Abstract
Objectives
Cerebral magnetic resonance imaging (cMRI) at term-equivalent age (TEA) can detect brain injury (BI) associated with adverse neurological outcomes in preterm infants. This study aimed to assess BI incidences in a large, consecutive cohort of preterm infants born < 32 weeks of gestation, the comparison between very (VPT, ≥ 28 + 0 to < 32 + 0 weeks of gestation) and extremely preterm infants (EPT, < 28 + 0 weeks of gestation) and across weeks of gestation.
Methods
We retrospectively analyzed cMRIs at TEA of VPT and EPT infants born at a large tertiary center (2009–2018). We recorded and compared the incidences of BI, severe BI, intraventricular hemorrhage (IVH), periventricular hemorrhagic infarction (PVHI), cerebellar hemorrhage (CBH), cystic periventricular leukomalacia (cPVL), and punctate white matter lesions (PWML) between VPTs, EPTs, and across weeks of gestation.
Results
We included 507 preterm infants (VPT, 335/507 (66.1%); EPT, 172/507 (33.9%); mean gestational age (GA), 28 + 2 weeks (SD 2 + 2 weeks); male, 52.1%). BIs were found in 48.3% of the preterm infants (severe BI, 12.0%) and increased with decreasing GA. IVH, PVHI, CBH, cPVL, and PWML were seen in 16.8%, 0.8%, 10.5%, 3.4%, and 18.1%, respectively. EPT vs. VPT infants suffered more frequently from BI (59.3% vs. 42.7%, p < 0.001), severe BI (18.6% vs. 8.7%, p = 0.001), IVH (31.9% vs. 9.0%, p < 0.001), and CBH (18.0% vs. 6.6%, p < 0.001).
Conclusion
Brain injuries are common cMRI findings among preterm infants with a higher incidence of EPT compared to VPT infants. These results may serve as reference values for clinical management and research.
Clinical relevance statement
Our results with regard to gestational age might provide valuable clinical insights, serving as a key reference for parental advice, structured follow-up planning, and enhancing research and management within the Neonatal Intensive Care Unit.
Key Points
• Brain injury is a common cMRI finding in preterm infants seen in 48.3% individuals.
• Extremely preterm compared to very preterm infants have higher brain injury incidences driven by brain injuries such as intraventricular and cerebellar hemorrhage.
• Reference incidence values are crucial for parental advice and structured follow-up planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preterm birth (before < 37 weeks of gestation) is common and affects around 15 million infants worldwide annually (10–12% of all live births, up to 18% in low-income settings [1]). Prematurity complications are the leading cause of mortality among young children (17.7% of children 0–5 years, 36.1% in neonates). Surviving very preterm (VPT, 28 to < 32 weeks of gestation) and extremely preterm (EPT, < 28 + 0 weeks of gestation) infants face an increased risk for major morbidities affecting them till adulthood [2]. Therefore, prematurity results in a major individual and socioeconomic burden [3, 4].
Although the prevalence of severe neurological impairment due to prematurity such as cerebral palsy has declined in recent years, the proportion of VPTs, particularly EPTs with neurologic impairment, has remained high (50%) [4,5,6]. There is a wide spectrum of neurodevelopmental disabilities including motor, cognitive, and neurosensory deficiencies, as well as emotional and behavioral problems, that are difficult to predict [7, 8]. Due to this high variability of neurological disorders, biomarkers for risk stratification, parental counseling, and therapeutic guidance are urgently needed.
Cerebral MRI (cMRI) at term-equivalent age (TEA) is a promising tool for predicting neurodevelopmental outcomes [2, 9]. CMRI detects precisely subtle changes such as focal or diffuse white matter lesions, low-grade intraventricular hemorrhage (IVH), and small cerebellar hemorrhages (CBH) beyond cranial ultrasound [10,11,12]. Accurate categorization of brain lesions is essential for risk estimation and identifying high-risk infants. More knowledge about incidences of structural brain lesions is needed to improve prevention, target interventions, and better apply supportive care to optimize functional outcomes in high-risk infants. While management strategies may differ across gestational age (GA) categories, the incidence of individual cMRI findings between VPTs and EPTs needs further exploration.
