Abstract
Objectives
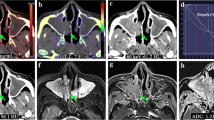
To explore the value of the synthetic MRI (SyMRI), combined with amide proton transfer–weighted (APTw) MRI for quantitative and morphologic assessment of sinonasal lesions, which could provide relative scale for the quantitative assessment of tissue properties.
Methods
A total of 80 patients (31 malignant and 49 benign) with sinonasal lesions, who underwent the SyMRI and APTw examination, were retrospectively analyzed. Quantitative parameters (T1, T2, proton density (PD)) and APT % were obtained through outlining the region of interest (ROI) and comparing the two groups utilizing independent Student t test or a Wilcoxon test. Receiver operating characteristic curve (ROC), Delong test, and logistic regression analysis were performed to assess the diagnostic efficiency of one-parameter and multiparametric models.
Results
SyMRI-derived mean T1, T2, and PD were significantly higher and APT % was relatively lower in benign compared to malignant sinonasal lesions (p < 0.05). The ROC analysis showed that the AUCs of the SyMRI-derived quantitative (T1, T2, PD) values and APT % ranged from 0.677 to 0.781 for differential diagnosis between benign and malignant sinonasal lesions. The T2 values showed the best diagnostic performance among all single parameters for differentiating these two masses. The AUCs of combined SyMRI-derived multiple parameters with APT % (AUC = 0.866) were the highest than that of any single parameter, which was significantly improved (p < 0.05).
Conclusion
The combination of SyMRI and APTw imaging has the potential to reflect intrinsic tissue characteristics useful for differentiating benign from malignant sinonasal lesions.
Clinical relevance statement
Combining synthetic MRI with amide proton transfer–weighted imaging could function as a quantitative and contrast-free approach, significantly enhancing the differentiation of benign and malignant sinonasal lesions and overcoming the limitations associated with the superficial nature of endoscopic nasal sampling.
Key Points
• Synthetic MRI and amide proton transfer–weighted MRI could differentiate benign from malignant sinonasal lesions based on quantitative parameters.
• The diagnostic efficiency could be significantly improved through synthetic MRI + amide proton transfer–weighted imaging.
• The combination of synthetic MRI and amide proton transfer–weighted MRI is a noninvasive method to evaluate sinonasal lesions.






Similar content being viewed by others
Abbreviations
- APTw:
-
Amide proton transfer–weighted
- AUC:
-
Area under the curve
- cMRI:
-
Conventional MRI
- CT:
-
Computed tomography
- H&E:
-
Hematoxylin-eosin staining
- ICC:
-
Intraclass correlation coefficient
- PD:
-
Proton density
- ROC:
-
Receiver operating characteristic curve
- ROIs:
-
Regions of interest
- SyMRI:
-
Synthetic MRI
References
Slootweg PJ, Ferlito A, Cardesa A et al (2013) Sinonasal tumors: a clinicopathologic update of selected tumors. Eur Arch Otorhinolaryngol 270:5–20. https://doi.org/10.1007/s00405-012-2025-4
Sen S, Chandra A, Mukhopadhyay S, Ghosh P (2015) Sinonasal tumors: computed tomography and MR imaging features. Neuroimaging Clin N Am 25:595–618. https://doi.org/10.1016/j.nic.2015.07.006
Eggesbø HB (2012) Imaging of sinonasal tumours. Cancer Imaging 12:136–152. https://doi.org/10.1102/1470-7330.2012.0015
Wang XY, Yan F, Hao H et al (2015) Improved performance in differentiating benign from malignant sinonasal tumors using diffusion-weighted combined with dynamic contrast-enhanced magnetic resonance imaging. Chin Med J (Engl) 128:586–592. https://doi.org/10.4103/0366-6999.151649
