Abstract
Background
Lung cancer, the second most common cancer, presents persistently dismal prognoses. Radiomics, a promising field, aims to provide novel imaging biomarkers to improve outcomes. However, clinical translation faces reproducibility challenges, despite efforts to address them with quality scoring tools.
Objective
This study had two objectives: 1) identify radiomics biomarkers in post-radiotherapy stage III/IV nonsmall cell lung cancer (NSCLC) patients, 2) evaluate research quality using the CLEAR (CheckList_for_EvaluAtion_of_Radiomics_research), RQS (Radiomics_Quality_Score) frameworks, and formulate an amalgamated CLEAR-RQS tool to enhance scientific rigor.
Materials and methods
A systematic literature review (Jun-Aug 2023, MEDLINE/PubMed/SCOPUS) was conducted concerning stage III/IV NSCLC, radiotherapy, and radiomic features (RF). Extracted data included study design particulars, such as sample size, radiotherapy/CT technique, selected RFs, and endpoints. CLEAR and RQS were merged into a CLEAR-RQS checklist. Three readers appraised articles utilizing CLEAR, RQS, and CLEAR-RQS metrics.
Results
Out of 871 articles, 11 met the inclusion/exclusion criteria. The Median cohort size was 91 (range: 10–337) with 9 studies being single-center. No common RF were identified. The merged CLEAR-RQS checklist comprised 61 items. Most unreported items were within CLEAR’s “methods” and “open-source,” and within RQS’s “phantom-calibration,” “registry-enrolled prospective-trial-design,” and “cost-effective-analysis” sections. No study scored above 50% on RQS. Median CLEAR scores were 55.74% (32.33/58 points), and for RQS, 17.59% (6.3/36 points). CLEAR-RQS article ranking fell between CLEAR and RQS and aligned with CLEAR.
Conclusion
Radiomics research in post-radiotherapy stage III/IV NSCLC exhibits variability and frequently low-quality reporting. The formulated CLEAR-RQS checklist may facilitate education and holds promise for enhancing radiomics research quality.
Clinical relevance statement
Current radiomics research in the field of stage III/IV postradiotherapy NSCLC is heterogenous, lacking reproducibility, with no identified imaging biomarker. Radiomics research quality assessment tools may enhance scientific rigor and thereby facilitate radiomics translation into clinical practice.
Key Points
-
There is heterogenous and low radiomics research quality in postradiotherapy stage III/IV nonsmall cell lung cancer.
-
Barriers to reproducibility are small cohort size, nonvalidated studies, missing technical parameters, and lack of data, code, and model sharing.
-
CLEAR (CheckList_for_EvaluAtion_of_Radiomics_research), RQS (Radiomics_Quality_Score), and the amalgamated CLEAR-RQS tool are useful frameworks for assessing radiomics research quality and may provide a valuable resource for educational purposes in the field of radiomics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the second most prevalent malignancy worldwide, with approximately 2.2 million newly diagnosed cases in 2020 [1], the majority of which are nonsmall cell lung cancer (NSCLC), comprising nearly 84% of cases [2]. NSCLC stage I and II are typically surgically managed, while treatment for locally advanced unresectable stage III and metastatic stage IV often necessitates adjuvant radiotherapy, frequently combined with chemotherapy and sometimes immunotherapy.
Despite therapeutic advancements, there has been only marginal improvement in the 5-year survival rates for stage III/IV from 24.6% in 2016 to 26.4% in 2020 [2]. Consequently, the research focus has shifted towards screening, diagnosis, and personalized management strategies to ameliorate both quality of life and survival outcomes.