Current cMRI studies on incidences of brain injuries (BIs) are rare and often derived from small samples or missing hemorrhage-sensitive sequences. In particular, data across different GA is lacking but highly clinically relevant. Therefore, the present study aims to describe the incidence and variety of structural brain abnormalities in a large well-defined cohort of preterm infants born at a large academic center. Moreover, this study aims to describe the incidences in subgroups of VPTs and EPTs across weeks of gestation.
Methods
Study design and patient characteristics
We conducted a retrospective observational cohort study assessing clinical and imaging data among all preterm infants born < 32 + 0 weeks of gestation at the level III Neonatal Intensive Care Unit (NICU) of the University Hospital Essen in Germany between 01.01.2009 and 31.12.2018. The Institutional Review Board and Ethics Committee approved the present study, and the need to obtain informed consent was waived due to its retrospective nature. We performed this study according to the Standards of Reporting of Diagnostic Accuracy Studies statement (STARD) [13].
Infants were included in this study if they met the following inclusion criteria: (1) in-house birth and treatment at the University Hospital Essen until discharge (and readmission at TEA) or TEA and (2) survival until TEA. Exclusion criteria were (1) diagnosed or suspected genetic disorders or congenital infections and (2) cMRI at TEA unavailable (lack of parental consent). Infants with genetic disorders and congenital infections (e.g., CMV infections) were excluded, as they can be associated with altered brain structure, with a possible overlap of neurological symptoms.
Clinical data (gestational age, birth weight, birth mode, multiples, sex, percentile, preterm premature rupture of membranes (PPROMs), small for gestational age (SGA, defined as infants with a birthweight < 10th percentile), admission temperature) of the infants with available cMRI were collected and reviewed from the medical reports retrospectively (K.D.).
Magnetic resonance imaging protocol
MR imaging was performed on a 3-T scanner in most of the infants (n = 400, Magnetom Skyra, Siemens Healthcare), if available with a MR-compatible incubator (Lammers Medical Technology (LMT) nomag IC) as previously described [14] or on 1.5-T scanners (n = 107, Magnetom Avanto or Magnetom Aera, Siemens Healthcare) (Table 1 suppl.) using a “feed and wrap” technique for immobilization. A more detailed description of the standardized patient preparation is available in our prior publication [14]. Imaging was routinely performed without sedation, and chloral hydrate (20–50 mg/kg) was administered orally if necessary. The routine imaging protocol contained T2-weighted turbo spin echo (TSE) imaging (transversal), a 3D T1-weighted imaging (fast low-angle shot (FLASH), scanned sagittally with transversal and coronal reconstructions), susceptibility-weighted imaging (SWI), and diffusion-weighted imaging (DWI (gradients: b0, b700, b1000)/DTI) as published before [14]. Depending on the underlying pathology, other/additional sequences were performed.
MRI image analysis
Qualitative and quantitative MR image analyses were independently performed by two pediatric radiologists with 14 and 27 years of experience (S.S., B.S.) blinded to clinical data. In case of discrepant results, the final diagnosis was determined by consensus. Interrater reliability was almost perfect for all brain injuries (Cohen’s kappa, 0.81–1; n = 38, Table 2 suppl.) [15]. Images were evaluated regarding the presence of IVH I°-III° [16], periventricular hemorrhagic infarction (PVHI) [17], CBH [18], diffuse excessive high signal intensity (DEHSI) [19, 20], ventricular dilatation (VD) [19], punctate white matter lesions (PWML [20, 21], uni-/bilateral and number of lesions/side), and cystic white matter lesions (cPVL, uni-/bilateral) (Fig. 1 suppl.). We defined BI and sBI as follows: BI (IVHI°-III°, PVHI, moderate/severe VD, CBH, PWML, cPVL) and severe BI (sBI) (IVH III°, PVHI, CBH III° + IV°, severe VD, PVL cysts). Presence and number were reported. A detailed description of the classification and grading of preterm brain injuries is given in Table 3 suppl.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical and ordinal variables are presented as absolute and relative frequencies. Comparisons were performed using an independent sample t-test or Wilcoxon rank-sum test for continuous variables, Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables. All statistical analyses (T.M.) were performed using Stata 16.1 (StataCorp LP). A 2-tailed p-value < 0.05 was required for all analyses to reject the null hypothesis.
