Abstract
Objective
Body composition assessment derived from cross-sectional imaging has shown promising results as a prognostic biomarker in several tumor entities. Our aim was to analyze the role of low skeletal muscle mass (LSMM) and fat areas for prognosis of dose-limiting toxicity (DLT) and treatment response in patients with primary central nervous system lymphoma (PCNSL).
Methods
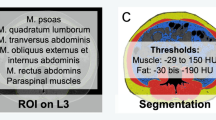
Overall, 61 patients (29 female patients, 47.5%) with a mean age of 63.8 ± 12.2 years, range 23–81 years, were identified in the data base between 2012 and 2020 with sufficient clinical and imaging data. Body composition assessment, comprising LSMM and visceral and subcutaneous fat areas, was performed on one axial slice on L3-height derived from staging computed tomography (CT) images. DLT was assessed during chemotherapy in clinical routine. Objective response rate (ORR) was measured on following magnetic resonance images of the head accordingly to the Cheson criteria.
Results
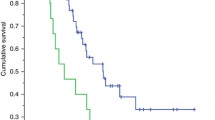
Twenty-eight patients had DLT (45.9%). Regression analysis revealed that LSMM was associated with objective response, OR = 5.19 (95% CI 1.35–19.94, p = 0.02) (univariable regression), and OR = 4.23 (95% CI 1.03- 17.38, p = 0.046) (multivariable regression). None of the body composition parameters could predict DLT. Patients with normal visceral to subcutaneous ratio (VSR) could be treated with more chemotherapy cycles compared to patients with high VSR (mean, 4.25 vs 2.94, p = 0.03). Patients with ORR had higher muscle density values compared to patients with stable and/or progressive disease (34.46 ± vs 28.18 ± HU, p = 0.02).
Conclusions
LSMM is strongly associated with objective response in patients with PCNSL. Body composition parameters cannot predict DLT.
Clinical relevance statement
Low skeletal muscle mass on computed tomography (CT) is an independent prognostic factor of poor treatment response in central nervous system lymphoma. Analysis of the skeletal musculature on staging CT should be implemented into the clinical routine in this tumor entity.
Key Points
• Low skeletal muscle mass is strongly associated with the objective response rate.
• No body composition parameters could predict dose-limiting toxicity.

Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- CR:
-
Complete response
- CT:
-
Computed tomography
- DLBCL:
-
Diffuse large B-cell lymphoma
- DLT:
-
Dose-limiting toxicity
- FFM:
-
Fat-free mass
- FM:
-
Fat mass
- HU:
-
Hounsfield unit
- IMAT:
-
Intramuscular adipose tissue
- L:
-
Lumbar
- LSMM:
-
Low skeletal muscle mass
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- ORR:
-
Objective response rate
- PCNSL:
-
Primary central nervous system lymphoma
- PD:
-
Progressive disease
- SAT:
-
Subcutaneous adipose tissue
- SMA:
-
Skeletal muscle area
- SMI:
-
Skeletal muscle index
- TAT:
-
Total adipose tissue
- VAT:
-
Visceral adipose tissue
- VSR:
-
Visceral to subcutaneous ratio
- WBRT:
-
Whole-brain radiotherapy
References
Schaff LR, Grommes C (2022) Primary central nervous system lymphoma. 140(9):971–979
Yuan Y, Ding T, Wang S, Chen H, Mao Y, Chen T (2021) Current and emerging therapies for primary central nervous system lymphoma. Biomark Res 9(1):32
Xia L, Zhao R, Wan Q et al (2020) Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med 9(21):7964–7978
Borggreve AS, den Boer RB, van Boxel GI et al (2020) The predictive value of low muscle mass as measured on CT scans for postoperative complications and mortality in gastric cancer patients: a systematic review and meta-analysis. J Clin Med 9(1):199
Yang Z, Zhou X, Ma B et al (2018) Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg 22(11):1890–1902
Albano D, Messina C, Vitale J, Sconfienza LM (2020) Imaging of sarcopenia: old evidence and new insights. Eur Radiol 30(4):2199–2208
Prado CM, Lieffers JR, McCargar LJ (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635
Surov A, Pech M, Gessner D et al (2021) Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis Clin Nutr 40(10):5298–5310
Jia S, Qiao R, Xiao Y et al (2020) Prognostic value of sarcopenia in survivors of hematological malignances undergoing a hematopoietic stem cell transplantation: a systematic review and meta-analysis. Support Care Cancer 28(8):3533–3542
Abrey LE, Batchelor TT, Ferreri AJ et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23(22):5034–43
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Saravana-Bawan B, Goplen M, Alghamdi M, Khadaroo RG (2021) The relationship between visceral obesity and post-operative complications: a meta-analysis. J Surg Res 267:71–81
Houillier C, Soussain C, Ghesquières H et al (2020) Management and outcome of primary CNS lymphoma in the modern era: an LOC network study. Neurology 94(10):e1027–e1039
Fu F, Sun X, Li Y et al (2021) Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict chemotherapeutic responses and survival in primary central-nervous-system lymphoma. Eur Radiol 31(4):1863–1871
Leone R, Sferruzza G, Calimeri T et al (2021) Quantitative muscle mass biomarkers are independent prognosis factors in primary central nervous system lymphoma: the role of L3-skeletal muscle index and temporal muscle thickness. Eur J Radiol 143:109945
Furtner J, Nenning KH, Roetzer T et al (2021) Evaluation of the temporal muscle thickness as an independent prognostic biomarker in patients with primary central nervous system lymphoma. Cancers (Basel) 13(3):566
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(4):601
Besutti G, Massaro F, Bonelli E et al (2021) Prognostic impact of muscle quantity and quality and fat distribution in diffuse large B-cell lymphoma patients. Front Nutr 8:620696
Gohmann RF, Temiz B, Seitz P et al (2021) Segmentation and characterization of visceral and abdominal subcutaneous adipose tissue on CT with and without contrast medium: influence of 2D- and 3D-segmentation. Quant Imaging Med Surg 11(10):4258–4268
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Alexey Surov.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surov, A., Meyer, H.J., Hinnerichs, M. et al. CT-defined sarcopenia predicts treatment response in primary central nervous system lymphomas. Eur Radiol 34, 790–796 (2024). https://doi.org/10.1007/s00330-023-09712-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09712-y