Radiomics, an emerging field, leverages noninvasive techniques to extract radiomic features (RFs) from medical images, surpassing standard radiology reporting. RFs, also known as texture analysis, capture grey-level intensities and spatial relationships within the region of interest (ROI) in two-dimensional (2D) pixel and three-dimensional (3D) voxel spaces, hypothesized to be associated with tissue heterogeneity and tumor microenvironment [3,4,5,6,7]. A primary objective of radiomics is to provide predictive imaging biomarkers that, in conjunction with clinical parameters, could improve diagnosis and treatment prognostication, quality of life, and overall survival (OS), aligning with personalized and precision medicine goals.
Despite the substantial volume of NSCLC radiomics research, the translation into clinical practice has been constrained by technical and methodological challenges, resulting in studies with low statistical power and decreased replicability, reproducibility, and generalizability [3, 8,9,10,11,12,13]. Quality scoring tools and checklists, such as the Radiomics Quality Score (RQS) with 16 items and a maximum point score of 36, and the CheckList for EvaluAtion of Radiomics Research (CLEAR) with 58 items but without point-scoring, have been developed to address these challenges [10, 14]. However, their adoption has been limited, and concerns persist regarding their reliability in uniformly assessing the quality of radiomics research [9, 15].
Our study aims to 1) identify promising radiomics biomarkers in stage III/IV NSCLC treated with radiation in the literature and 2) critically appraise the research pipeline using the recently published CLEAR and longer-existing RQS systems, and merge the wording of both CLEAR and RQS frameworks into a comprehensive checklist (CLEAR-RQS) allowing a comparison between CLEAR-RQS point-scoring against CLEAR and RQS [9, 10]. CLEAR-RQS aims to serve as a valuable resource to radiomics researchers and educators across various disciplines.
Materials and methods
For this research, IRB approval was not required since it does not include any human subjects or include any identifiable private information.
Objective 1: PRISMA literature search to identify radiomics studies in stage III/IV NSCLC patients treated with radiotherapy
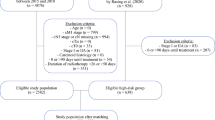
We conducted a literature search of online databases MEDLINE, PubMed, and SCOPUS from June to August 2023. Search fields comprised of [Stage III NSCLC OR Stage IV NSCLC OR nonsmall cell lung cancer] AND [radiotherapy OR SABR (stereotactic ablative body radiation) OR SBRT (stereotactic body radiation therapy)] AND [CT radiomic OR [quantitative AND imaging] OR [texture AND feature]]. Initial title and abstract analyses were performed by K.T. (3rd-year graduate medical student) with subsequent full-text screening assessment by K.T. and H.S.K. (radiologist with 20 years of general and 16 years of oncological imaging subspecialty knowledge). The final article selection comprised original research in human studies with articles written in the English language on CT radiomics in post-radiotherapy stage III/IV NSCLC (Table 1). Figure 1 shows the PRISMA flow diagram of the literature search.
Literature data extraction and analysis
Article data extraction included cohort size, radiotherapy/ CT technique, utilized radiomics software, selected RFs, and study endpoints.
Critical appraisal of full-text articles was performed regarding the following research questions: 1) are there commonly selected RFs for treatment response, adverse events, and/ or outcomes in patients undergoing radiotherapy? 2) are there factors within the research study design that would impede reproducibility?
Objective 2: critical appraisal of selected articles applying CLEAR and RQS frameworks and development of a comprehensive radiomics assessment checklist (CLEAR-RQS)
All articles were assessed by three readers, D.G. (radiologist with 4 years of general radiology experience), K.T., and H.S.K., utilizing the RQS metrics and the CLEAR/ CLEAR-RQS criteria [10, 14]. To facilitate a direct comparison between RQS and CLEAR/ CLEAR-RQS, a point score of 1 for “yes” and of 0 for “no” or “NA” responses was assigned to each CLEAR/ CLEAR-RQS item, resulting in a maximal possible score of 58 for CLEAR and 61 for CLEAR-RQS.
The mean score from all three readers was utilized to compare the RQS, CLEAR, and CLEAR-RQS frameworks. To enable a relative comparison between frameworks, the score of each tool was proportionally converted to a percentage based on its metric (e.g., 100% equated to a CLEAR point score of 58, a CLEAR-RQS score of 61, and an RQS score of 36).