Results
Study group
The study population is presented in a flowchart (Fig. 1). Of 650 consecutively born infants in 2009–2018 at our hospital, 560 met the inclusion criteria (77 patients died before TEA, 13 were transferred to other hospitals before TEA), further 53 patients were excluded due to known neurological disease (n = 7) or missing cMRI (n = 46). MRI scans were performed at TEA at a median of 40 + 1 weeks of gestation (IQR, 0–4) and cMRIs were available in 91.7% (507/553; EPT, 97.2% (172/177); VPT, 89.1% (335/376)). All EPTs from 23, 25, and 26 weeks of gestation were included (100%) and received cMRI (Table 1).
The clinical characteristics of the 507 infants are shown in Table 2. Mean GA was 28 + 2 weeks (SD 2 + 2 weeks, range 23 + 2–31 + 6 weeks), mean birth weight 1170 g ± 387 g (range 420–2200 g) with significantly lower birth weight in the EPTs compared to VPTs (793 g ± 214 g vs. 1364 g ± 303 g, p < 0.001). Sex was relatively balanced overall (52.1% boys vs. 47.9% girls) with significantly less boys in the EPTs (43.6% vs. 56.4%, p = 0.007). While vaginal delivery rate was lower in the EPTs (2.9% vs. 4.2%, p = 0.047), there was no significant difference between the groups regarding multiple births, PPROM, small for gestational age (SGA), and admission temperature.
cMRI findings at TEA
Diagnostic cMRI was available in all 507 children. The presence of brain injuries according to weeks of gestation is shown in Fig. 2. EPTs showed significantly higher rates of IVH, CBH, and VD compared to VPTs (Table 3/Fig. 2/Fig. 3).
cMRI brain injuries at term-equivalent age stratified by weeks of gestation. CBH, cerebellar hemorrhage; cPVL, cystic periventricular leukomalacia; IVH, intraventricular hemorrhage; number of brain injuries (IVH I°-III°, PVHI, moderate and severe VD, CBH, punctate white matter lesions, cPVL); and number of severe brain injuries (IVH III°, PVHI, CBH III° + IV°, severe VD, cPVL); PVHI, periventricular hemorrhagic infarction; VD, ventricular dilatation (mild, moderate, and severe)
cMRI brain lesions at term-equivalent age stratified by extremely preterm (EPT, < 28 + 0 weeks of gestation) and very preterm (VPT, 28 + 0 to < 32 + 0 weeks of gestation) infants. BI, brain injuries (IVH I°-III°, PVHI, moderate and severe VD, CBH, PWML, cPVL); CBH, cerebellar hemorrhage; cPVL, cystic periventricular leucomalacia; IVH, intraventricular hemorrhage; PWML, punctate white matter lesions; sBI, severe brain injuries (IVH III°, PVHI, CBH III° + IV°, severe VD, cPVL), ventricular dilatation (VD), significant: p < 0.05
Frequency of brain injury
A total of 245 (48.3%) infants showed BI at cMRI (Table 3), and the incidence decreased with increasing GA (Fig. 2). 93.7% of the infants born at 23 weeks of gestation had at least one, and 62.5% had two or more BIs, whereas 44.7% of the infants born at 31 weeks of gestation had at least one, and 15.5% had two or more BIs. EPTs are significantly more often affected by BI (> 1 BI) and showed a significantly higher number of concomitant BIs compared to VPTs (both p < 0.001).
At least one form of sBI was present in 61 (12.0%) infants with higher incidences and numbers of sBI with decreasing weeks of gestation, particularly in infants born at 23 and 24 weeks of gestation (Fig. 2). EPTs suffered significantly more often from sBI compared to VPTs (18.6% vs. 8.7%, p = 0.001). At least one form of intracranial hemorrhage (IVH I°-III°, PVHI, CBH) was present in 22.9%, and significantly more often in EPTs compared to VPTs (40.7% vs. 13.7%, p < 0.001).
Intraventricular hemorrhage
Overall, 16.8% (n = 85) of the infants suffered from IVH. The incidence decreased with increasing GA, particularly for IVH II°/III° (Fig. 4). IVH II° decreased from 31.3% at 23 weeks of gestation to 20.7–22.2% at 24–25 weeks of gestation to 8.3–6.7% at 26–28 weeks of gestation to < 5% at 28–32 weeks of gestation. The highest incidence of IVH III° was found in infants born at 23 weeks of gestation (18.8%). It decreased between 24 and 30 weeks of gestation (1.8–5.6%) and was not present in infants born at 31 weeks of gestation. EPTs were significantly more often affected by IVH than VPTs. Incidence of PVHI was rare and did not differ between EPTs and VPTs; concomitant IVH II° or III° was present in all infants.
