaging Clin N Am 22:373–395. https://doi.org/10.1016/j.mric.2014.04.009
Jiang H, Chen J, Gao R et al (2017) Liver fibrosis staging with diffusion-weighted imaging: a systematic review and meta-analysis. Abdom Radiol (NY) 42:490–501. https://doi.org/10.1007/s00261-016-0913-6
Gordic S, Ayache JB, Kennedy P et al (2017) Value of tumor stiffness measured with MR elastography for assessment of response of hepatocellular carcinoma to locoregional therapy. Abdom Radiol (NY) 42:1685–1694. https://doi.org/10.1007/s00261-017-1066-y
Dong Y, Zheng Q, Wang Z et al (2019) Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol 12:112. https://doi.org/10.1186/s13045-019-0795-5
Schrader J, Gordon-Walker TT, Aucott RL et al (2011) Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53:1192–1205. https://doi.org/10.1002/hep.24108
Funding
This study has received grant funding from the Science and Technology Support Program of Sichuan Province (Grant number 2022YFS0072 & 2022YFS0071), the National Nature Science Foundation of China (Grant number 82302155 & U22A20343); the 1.3.5 project for disciplines of excellence at West China Hospital, Sichuan University (Grant No. ZYJC21012 & ZYGD22004); the Department of Defense (DoD) award (Grant number PR181303 & W81XWH-19-1-0583), the US National Institutes of Health (NIH) (Grant number R01 EB017197 & R01 DK132718 & R01 DK136731 & R37 EB001981) and have intellectual property and a financial interest related to MRE technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bin Song (songlab_radiology@163.com, Tel: +86 18980601592, No.37 Guoxue Alley, Chengdu, China, 610041).
Conflict of interest
The Mayo Clinic and RLE have intellectual property and a financial interest related to Magnetic resonance elastography technology in this work. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
None.
Methodology
-
Prospective
-
Cross sectional study
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Wu, Z., Zhang, Z. et al. Apparent diffusion coefficient and tissue stiffness are associated with different tumor microenvironment features of hepatocellular carcinoma. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10743-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10743-2