K.T. and H.S.K. systematically compared the wording and interpretation of all 58 CLEAR and 16 RQS items (Table 2). To prevent redundancy, identical and very similar items were merged, retaining the wording of the more specific source framework (CLEAR or RQS). No new wording was introduced to ensure adherence to the respective source framework.
Results
Objective 1: PRISMA literature search
Figure 1 demonstrates the PRISMA diagram, which outlines the literature search. In total, 871 articles were found (PubMed n = 403, MEDLINE n = 249, SCOPUS n = 219). After the exclusion of 462 duplicates, 409 article abstracts were screened. This resulted in 22 identified articles that underwent full-text assessment, of which a further 11 were excluded based on inclusion and exclusion criteria (Table 1). Finally, 11 articles were included in the systematic review (Supplemental Table S1).
Cohort specifics
Retrospective patient cohort sizes ranged from 10 to 337 (median = 91, mean =114), with 7 studies comprising smaller cohort sizes of less than 100 [11, 16,17,18,19,20,21]. All studies except for 2 analyzed single-center patient cohorts [11, 22].
Study endpoints of selected radiomic features
Study endpoints varied with selected RFs relating to OS in three studies [17, 23, 24] and to treatment response in two studies [19, 25]. Three studies analyzed both OS and progression-free survival [11, 21, 22], and two studies examined the treatment-related complication of radiation pneumonitis [18, 20]. One study measured RF changes in the NSCLC tumor before and during radiotherapy without association with any clinical endpoint [16].
Radiotherapy regimen
Applied radiotherapy methods varied, with intensity-modulated radiotherapy (IMRT) utilized in three studies, IMRT or stereotactic body radiotherapy in one study, volumetric modulated arc therapy (VMAT) used in two studies [16, 18, 21, 23, 25, 26]. One study employed stereotactic ablative radiotherapy (SBRT) in a subset of its patient cohort [23]. Four studies did not mention specific radiotherapy delivery methods [17, 19, 20, 22].
CT imaging protocol
CT vendor/ scanner type and scanning technique varied or were not disclosed in multiple aspects.
Regarding CT vendor and scanner models, 6 out of 11 articles mentioned the scanner type model, and out of these 6, 5 used a single CT scanner model.
Two studies used noncontrast cone beam CT images [17, 22].
Three studies used contrast-enhanced CT images [11, 21, 24], and the remaining 6 studies did not mention specific contrast phases [16, 18,19,20, 23, 25].
Three studies specified the respiratory cycle timepoint of image acquisition, with 2 at free breathing cycles [18, 20] and 1 at the end-expiratory phase [21].
Three studies did not specify the CT slice thickness [19, 24, 25], and 4 studies reported a CT slice thickness of 2.5 mm [11, 16, 20, 22].
One study each analyzed 1 or 2 mm [23], 1 or 3 mm [17], 2.5 or 3.0 mm [21], and 5 mm [18] CT slice thicknesses, respectively.
Radiomic feature extraction
RF extraction software was highly variable among the studies. Eight studies extracted features utilizing common software tools (1 AnalysisKit [23], 2 PyRadiomics [18, 20], 2 IBEX [16, 17], 3 MATLAB [11, 19, 22], 1 LIFEx [25]). One study employed an in-house software to extract radiomic features [21], and 1 study did not disclose the utilized software [5].
Radiomic feature selection
Full-text analysis scoring revealed a lack of similarities to identify common RFs given the variability of study endpoints (e.g., treatment response, OS, radiotherapy-related pneumonitis), along with differing data sets. Grey-Level Co-occurrence Matrix (GLCM)[11, 16-20; 22, 24, 25], first-order RFs intensity [16, 17, 20, 22, 23] and shape [17, 20, 22, 23], and higher order RF Grey-Level Size Zone Matrix (GLSZM) [18, 23, 25], were among the selected RFs described.