Intraventricular hemorrhage I°-III°. The T2 (A) and SWI (D) images of a male extremely preterm infant born at 25 weeks of gestation showed IVH I°. IVH I° is visible bilaterally on SWI (D), on T2 only on the left side (white arrows). T2 (B)- and SWI (E)-weighted images of a female very preterm infant (31 weeks of gestation) with IVH II° visible at SWI-weighted image (E, white arrows). T2 (C) and SWI (F) images of a female preterm infant (25 weeks of gestation) with IVH III° (white arrows)
Cerebellar hemorrhage
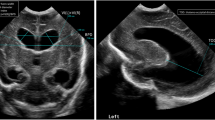
CBH was present in 10.5% (n = 53) of the infants with a decreasing incidence with increasing GA at birth (25% in infants born at 23, 8.7% at 31 weeks of gestation). It was also significantly higher in EPTs compared to VPTs (18% and 6.6%, respectively, p < 0.001, Fig. 5).
Cystic periventricular leucomalacia
Cystic PVL was present in 3.4% of the infants, with no significant difference between EPTs and VPTs (4.7% and 2.7%, p = 0.3, Fig. 6). There was no significant difference in the incidence of unilateral or bilateral cysts between EPTs and VPTs.
White matter damage. T2 (A) and T1 (B transverse reconstruction of 3D FLASH, C FLASH sequence sagittal) of a female very preterm infant born at 27 weeks of gestation with multiple, bilateral punctate white matter lesions demonstrated as hypointensities (white arrows in A) and hyperintensities (white arrows in B and C), bilateral cystic PVL (black arrows in A) and diffuse excessive high signal intensity (DEHSI) (fat black arrow in A)
Punctate white matter lesions
PWML were found in 18.1% (n = 92) of the infants with a higher percentage in VPTs compared to EPTs (20.9% and 12.8%, p = 0.028). Bilateral PWML were more frequent than unilateral lesions in both groups (Fig. 6). We detected 50 preterm infants with ≥ 6 PWML (VPT 12.8% vs. EPT 4.1%, p = 0.001).
Diffuse excessive high signal intensity
DEHSI were present in 96.7% (n = 490) of the infants, with no significant difference between EPTs and VPTs.
Ventricular dilatation
In 91.9% (n = 467) of the infants, a VD was present with a decrease in incidence and severity with increasing GA. VD was present in all infants born at 23 weeks of gestation, with a high percentage of high-grade dilatation (severe, 25%), which was rare in infants born at 31 weeks of gestation (1%). A significantly higher average degree of dilatation was present in EPTs compared to VPTs (p = 0.001).
Discussion
We report on brain injury incidences in preterm infants born below 32 weeks of gestation, as determined by cMRI at TEA. Our data covers a near-complete, unselected, and consecutive cohort of preterm infants born at a large tertiary neonatal care center over 10 years. An additional subgroup analysis compared extremely preterm to very preterm infants, and each week of gestation separately. We found that brain injury is a common cMRI finding among preterm infants with a higher incidence in extremely preterm compared to very preterm infants. We also show the distribution of brain injuries across weeks of gestation. These incidences of brain injuries across gestational age can help to improve risk stratification, parental counseling, decision-making, and medical support planning.
Adverse neurological outcome is frequent in preterm infants and ranges from mild cognitive disorders to severe motor deficits like cerebral palsy. In a recent review, the neurological consequences of prematurity and clinical implications were published by Inder et al based on brain injuries detected by cMRI [2], which has been proven to be a promising imaging tool for predicting neurodevelopmental outcomes for many years. In 2006, Woodward et al found that abnormal findings identified by cMRI at TEA in very preterm infants strongly predicted adverse neurodevelopmental outcomes at two years of age [9], which was also confirmed in other studies [10, 18]. As a result, cMRI was implemented in our hospital for all infants born < 32 weeks of gestation as a part of standard clinical practice. Although published cohorts with routine cMRI at TEA already exist, incidences of cMRI findings separately for each week of gestation have, to our knowledge, not been published yet. Additionally, published studies on cohorts with routine cMRI at TEA have been smaller (n = 300 [22], n = 247 [23]), had more dropouts (84.5% [22]), used only conventional sequences or irregular use of SWI [22, 24], included infants > 32 + 0 weeks of gestation [22, 24], only EPTs [25], or did not differentiate between EPTs and VPTs. Therefore, the presented incidences of BI in preterm infants with regard to GA offer valuable reference data for clinical management and research.