Jégoux F, Métreau A, Louvel G, Bedfert C (2013) Paranasal sinus cancer. Eur Ann Otorhinolaryngol Head Neck Dis 130:327–335. https://doi.org/10.1016/j.anorl.2012.07.007
Carta F, Blancal JP, Verillaud B et al (2013) Surgical management of inverted papilloma: approaching a new standard for surgery. Head Neck 35:1415–1420. https://doi.org/10.1002/hed.23159
Valente G, Mamo C, Bena A et al (2006) Prognostic significance of microvessel density and vascular endothelial growth factor expression in sinonasal carcinomas. Hum Pathol 37:391–400. https://doi.org/10.1016/j.humpath.2005.11.021
Airoldi M, Garzaro M, Valente G et al (2009) Clinical and biological prognostic factors in 179 cases with sinonasal carcinoma treated in the Italian Piedmont region. Oncology 76:262–269. https://doi.org/10.1159/000206140
Cellina M, Gibelli D, Floridi C et al (2020) Sphenoid sinuses: pneumatisation and anatomical variants-what the radiologist needs to know and report to avoid intraoperative complications. Surg Radiol Anat 42:1013–1024. https://doi.org/10.1007/s00276-020-02490-y
Madani G, Beale TJ, Lund VJ (2009) Imaging of sinonasal tumors. Semin Ultrasound CT MR 30:25–38. https://doi.org/10.1053/j.sult.2008.10.013
Wang P, Tang Z, Xiao Z et al (2022) Dual-energy CT in differentiating benign sinonasal lesions from malignant ones: comparison with simulated single-energy CT, conventional MRI, and DWI. Eur Radiol 32:1095–1105. https://doi.org/10.1007/s00330-021-08159-3
Razek AA, Sieza S, Maha B (2009) Assessment of nasal and paranasal sinus masses by diffusion-weighted MR imaging. J Neuroradiol 36:206–211. https://doi.org/10.1016/j.neurad.2009.06.001
Martínez Barbero JP, Rodríquez Jiménez I, Martin Noguerol T, Luna Alcalá A (2013) Utility of MRI diffusion techniques in the evaluation of tumors of the head and neck. Cancers (Basel) 5:875–889. https://doi.org/10.3390/cancers5030875
Abdel Razek AA, Gaballa G, Elhawarey G et al (2009) Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol 19:201–208. https://doi.org/10.1007/s00330-008-1123-6
Xiao Z, Tang Z, Qiang J et al (2018) Intravoxel incoherent motion MR imaging in the differentiation of benign and malignant sinonasal lesions: comparison with conventional diffusion-weighted MR imaging. AJNR Am J Neuroradiol 39:538–546. https://doi.org/10.3174/ajnr.A5532
Xiao Z, Zhong Y, Tang Z et al (2018) Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 28:2923–2933. https://doi.org/10.1007/s00330-017-5286-x
Jiang JX, Tang ZH, Zhong YF, Qiang JW (2017) Diffusion kurtosis imaging for differentiating between the benign and malignant sinonasal lesions. J Magn Reson Imaging 45:1446–1454. https://doi.org/10.1002/jmri.25500
Su GY, Xu YK, Liu J et al (2023) Texture analysis of diffusion kurtosis imaging for differentiating malignant from benign sinonasal lesions: added value to conventional imaging features. Br J Radiol 96:20220806. https://doi.org/10.1259/bjr.20220806
Karkuzhali P, Gnanaguruparan A, Bhattachryya M (2006) Psammomatoid ossifying fibroma of sinonasal tract. Otolaryngol Head Neck Surg 134:705–707. https://doi.org/10.1016/j.otohns.2005.03.077
van Rijswijk CS, Kunz P, Hogendoorn PC et al (2002) Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imaging 15:302–307. https://doi.org/10.1002/jmri.10061
White ML, Zhang Y, Robinson RA (2006) Evaluating tumors and tumorlike lesions of the nasal cavity, the paranasal sinuses, and the adjacent skull base with diffusion-weighted MRI. J Comput Assist Tomogr 30:490–495. https://doi.org/10.1097/00004728-200605000-00023