Model building
Model or nomogram building with non-RF parameters was described in 8 out of 11 studies [11, 18, 19, 21,22,23,24,25]. Available model/ nomogram performance varied, with three studies demonstrating borderline significant p values of 0.048, 0.049, and 0.046, respectively [11, 21, 23]. Most common utilized clinicopathological parameter for model building was smoking [18, 19, 21, 25], T- and N-stage [19, 21, 22, 25], with each factor observed in four studies, followed by tumor histology incorporated in three studies [19, 21, 22].
Supplemental Table S1 describes the articles’ detailed data extraction.
Objective 2: applying CLEAR and RQS point-scoring to selected articles (n = 11) and development of a comprehensive radiomics assessment checklist (CLEAR-RQS)
CLEAR metrics
The median CLEAR point score was 32.33 (55.74%, range: 25.33–48 [47.7–82.75%]). Across all three readers, all studies fulfilled the “manuscript preparation” CLEAR criteria of providing a title, abstract, keywords, introduction, and discussion. All articles failed to report details regarding the items “sample size calculation” and “flowchart for eligibility criteria”, and the entire domain of “open science.”
Table 3 summarizes the 44 items in detail where two or all three readers identified missing data pertaining to the respective CLEAR item.
RQS metrics
The median RQS point-score was 6.33 (17.59%) with a range of 0-16 points (0–44.44%) out of a maximal possible 36-point-score. Many criteria scored 0 or below by all readers as illustrated in Table 4, e.g., no study contained “phantom calibrations”, were “prospective studies registered with a database”, or performed a “cost-effective analysis”.
Comparing CLEAR and RQS point distribution
Table 5 demonstrates the point distribution for papers evaluated using the CLEAR and RQS criteria. Ranking differed for the top 3 articles when using the CLEAR versus RQS systems, for example, Chen et al [23] ranked 1st on the RQS but 4th according to CLEAR metrics, whereas Van Timmeren et al [22] ranked 1st on the CLEAR but 2nd according to the RQS framework.
Figure 2 shows the score point values and respective ranking of appraised articles according to the CLEAR and RQS metrics.
RQS and CLEAR percentage score distributions of assessed radiomics articles in post-radiotherapy stage III/IV NSCLC (n = 11). Red bars representing the RQS, and green bars representing the CLEAR, frameworks. Numbers on top of the bars represent the RQS and CLEAR rank, respectively. The horizontal red bar delineates 50% percent highlighting that no RQS score was above 50%. Articles are listed in alphabetical order
Amalgamation of CLEAR and RQS items into a comprehensive assessment checklist (CLEAR-RQS) and comparing CLEAR-RQS with CLEAR and RQS
The 58 CLEAR and 16 RQS items’ wording was compared and identical or similar, resulted in the merging of items and the development of a 61-item CLEAR-RQS checklist (Table 2).
When applying the newly developed CLEAR-RQS checklist, the scoring percentage of each article was between its CLEAR and RQS score, with CLEAR-RQS adhering closer to the CLEAR checklist (Supplemental Fig. S1). This is easily explained, given that the CLEAR-RQS checklist contains 61 items, which is much more aligned with the 58-item containing CLEAR checklist compared to the RQS framework which only contains 16 items.
Discussion
This systematic literature review on radiomic features in post-radiotherapy stage III/IV NSCLC patients yielded 11 retrospective studies, exhibiting substantial variations in their study design, rendering them incomparable, and failing to identify an RF suitable for clinical translation. Moreover, there was low reporting quality when applying both the CLEAR and RQS frameworks, consistent with findings from other radiomics data reviews and meta-analyses [8, 15, 27, 28]. Merging the CLEAR and RQS frameworks into a comprehensive CLEAR-RQS checklist aimed to provide a comprehensive yet detailed guide for designing and critically appraising published research to the radiomics research community.