Early identification of high-risk infants for adverse neurological outcomes is essential, as it allows for better obstetrical management and neurorehabilitative support [26], as well as targeted intervention to counteract brain injury [2] throughout the neonatal period as a critical stage of brain plasticity and beyond. It has been shown that the degree of immaturity is the key factor influencing the occurrence of BI. In accordance with previous research, we found an increased incidence of BI with lower GA [5, 27,28,29]. In our study, infants born < 28 weeks of gestation suffered significantly more frequently from BI, severe BI, IVH, and CBH. Given the vulnerability of this patient group, it is essential to conduct further neonatal research into the variety and frequency of structural brain injuries, in order to improve treatment and outcome.
Intracranial hemorrhage is common in preterm infants and is associated with worse neurological outcomes [2, 23, 30]. As preterm infants often suffer from small or residual hemorrhages, we utilized the highly sensitive SWI sequence to improve hemosiderin detection compared to conventional imaging [31], facilitating an improved classification and detection of hemorrhages. Using SWI may lead to a higher detection rate of small hemorrhages (e.g., punctate hemorrhages, intraventricular blood for differentiation IVH I°/II°) as published before in diverging clinical settings [32, 33]. The incidence of IVH in our study group (16.8%) was in line with published data (14–16.2% [22,23,24]), as it was for the EPTs separately (32% vs. 32.8% [25]). Incidence of PVHI was low in our group (0.8% vs. 3% [24]). Mostly, incidences of IVH are reported without further differentiation, losing detailed information [18, 22, 34]. In contrast to Buchmayer et al, who also used SWI, we found a lower incidence of IVH I° and a higher percentage of IVH II° in the EPTs (IVH I°, 11.6%/IVH II°, 15.1% versus 14.1%/5.6% [25]), and a lower incidence of IVH III° and PVHI (IVH III°, 5.2%/PVHI, 1.2% versus 8.6%/4.5% [25]). As the impact of IVH I°/II° in opposition to IVH III°/PVHI for the neurodevelopmental outcome is discussed controversially [35, 36] and lacks MRI data [24], we are convinced that a detailed classification should be the basis for further evaluation of the impact of intracranial hemorrhage on neurodevelopmental outcome.
CBH has an substantial, previously underestimated, impact on neurodevelopmental outcomes [2, 37]. Our results corroborate prior studies with an incidence of 10.5% for CBH in infants born < 32 weeks of gestation (10% [18], 8% [22]) and for EPTs (18% vs. 20.7% [25]/19% [38]), even though higher rates of CBHs (30%) are published [24]. In conclusion, CBH is a frequent finding, particularly in EPTs, which may reflect that the third trimester is the critical developmental phase (volumetric growth, 600%) of the cerebellum [39].
White matter damage predicts neurological impairment in preterm infants associated with cognitive and motor delay [2, 9]. CPVL, PWML, and VD are a sign of white matter loss, and DEHSI belong to the preterm-specific injuries and abnormalities of the white matter with multifactorial pathogenesis [40]. CPVL is one of the strongest predictors of adverse neurological outcomes and may result in cerebral palsy, particularly in EPTs [4, 18, 41]. We detected comparable incidences of cPVL with 3.4%, of whom 1.8% were located bilaterally (1.3–3% [24, 42]). Another pathology with presumed association with adverse neurodevelopmental outcome is PWML. It is known that number (> 6) and localization of PWML have an impact on motor, cognitive, and behavioral outcomes of preterm infants [19, 41]. Interestingly, we found a higher incidence in the VPTs compared to the EPTs, which may be caused by the disappearance of these lesions over time until TEA as shown in longitudinal studies [43]. White matter volume loss resulting in VD is supposed to be responsible for neurodevelopmental impairment, particularly motor delay [2, 19]. VD was frequent in our study group ranging from mild to severe degrees. Corroborating previous publications, DEHSI were common in our cohort (96.7%) and we presume these to be temporary prematurity-related brain lesions without pathological impact [19, 44]. Further studies are necessary to discriminate the impact of the different white matter pathologies and to clarify the potential clinical implications of the obtained results.
