Abstract
Objectives
The aims of the present study were to investigate a combination of magnetic resonance elastography (MRE) and vibration-controlled transient elastography (VCTE) or MRE and fibrosis score 4 (FIB-4) in the detection of significant fibrosis in patients with metabolic dysfunction-associated steatotic liver disease (MASLD).
Methods
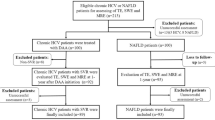
Between November 5, 2021, and March 4, 2022, a total of 119 consecutive patients with MASLD were included. Liver stiffness was measured using liver biopsy, MRE, VCTE, and FIB-4. Data were collected from outpatient visit charts. Significant fibrosis was defined as ≥ stage 2 fibrosis.
Results
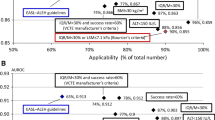
All 119 MASLD patients were Caucasian, and their median age was 55 years. MRE, VCTE, and FIB-4 demonstrated significant accuracy in the detection of significant fibrosis with an area under the ROC curve (AUC) of 0.848 ± 0.036 (p < 0.001), 0.632 ± 0.052 (p = 0.012), and 0.664 ± 0.051 (p = 0.001), respectively. However, the diagnostic performance of MRE was superior compared to that of VCTE (AUC difference: 0.216 ± 0.053, p < 0.001) and FIB-4 (AUC difference: 0.184 ± 0.058, p = 0.001). With logistic regression analysis, it was determined that when compared to MRE alone, a combination of MRE and TE (p = 0.880) or MRE and FIB-4 (p = 0.455) were not superior for detecting significant fibrosis.
Conclusions
MRE alone is an accurate and non-invasive method for the identification of MASLD patients with significant fibrosis.
Clinical relevance statement
Magnetic resonance elastography alone accurately detects significant fibrosis in patients with metabolic dysfunction-associated steatotic liver disease.
Key Points
• In routine clinical practice, several non-invasive biochemical-based biomarkers and imaging methods are widely used to assess liver fibrosis in patients with metabolic dysfunction-associated steatotic liver disease.
• Magnetic resonance elastography (MRE) is more accurate than vibration-controlled transient elastography (VCTE) or fibrosis score 4 (FIB-4) for assessing liver fibrosis and identifying significant fibrosis in patients with metabolic dysfunction-associated steatotic liver disease.
• The combination of MRE and VCTE or MRE and FIB-4 was not superior to MRE alone.

Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the ROC curve
- CAP:
-
Controlled attenuation parameter
- CI:
-
Confidence interval
- CRN:
-
NASH-Clinical Research Network
- FIB-4:
-
Fibrosis score 4
- GGT:
-
Gamma-glutamyl transpeptidase
- IQR/M:
-
Interquartile range/median value
- IQR:
-
Interquartile range
- kPa:
-
Kilopascal
- LDL:
-
Low-density lipoprotein
- MAS:
-
Metabolic dysfunction-associated steatosis
- MASH:
-
Metabolic dysfunction-associated steatohepatitis
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MRE:
-
Magnetic resonance elastography
- MR-PDFF:
-
MR-proton density fat fraction
- NAFL:
-
Nonalcoholic fatty liver
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- OR:
-
Odds ratio
- PPV:
-
Positive predictive value
- SLD:
-
Steatotic liver disease
- VCTE:
-
Vibration-controlled transient elastography
References
Younossi ZM, Koenig AB, Abdelatif D et al (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 64:73–84
Wong RJ, Aguilar M, Cheung R et al (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148:547–555
Singh S, Allen AM, Wang Z et al (2015) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13:643–654
Rinella ME, Lazarus JV, Ratziu V et al (2023) A multi-society delphi consensus statement on new fatty liver disease nomenclature. Hepatology. https://doi.org/10.1097/HEP.0000000000000520
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344(7):495–500
Ratziu V, Charlotte F, Heurtier A et al (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128(7):1898–1906
Castera L, Vergniol J, Foucher J et al (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128:343–350
Shah AG, Lydecker A, Murray K et al (2009) Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7:1104–1112
Zarski J-P, Sturm N, Guechot J et al (2012) Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C. The ANRS HCEP-23 study. J Hepatol 56:55–62
Idilman IS, Aniktar H, Idilman R et al (2013) Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267:767–775
Loomba R, Adams LA (2020) Advances in non-invasive assessment of hepatic fibrosis. Gut 69:1343–1352
Hsu C, Caussy C, Imajo K et al (2019) Magnetic resonance vs transient elastography analysis of patients with non-alcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 17:630–637
Cui J, Heba E, Hernadez C et al (2016) Magnetic resosnance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. A prospective study. Hepatology 63:453-461
Park CC, Nguyen P, Hernandez C et al (2017) Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152:598–607
Xiao G, Zhu S, Xiao X et al (2017) Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease A metanalysis. Hepatology 66:1486–1501
Jung J, Loomba RR, Imajo K et al (2021) MRE combined with FIB-4 (MEFIB) index in the detection of candidates for pharmacologic treatment of NASH-related fibrosis. Gut 70:1946–1953
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology 43:1317–1325
Melekoglu Ellik Z, Idilman IS, Kartal A et al (2022) Evaluation of magnetic resonance elastography and transient elastography for liver fibrosis and steatosis assessment in the liver transplant setting. Turk J Gastroenterol 33:153–160
Kleiner DE, Brunt EM, Van Natta M et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313-1321
Hanley JA, McNeil BJ (1983) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839
Altamirano-Barrera A, Barranco-Fragoso B, Mendez-Sanchez N (2017) Management strategies for liver fibrosis. Ann Hepatol 16:48–56
Hagström H, Nasr P, Ekstedt M et al (2017) Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 67:1265–1273
Ajmera V, Kim BK, Yang K et al (2022) Liver stiffness on magnetic resonance elastography and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology 163:1079–1089
Sheka AC, Adeyi O, Thompson J et al (2020) Nonalcoholic steatohepatitis: a review. JAMA 24:1175–1183
Albhaisi SAM, Sanyal AJ (2021) New drugs for NASH. Liver Int S1:112–118
Acknowledgements
The authors would like to thank all the radiology team, gastroenterology staff, and pathologists at Ankara University School of Medicine, Ankara, Turkey.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ramazan Idilman.
Conflict of interest
For all authors, except Rohit Loomba, there are none to declare conflicts of interest, no financial support was received for the conduct of this study. Rohit Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bios, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Ramazan Idilman is a member of the Science Academy.
Statistics and biometry
One of the authors (Atilla H Elhan) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
This study was approved by the ethics committee of the Ankara University School of Medicine.
Study subjects or cohorts overlap
None of these study subjects were previously reported.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duman, S., Kuru, D., Gumussoy, M. et al. A combination of non-invasive tests for the detection of significant fibrosis in patients with metabolic dysfunction-associated steatotic liver disease is not superior to magnetic resonance elastography alone. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10441-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10441-5