Limitations in radiomics study design
This review revealed several shortcomings in research design, potentially diminishing the generalizability and reproducibility of identified RFs.
The heterogeneity of study cohorts and relatively small sample sizes may limit comparability. Notably, two studies featured small sample sizes (n = 10, n = 23), rendering validation nearly unfeasible [16, 17].
Data harmonization, particularly image acquisition and reconstruction settings (referred to as “pre-processing” by CLEAR and RQS), emerged as a key requirement in radiomics research [29, 30]. Three studies did not disclose whether CT slice thickness harmonization was performed [19, 24, 25]. Body habitus, scanner models, and demographic parameters may influence radiomic analysis, necessitating their specifications for future validation [30]. This may require further data postprocessing to ensure reproducibility [29]. Two studies [17, 22] used cone-beam CT (CBCT) images, introducing challenges related to radiomic region-of-interest delineation caused by scattered radiation artifacts [31, 32]. Only three studies detailed the use of free breathing CT images [17, 18, 20], with the remaining studies neglecting to specify the CT acquisition breathing cycle point [11, 16, 19, 21,22,23,24,25]. Free-breathing studies introduce image blurring due to movement artefacts, acknowledged to impact radiomics analysis [33]. Consequently, RF extraction from inherently inconsistent or highly variable CT scanning protocols may compromise result interpretation and reproducibility.
Seven studies omitted reporting of image pre-processing resampling techniques and associated parameters [11, 16,17,18,19, 24, 25]. Eight studies failed to describe discretization methods [11, 16,17,18,19,20, 24, 25]. Image resampling, particularly downsampling and interpolating images in a manner that preserves spatial detail while avoiding overfitting, is critical for data harmonization. Shafiq-ul-Hassan et al demonstrated that resampling could reduce feature variability, therefore enhancing RF robustness [34].
Only 2 studies reported details of feature extraction segmentation of reliability analysis [11, 21]. Description of this step is important, as manual or semi-automated segmentation methods may introduce intra- and inter-observer variability, impacting reproducibility [35].
Certain categories of RFs, including first-order (intensity, shape) and higher-order (GLCM (Grey-Level Co-Occurrence Matrix), GLSZM (Grey-Level Size Zone Matrix)) groups, were more commonly investigated [11, 16,17,18,19,20, 22, 24, 25].
CLEAR and RQS metrics to assess the quality of radiomics research reporting
Item weighting
Assessing study quality depends on robust research design and comprehensive reporting of methodology, statistical parameters, and results. Both CLEAR criteria and RQS scores indicated suboptimal reporting quality, with variations in study rankings. No study fully met all CLEAR items, and RQS scores ranged from 0 to +16 points, less than 50% of the maximum achievable +36 points. Our analysis suggests that these assessment tools offer complementary critiques for identifying methodological challenges hindering the reproducibility and clinical application of radiomic results.
The CLEAR checklist offers a general guideline covering all aspects of the radiomics workflow, while the RQS framework comprises 16 criteria with varying weighted point scores. Certain domains, such as “prospective validation in an appropriate trial” (0 or +7 points) and “validation cohorts” (-5, +2, +3, +4, +5 points), are assigned more points compared to others. These items contributed most to top-scoring papers on RQS, which did not align with their CLEAR ranking. For instance, the RQS item “validation” negatively impacted the scores of Yang et al (3.67 points) [18], Shi et al (0.67 points) [17], and Zhang et al (0.67 points) [16], ranking them 7th, 10th, and 11th out of 11 articles, respectively. Such large point score disparities were not observed with CLEAR criteria, as exemplified by the comparison of Wang et al and Fried et al [21, 24]. With RQS, Wang et al ranked 5th (12.67 points) while Fried et al ranked 6th (6.33 points), whereas in the CLEAR metric, the point scoring disparity was less evident, and with Wang et al ranking lower (rank 7, 31.33 points) than Fried et al (rank 5, 35.67 points [21, 24].