Wang P, Hu S, Wang X et al (2023) Synthetic MRI in differentiating benign from metastatic retropharyngeal lymph node: combination with diffusion-weighted imaging. Eur Radiol 33:152–161. https://doi.org/10.1007/s00330-022-09027-4
Yang F, Li Y, Lei H et al (2023) Histogram analysis of synthetic magnetic resonance imaging: correlations with histopathological factors in head and neck squamous cell carcinoma. Eur J Radiol 160:110715. https://doi.org/10.1016/j.ejrad.2023.110715
Yang F, Li X, Li Y et al (2023) Histogram analysis of quantitative parameters from synthetic MRI: correlations with prognostic factors in nasopharyngeal carcinoma. Eur Radiol 33:5344–5354. https://doi.org/10.1007/s00330-023-09553-9
Li M, Fu W, Ouyang L et al (2023) Potential clinical feasibility of synthetic MRI in bladder tumors: a comparative study with conventional MRI. Quant Imaging Med Surg 13:5109–5118. https://doi.org/10.21037/qims-22-1419
Li Q, Xiao Q, Yang M et al (2021) Histogram analysis of quantitative parameters from synthetic MRI: correlations with prognostic factors and molecular subtypes in invasive ductal breast cancer. Eur J Radiol 139:109697. https://doi.org/10.1016/j.ejrad.2021.109697
Cui Y, Han S, Liu M et al (2020) Diagnosis and grading of prostate cancer by relaxation maps from synthetic MRI. J Magn Reson Imaging 52:552–564. https://doi.org/10.1002/jmri.27075
Zhu K, Chen Z, Cui L et al (2022) The preoperative diagnostic performance of multi-parametric quantitative assessment in rectal carcinoma: a preliminary study using synthetic magnetic resonance imaging. Front Oncol 12:682003. https://doi.org/10.3389/fonc.2022.682003
Konar AS, Paudyal R, Shah AD et al (2022) Qualitative and quantitative performance of magnetic resonance image compilation (MAGiC) method: an exploratory analysis for head and neck imaging. Cancers (Basel) 14:3624. https://doi.org/10.3390/cancers14153624
Peng Y, Zou X, Chen G et al (2023) Chemical shift-encoded sequence (IDEAL-IQ) and amide proton transfer (APT) MRI for prediction of histopathological factors of rectal cancer. Bioengineering (Basel) 10:720. https://doi.org/10.3390/bioengineering10060720
Wang HJ, Cai Q, Huang YP et al (2022) Amide proton transfer-weighted MRI in predicting histologic grade of bladder cancer. Radiology 305:127–134. https://doi.org/10.1148/radiol.211804
Yuan J, Chen S, King AD et al (2014) Amide proton transfer-weighted imaging of the head and neck at 3 T: a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed 27:1239–1247. https://doi.org/10.1002/nbm.3184
Law BKH, King AD, Ai QY et al (2018) Head and neck tumors: amide proton transfer MRI. Radiology 288:782–790. https://doi.org/10.1148/radiol.2018171528
Ma C, Tian S, Song Q et al (2023) Amide proton transfer-weighted imaging combined with intravoxel incoherent motion for evaluating microsatellite instability in endometrial cancer. J Magn Reson Imaging 57:493–505. https://doi.org/10.1002/jmri.28287
Chen W, Liu G, Chen J et al (2023) Whole-tumor amide proton transfer-weighted imaging histogram analysis to predict pathological extramural venous invasion in rectal adenocarcinoma: a preliminary study. Eur Radiol 33:5159–5171. https://doi.org/10.1007/s00330-023-09418-1
Yu L, Li C, Luo X et al (2019) Differentiation of malignant and benign head and neck tumors with amide proton transfer-weighted MR imaging. Mol Imaging Biol 21:348–355. https://doi.org/10.1007/s11307-018-1248-1
Han Y, Wang W, Yang Y et al (2020) Amide proton transfer imaging in predicting isocitrate dehydrogenase 1 mutation status of grade II/III gliomas based on support vector machine. Front Neurosci 14:144. https://doi.org/10.3389/fnins.2020.00144