Our study has several limitations, particularly the retrospective single-center character of our study design. However, the rigorous data collection and curation at our academic center led to only a relatively small number of missing data, particularly in the EPT group, limiting the selection bias and allowing a uniform imaging acquisition (e.g., standardized high-quality sequences, SWI, 3-T scanners) and interpretation by two specialized pediatric radiologists. Additionally, the cross-sectional design of the study does not provide information on the prognostic value of the individual imaging findings. Further, prospective, if possible, multicenter clinical studies are necessary to confirm our results and to determine the prognostic value of imaging findings beyond clinical assessment and conventional imaging.
In conclusion, this study highlights the high prevalence of brain injury in preterm infants, with cMRI revealing more brain injuries in EPT compared to VPT infants and a strong association between lower gestational age and increased brain injury incidences. Knowledge of the frequency of brain injuries, such as intraventricular and cerebellar hemorrhages, is essential for outcome evaluation and is the basis for improving patient management, in particular individually tailored strategies for care in infants born below 32 weeks of gestation.
Abbreviations
- BI:
-
Brain injury
- CBH:
-
Cerebellar hemorrhage
- cMRI:
-
Cerebral magnetic resonance imaging
- cPVL:
-
Cystic periventricular leucomalacia
- DEHSI:
-
Diffuse excessive high signal intensity
- EPT:
-
Extremely preterm infants
- GA:
-
Gestational age
- IVH:
-
Intraventricular hemorrhage
- PPROM:
-
Preterm premature rupture of membranes
- PVHI:
-
Periventricular hemorrhagic infarction
- PWML:
-
Punctate white matter lesions
- sBI:
-
Severe brain injury
- SGA:
-
Small for gestational age
- SWI:
-
Susceptibility-weighted imaging
- TEA:
-
Term-equivalent age (the regular time point at which the infant would have been born had it not been prematurely delivered)
- VD:
-
Ventricular dilatation
- VPT:
-
Very preterm infants
References
Blencowe H, Cousens S, Oestergaard MZ et al (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379:2162–2172
Inder TE, Volpe JJ, Anderson PJ (2023) Defining the Neurologic Consequences of Preterm Birth. N Engl J Med 389:441–453
Beck S, Wojdyla D, Say L et al (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88:31–38
Finch-Edmondson M, Morgan C, Hunt RW, Novak I (2019) Emergent prophylactic, reparative and restorative brain interventions for infants born preterm with cerebral palsy. Front Physiol 10:15
Serenius F, Ewald U, Farooqi A et al (2016) Extremely preterm infants in Sweden study group neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr 170:954–963
Arthur R (2006) Magnetic resonance imaging in preterm infants. J Pediatr Radiol 36:593–607
Anderson PJ, Doyle LW (2008) Cognitive and educational deficits in children born extremely preterm. Semin Perinatol 32:51–58
Johnson S, Wolke D, Hennessy E, Marlow N (2011) Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol 36:74–95
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355:685–694
Woodward LJ, Clark CA, Bora S, Inder TE (2012) Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One 7:e51879
Melbourne L, Chang T, Murnick J, Zaniletti I, Glass P, Massaro AN (2016) Clinical impact of term-equivalent magnetic resonance imaging in extremely low-birth-weight infants at a regional NICU. J Perinatol 36:985–989
Nataraj P, Svojsik M, Sura L et al (2021) Comparing head ultrasounds and susceptibility-weighted imaging for the detection of low-grade hemorrhages in preterm infants. J Perinatol 41:736–742
Bossuyt PM, Reitsma JB, Bruns DE et al (2003) The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138:W1-12
Sirin S, Goericke SL, Huening BM et al (2013) Evaluation of 100 brain examinations using a 3 Tesla MR-compatible incubator-safety, handling, and image quality. Neuroradiology 55:1241–1249
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159174
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534
Volpe JJ, Inder TE, Darras BT et al (2018) Volpe’s Neurology of the Newborn. Elsevier
Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE (2014) Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134:e444-453
de Bruïne FT, van den Berg-Huysmans AA, Leijser LM et al (2011) Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 261:899–906
Rutherford MA, Supramaniam V, Ederies A et al (2010) Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52:505–521
Martinez-Biarge M, Groenendaal F, Kersbergen KJ et al (2016) MRI based preterm white matter injury classification: the importance of sequential imaging in determining severity of injury. PLoS One 11:e0156245