A recently published quality scoring tool for radiomics research, METRICS (METhodological RadiomICs Score), has been developed by an international panel and has been endorsed by the European Society of Medical Imaging Informatics (EUSoMII). METRICS contains weighted items carefully selected and discussed via a modified Delphi process to ensure a balanced consensus among panelists [36]. This new point-scoring framework aims to facilitate critical appraisal of a broad range of radiomics research, from the manual data labeling and extraction to deep learning artificial intelligence (AI) pipelines.
Inter-rater variability
D’Antonoli et al’s study revealed that the RQS metric is susceptible to inter-rater biases, as its domains can be construed differently depending on raters’ backgrounds [9]. This corresponds to our findings, as our three raters – a graduate medical student, a junior radiologist, and a senior radiologist – exhibited minor discrepancies in RQS scores, which were reconciled through consensus. This variability aligns with prior research indicating low RQS scores and poor inter-rater reliability [9, 27, 28].
Creating a comprehensive CLEAR-RQS checklist to aid future education and research
Efforts aim to develop a robust tool for assessing radiomics research quality, with a focus on machine learning and other AI models [37,38,39]. The RQS and CLEAR frameworks specifically address radiomics methodology [10, 14], which has garnered attention from the Society of Nuclear Medicine and Molecular Imaging, the European Association of Nuclear Medicine [39], and the Scientific Editorial Board of European Radiology [40].
The herein presented CLEAR-RQS checklist, developed by an international research group from two academic tertiary institutions, aims to comprehensively evaluate radiomics methodologies, without sacrificing specificity. It integrates standards from both CLEAR and RQS tools, preserving their detailed wording catering to radiomics researchers, while also serving educational purposes across various disciplines. The application of a point-scoring system to the CLEAR-RQS checklist should be avoided, given the intricate complexities inherent in real-world research scenarios, which may not be granular enough to adequately capture the nuanced quality of the assessed research investigations.
In conclusion, stage III/IV NSCLC radiomics research suffers from suboptimal reporting quality, hindering the discovery of validated predictive RFs. Technical challenges and lack of access to source images and model files impede reproducibility. Thorough validation and open access to data and code are essential to increase transparency and raise reporting standards [41, 42]. Adoption of the CLEAR-RQS checklist could accelerate the translation of radiomics research into clinical practice. Furthermore, sustained multi-disciplinary collaboration for continuous assessment and improvement in this rapidly evolving field is required to ultimately benefit patient outcomes in personalized medicine.
Change history
22 April 2024
Source Line layout was corrected.
Abbreviations
- CLEAR:
-
Checklist for evaluAtion of radiomics research
- GLCM:
-
Grey-level co-occurrence matrix
- GLSZM:
-
Gray-level size zone matrix
- IMRT:
-
Intensity-modulated radiotherapy
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- RF:
-
Radiomic feature
- RQS:
-
Radiomics quality score
- SBRT:
-
Stereotactic body radiation therapy
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Ganti AK, Klein AB, Cotarla I, Seal B, Chou E (2021) Update of incidence, prevalence, survival, and initial treatment in patients with non–small cell lung cancer in the US. JAMA Oncol 7:1824–1832
Grossmann P, Stringfield O, El-Hachem N et al (2017) Defining the biological basis of radiomic phenotypes in lung cancer. Elife 6:e23421
Hyun SH, Ahn MS, Koh YW, Lee SJ (2019) A machine-learning approach using PET-based radiomics to predict the histological subtypes of lung cancer. Clin Nucl Med 44:956–960