Zhang Z, Li S, Wang W et al (2023) Synthetic MRI for the quantitative and morphologic assessment of head and neck tumors: a preliminary study. Dentomaxillofac Radiol 52:20230103. https://doi.org/10.1259/dmfr.20230103
Meng N, Wang X, Sun J et al (2020) Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer. Eur Radiol 30:5758–5767. https://doi.org/10.1007/s00330-020-06884-9
Bobak CA, Barr PJ, O’Malley AJ (2018) Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med Res Methodol 18:93. https://doi.org/10.1186/s12874-018-0550-6
Zhao L, Liang M, Xie L et al (2021) Prediction of pathological prognostic factors of rectal cancer by relaxation maps from synthetic magnetic resonance imaging. Eur J Radiol 138:109658. https://doi.org/10.1016/j.ejrad.2021.109658
Zhao L, Liang M, Wang S et al (2021) Preoperative evaluation of extramural venous invasion in rectal cancer using radiomics analysis of relaxation maps from synthetic MRI. Abdom Radiol (NY) 46:3815–3825. https://doi.org/10.1007/s00261-021-03021-y
Yang F, Wei H, Li X et al (2023) Pretreatment synthetic magnetic resonance imaging predicts disease progression in nonmetastatic nasopharyngeal carcinoma after intensity modulation radiation therapy. Insights Imaging 14:59. https://doi.org/10.1186/s13244-023-01411-y
Duchaussoy T, Budzik JF, Norberciak L et al (2019) Synthetic T2 mapping is correlated with time from stroke onset: a future tool in wake-up stroke management? Eur Radiol 29:7019–7026. https://doi.org/10.1007/s00330-019-06270-0
Jung Y, Gho SM, Back SN et al (2018) The feasibility of synthetic MRI in breast cancer patients: comparison of T(2) relaxation time with multiecho spin echo T(2) mapping method. Br J Radiol 92:20180479. https://doi.org/10.1259/bjr.20180479
Cai Q, Wen Z, Huang Y et al (2021) Investigation of synthetic magnetic resonance imaging applied in the evaluation of the tumor grade of bladder cancer. J Magn Reson Imaging 54:1989–1997. https://doi.org/10.1002/jmri.27770
Raz E, Win W, Hagiwara M et al (2015) Fungal sinusitis. Neuroimaging Clin N Am 25:569–576. https://doi.org/10.1016/j.nic.2015.07.004
Wang YZ, Yang BT, Wang ZC, Song L, Xian JF (2012) MR evaluation of sinonasal angiomatous polyp. AJNR Am J Neuroradiol 33:767–772. https://doi.org/10.3174/ajnr.A2856
Schmidt H, Schwenzer NF, Gatidis S et al (2016) Systematic evaluation of amide proton chemical exchange saturation transfer at 3 T: effects of protein concentration, pH, and acquisition parameters. Invest Radiol 51:635–646. https://doi.org/10.1097/rli.0000000000000292
Wang F, Xiang YS, Wu P, Shen AJ, Wang PJ (2023) Evaluation of amide proton transfer imaging for bladder cancer histopathologic features: a comparative study with diffusion- weighted imaging. Eur J Radiol 159:110664. https://doi.org/10.1016/j.ejrad.2022.110664
Yamada I, Yoshino N, Hikishima K et al (2017) Colorectal carcinoma: ex vivo evaluation using 3-T high-spatial-resolution quantitative T2 mapping and its correlation with histopathologic findings. Magn Reson Imaging 38:174–181. https://doi.org/10.1016/j.mri.2016.12.028
Acknowledgements
The authors acknowledge the support received from the GE Healthcare.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Xiaoyong Ren, MD, The Second Affiliated Hospital of Xi’an Jiaotong University.
Conflict of interest
One of the authors of this manuscript (X.W.) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, Y., Zhang, Q., Chen, X. et al. Synthetic MRI and amide proton transfer–weighted MRI for differentiating between benign and malignant sinonasal lesions. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10696-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10696-6