Neubauer V, Djurdjevic T, Griesmaier E, Biermayr M, Gizewski ER, Kiechl-Kohlendorfer U (2017) Routine magnetic resonance imaging at term-equivalent age detects brain injury in 25% of a contemporary cohort of very preterm infants. PLoS One 12:e0169442
Hayakawa K, Tanda K, Nishimoto M, Nishimura A, Kinoshita D, Sano YJN (2022) The incidence of intraventricular hemorrhage in low-birth-weight infants: assessment by magnetic resonance imaging. Neuropediatrics 53:246–250
Arulkumaran S, Tusor N, Chew A et al (2020) MRI findings at term-corrected age and neurodevelopmental outcomes in a large cohort of very preterm infants. AJNR Am J Neuroradiol 41:1509–1516
Buchmayer J, Kasprian G, Giordano V et al (2022) Routine use of cerebral magnetic resonance imaging in infants born extremely preterm. J Pediatr 248(74–80):e71
Hughes AJ, Redsell SA, Glazebrook C (2016) Motor development interventions for preterm infants: a systematic review and meta-analysis. Pediatrics 138(4):e20160147
Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P (2008) Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol 50:334–340
Hinojosa-Rodriguez M, Harmony T, Carrillo-Prado C et al (2017) Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin 16:355–368
Spittle AJ, Morgan C, Olsen JE, Novak I, Cheong JL (2018) Early diagnosis and treatment of cerebral palsy in children with a history of preterm birth. Clin Perinatol 45:409–420
Goeral K, Kasprian G, Huning BM et al (2022) A novel magnetic resonance imaging-based scoring system to predict outcome in neonates born preterm with intraventricular haemorrhage. Dev Med Child Neurol 64:608–617
Dewan MV, Herrmann R, Schweiger B et al (2019) Are simple magnetic resonance imaging biomarkers predictive of neurodevelopmental outcome at two years in very preterm infants? Neonatology 116:331–340
Goos JD, van der Flier WM, Knol DL et al (2011) Clinical relevance of improved microbleed detection by susceptibility-weighted magnetic resonance imaging. Stroke 42:1894–1900
Haller S, Vernooij MW, Kuijer JP, Larsson E-M, Jäger HR, Barkhof F (2018) Cerebral microbleeds: imaging and clinical significance. Radiology 287:11–28
Whitelaw A (2007) A different view: there is value in grading intraventricular hemorrhage. Acta Paediatr 96:1257–1258
Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M (2006) Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr 149:169–173
Sherlock RL, Anderson PJ, Doyle LW, Group VICS (2005) Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Human Dev 81:909–916
Volpe JJ (2021) Commentary—cerebellar underdevelopment in the very preterm infant: Important and underestimated source of cognitive deficits. J Neonatal Perinatal Med 14:451
Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G (2009) Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 252:190–199
Volpe JJ (2009) Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 24:1085–1104
Counsell S, Rutherford M, Cowan F, Edwards A (2003) Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed 88:F269–F274
de Bruijn CAM, Di Michele S, Tataranno ML et al (2022) Neurodevelopmental consequences of preterm punctate white matter lesions: a systematic review. Pediatr Res. https://doi.org/10.1038/s41390-022-02232-3
van Haastert IC, Groenendaal F, Uiterwaal CS et al (2011) Decreasing incidence and severity of cerebral palsy in prematurely born children. J Pediatr 159:86-91 e81
Dyet LE, Kennea N, Counsell SJ et al (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Morel B, Bertault P, Favrais G et al (2021) Automated brain MRI metrics in the EPIRMEX cohort of preterm newborns: correlation with the neurodevelopmental outcome at 2 years. Diagn Interv Imaging 102:225–232
Funding
Open access funding provided by University of Zurich The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Selma Sirin, Department of Diagnostic Imaging, University Children’s Hospital Zürich, University of Zürich, Zürich, Switzerland.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Anja Stein: lecture fees for Chiesi.
Borek Foldyna is a member of the European Radiology Editorial Board. They have not taken part in the review or selection process of this article.
Statistics and biometry
Prof. Thomas Mayrhofer kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No previously reported subjects or cohorts.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Drommelschmidt, K., Mayrhofer, T., Hüning, B. et al. Incidence of brain injuries in a large cohort of very preterm and extremely preterm infants at term-equivalent age: results of a single tertiary neonatal care center over 10 years. Eur Radiol 34, 5239–5249 (2024). https://doi.org/10.1007/s00330-024-10592-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-024-10592-z