Wang JH, Wahid KA, van Dijk LV, Farahani K, Thompson RF, Fuller CD (2021) Radiomic biomarkers of tumor immune biology and immunotherapy response. Clin Transl Radiat Oncol 28:97–115
Yu X, Zhang S, Xu J et al (2023) Nomogram using CT radiomics features for differentiation of pneumonia-type invasive mucinous adenocarcinoma and pneumonia: multicenter development and external validation study. AJR Am J Roentgenol 220:224–234
Rosenthal MH, Schawkat K (2023) Beyond the AJR: CT radiomic features of the pancreas predict development of pancreatic cancer. AJR Am J Roentgenol 220:763
Malcolm JA, Tacey M, Gibbs P, Lee B, Ko HS (2023) Current state of radiomic research in pancreatic cancer: focusing on study design and reproducibility of findings. Eur Radiol 33:6659–6669
Akinci D’Antonoli T, Cavallo AU, Vernuccio F et al (2024) Reproducibility of radiomics quality score: an intra- and inter-rater reliability study. Eur Radiol. 34:2791–2804
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Coroller TP, Grossmann P, Hou Y et al (2015) CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol 114:345–350
Huynh E, Coroller TP, Narayan V et al (2016) CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer. Radiother Oncol 120:258–266
Sollini M, Antunovic L, Chiti A, Kirienko M (2019) Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging 46:2656–2672
Kocak B, Baessler B, Bakas S et al (2023) CheckList for EvaluAtion of Radiomics research (CLEAR): a step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 14:75
Spadarella G, Stanzione A, Akinci D’Antonoli T et al (2023) Systematic review of the radiomics quality score applications: an EuSoMII Radiomics Auditing Group Initiative. Eur Radiol 33:1884–1894
Zhang R, Cai Z, Luo Y, Wang Z, Wang W (2022) Preliminary exploration of response the course of radiotherapy for stage III non-small cell lung cancer based on longitudinal CT radiomics features. Eur J Radiol Open 9:100391
Shi L, Rong Y, Daly M et al (2020) Cone-beam computed tomography-based delta-radiomics for early response assessment in radiotherapy for locally advanced lung cancer. Phys Med Biol 65:015009
Yang S, Huang S, Ye X, Xiong K, Zeng B, Shi Y (2022) Risk analysis of grade ≥ 2 radiation pneumonitis based on radiotherapy timeline in stage III/IV non-small cell lung cancer treated with volumetric modulated arc therapy: a retrospective study. BMC Pulm Med 22:402
Ramella S, Fiore M, Greco C et al (2018) A radiomic approach for adaptive radiotherapy in non-small cell lung cancer patients. PLoS One 13:e0207455
Kawahara D, Imano N, Nishioka R et al (2021) Prediction of radiation pneumonitis after definitive radiotherapy for locally advanced non-small cell lung cancer using multi-region radiomics analysis. Sci Rep 11:16232
Fried DV, Tucker SL, Zhou S et al (2014) Prognostic value and reproducibility of pretreatment CT texture features in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 90:834–842
van Timmeren JE, van Elmpt W, Leijenaar RTH et al (2019) Longitudinal radiomics of cone-beam CT images from non-small cell lung cancer patients: Evaluation of the added prognostic value for overall survival and locoregional recurrence. Radiother Oncol 136:78–85
Chen W, Wang L, Hou Y et al (2022) Combined Radiomics-Clinical Model to Predict Radiotherapy Response in Inoperable Stage III and IV Non-Small-Cell Lung Cancer. Technol Cancer Res Treat 21:15330338221142400
Wang L, Dong T, Xin B et al (2019) Integrative nomogram of CT imaging, clinical, and hematological features for survival prediction of patients with locally advanced non-small cell lung cancer. Eur Radiol 29:2958–2967
Yan M, Wang W (2021) A radiomics model of predicting tumor volume change of patients with stage III non-small cell lung cancer after radiotherapy. Sci Prog 104:36850421997295
Oberije C, De Ruysscher D, Houben R et al (2015) A Validated Prediction Model for Overall Survival From Stage III Non-Small Cell Lung Cancer: Toward Survival Prediction for Individual Patients. Int J Radiat Oncol Biol Phys 92:935–944
Zhong J, Hu Y, Si L et al (2021) A systematic review of radiomics in osteosarcoma: utilizing radiomics quality score as a tool promoting clinical translation. Eur Radiol 31:1526–1535
Park JE, Kim D, Kim HS et al (2020) Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol 30:523–536
Zwanenburg A, Vallieres M, Abdalah MA et al (2020) The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 295:328–338
He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z (2016) Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Sci Rep 6:34921
Kurz C, Kamp F, Park YK et al (2016) Investigating deformable image registration and scatter correction for CBCT-based dose calculation in adaptive IMPT. Med Phys 43:5635
Song J-Y, Nam T-K, Ahn S-J, Chung W-K, Yoon M-S, Nah B-S (2009) Respiratory Motional Effect on Cone-Beam CT in Lung Radiation Surgery. Med Dosim 34:117–125
Huynh E, Coroller TP, Narayan V et al (2017) Associations of Radiomic Data Extracted from Static and Respiratory-Gated CT Scans with Disease Recurrence in Lung Cancer Patients Treated with SBRT. PLoS One 12:e0169172
Shafiq-Ul-Hassan M, Zhang GG, Latifi K et al (2017) Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys 44:1050–1062
Kothari G, Woon B, Patrick CJ et al (2022) The impact of inter-observer variation in delineation on robustness of radiomics features in non-small cell lung cancer. Sci Rep 12:12822
Kocak B, Akinci D’Antonoli T, Mercaldo N et al (2024) METhodological RadiomICs Score (METRICS): a quality scoring tool for radiomics research endorsed by EuSoMII. Insights Imaging 15:8
Mongan J, Moy L, Charles E, Kahn J (2020) Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell 2:e200029
Cerdá-Alberich L, Solana J, Mallol P et al (2023) MAIC–10 brief quality checklist for publications using artificial intelligence and medical images. Insights Imaging 14:11
Hatt M, Krizsan AK, Rahmim A et al (2023) Joint EANM/SNMMI guideline on radiomics in nuclear medicine. Eur J Nucl Med Mol Imaging 50:352–375
Kocak B, Chepelev LL, Chu LC et al (2023) Assessment of RadiomIcS rEsearch (ARISE): a brief guide for authors, reviewers, and readers from the Scientific Editorial Board of European Radiology. Eur Radiol 33:7556–7560
Ko HS (2024) Advancing radiomics research translation through a public database. Eur Radiol 34:433–435
Akinci D’Antonoli T, Cuocolo R, Baessler B, Pinto Dos Santos D (2024) Towards reproducible radiomics research: introduction of a database for radiomics studies. Eur Radiol 34:436–443
Acknowledgements
The authors are grateful for Prof Stephen Stuckey, director of Cancer Imaging at Peter MacCallum Cancer Centre (Peter Mac), and for family and friends of MD candidate Kevin Tran providing ongoing invaluable support and guidance. Furthermore, the authors acknowledge Rachel Ko who performed the exploratory literature search during the inception of the study as a Peter Mac research summer student.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Hyun Soo Ko
Conflict of interest
H.S.K. is a member of the Scientific Editorial Board for European Radiology (Imaging Informatics and Artificial Intelligence). They have not participated in the selection or review process for this article. The remaining authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr Wei Hong kindly provided statistical advice for this manuscript who is a medical oncologist and a certified biostatistician. However, no complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was not required because this study did not include any intervention or extraction of private information of human subjects.
Study subjects or cohorts overlap
There are no study subjects or cohorts overlap.
Methodology
-
Observational
-
Performed at two institutions
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, K., Ginzburg, D., Hong, W. et al. Post-radiotherapy stage III/IV non-small cell lung cancer radiomics research: a systematic review and comparison of CLEAR and RQS frameworks. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10736-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10736-1