Abstract
Objectives
To find simple imaging-based features on cardiac magnetic resonance (CMR) that are associated with major adverse cardiovascular events (MACE) in takotsubo syndrome (TTS).
Methods
Patients with TTS referred for CMR between 2007 and 2021 were retrospectively evaluated. Besides standard CMR analysis, commonly known complications of TTS based on expert knowledge were assessed and summarised via a newly developed PE2RT score (one point each for pleural effusion, pericardial effusion, right ventricular involvement, and ventricular thrombus). Clinical follow-up data was reviewed up to three years after discharge. The relationship between PE2RT features and the occurrence of MACE (cardiovascular death or new hospitalisation due to acute myocardial injury, arrhythmia, or chronic heart failure) was examined using Cox regression analysis and Kaplan–Meier estimator.
Results
Seventy-nine patients (mean age, 68 ± 14 years; 72 women) with TTS were included. CMR was performed in a median of 4 days (IQR, 2–6) after symptom onset. Over a median follow-up of 13.3 months (IQR, 0.4–36.0), MACE occurred in 14/79 (18%) patients: re-hospitalisation due to acute symptoms (9/79, 11%) or chronic heart failure symptoms (4/79, 5%), and cardiac death (1/79, 1%). Patients with MACE had a higher PE2RT score (median [IQR], 2 [2–3] vs 1 [0–1]; p < 0.001). PE2RT score was associated with MACE on Cox regression analysis (hazard ratio per PE2RT feature, 2.44; 95%CI: 1.62–3.68; p < 0.001). Two or more PE2RT complications were strongly associated with the occurrence of MACE (log-rank p < 0.001).
Conclusions
The introduced PE2RT complication score might enable an easy-to-assess outcome evaluation of TTS patients by CMR.
Key Points
• Complications like pericardial effusion, pleural effusion, right ventricular involvement, and ventricular thrombus (summarised as PE 2 RT features) are relatively common in takotsubo syndrome.
• The proposed PE 2 RT score (one point per complication) was associated with the occurrence of major adverse cardiac events on follow-up.
• Complications easily detected by cardiac magnetic resonance imaging can help clinicians derive long-term prognostic information on patients with takotsubo syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Takotsubo syndrome (TTS), also known as broken heart syndrome or stress-induced cardiomyopathy, is an acute transient cardiomyopathy characterised by left ventricular (LV) systolic dysfunction with reversible regional wall motion abnormalities (RWMA) [1]. The apical segments are most commonly affected by circumferential RWMA (“apical ballooning”); however, diverse atypical forms including midventricular, basal, and focal types exist [2]. TTS is commonly associated with emotional or physical triggers and its clinical presentation often mimics acute coronary syndrome (ACS) [3].
While the clinical spectrum and epidemiological features of TTS have become increasingly well characterized in recent decades, the data on its long-term prognosis is conflicting: TTS was widely believed to be a benign disease with favourable prognosis and complete recovery of LV systolic function [4]. However, recent studies indicate that the long-term mortality rate of patients with TTS is much higher than expected and is comparable to or even exceeds that of patients with acute myocardial infarction [2, 5, 6]. Previous outcome studies demonstrated that clinical and imaging parameters such as male sex, age, LV ejection fraction, physical trigger, right ventricular (RV) involvement, and non-apical ballooning were factors related to worse long-term prognosis [5, 7,8,9,10].
Cardiac magnetic resonance (CMR) is an important tool in the diagnostic work-up of suspected TTS as it can confirm its diagnosis and differentiate it from other causes of acute cardiac symptoms, such as acute myocarditis [1, 11]. CMR features of TTS are typically circumferential RWMA with corresponding myocardial oedema and absent ischemic or inflammatory late gadolinium enhancement (LGE) in regions of abnormal function [12,13,14]. In addition, concomitant complications such as pericardial effusion, pleural effusion, RV involvement, or intraventricular thrombus, which have been described with poor outcomes [15,16,17], can be easily assessed on CMR. While the diagnostic utility of CMR in TTS is well established, its prognostic value remains poorly studied.
In this study, we explored the association between pathological CMR findings and the occurrence of long-term major adverse cardiovascular events (MACE) in TTS patients. In particular, easy-to-assess complications like the presence of pericardial and pleural effusions, RV involvement, or intraventricular thrombus, were analysed. The aim of the study was to find simple CMR imaging features associated with TTS outcomes that could be incorporated into an imaging-based score in the daily clinical routine of any cardiovascular imaging centre.
Materials and methods
Study population
This retrospective, observational single-centre study was conducted at a tertiary care centre. From November 2007 to January 2021, patients with clinical suspicion of TTS referred for CMR were identified. TTS was defined based on the International Takotsubo Diagnostic Criteria including CMR results and standard clinical work-up [18]. According to these recommendations, the main diagnostic criterion was the presence of (transient) left ventricular RWMA extending beyond a single coronary vascular distribution and presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Also, concomitant coronary artery disease was not a general contradiction for takotsubo syndrome. Patients with positive LGE who had typical RWMA and fulfilled diagnostic criteria for TTS were also included (e.g., RWMA beyond myocardial scarring) [18]. Patients with other CMR diagnosis were excluded (e.g., infectious myocarditis). Clinical characteristics were received from in-house medical records. The study complies with the principles of the Helsinki Declaration and was approved by the local institutional review board that waived informed consent due to retrospective study design (number: 303/16).
Cardiac magnetic resonance
CMR was performed using 1.5-Tesla systems (1.5 Ingenia and 1.5 Intera, Philips Healthcare). For signal reception, a 16-channel body array coil with a digital interface was used. The CMR protocol consisted of electrocardiogram-gated steady-state free-precession cine imaging (four-chamber, two-chamber, short-axis, and LV outflow tract view) for functional analyses, T2-weighted short-tau inversion-recovery imaging (short-axis, two-chamber, and transversal view) for the assessment of myocardial oedema, and LGE imaging using segmented inversion-recovery gradient-echo sequence (short-axis, two-chamber, and four-chamber view) for the detection of myocardial scarring, necrosis or fibrosis. In a subgroup of patients, additional parametric mapping sequences were available (T1 mapping based on a standard 3(3)3(3)5 modified Look-Locker inversion recovery (MOLLI) acquisition scheme and T2 mapping based on a 6-echo gradient spin echo (GraSE) sequence). Mapping sequences were performed as previously described [14, 19, 20]. Contrast was given as a single bolus of 0.2 mmol/kg body weight of gadobutrol (Gadovist, Bayer Healthcare).
CMR analysis
Images were analysed in consensus by two radiologists (J.A.L. with 10 years of CMR experience, A.I. with 5 years of CMR experience) using certified software (IntelliSpace Portal Version 12, Philips Medical System). Readers were blinded to clinical outcomes. Functional analysis (LV end-diastolic volume, ejection fraction, and longitudinal/circumferential/radial strain), assessment of myocardial oedema by visual and semi-quantitative approach (T2 signal intensity ratio), presence of myocardial scars by LGE imaging, and quantitative assessment of global myocardial T1 and T2 relaxation times, and haematocrit corrected extracellular volume fraction (ECV) values were performed standardised according to current recommendations including centre-specific reference values as previously described [19,20,21,22]. RWMA type was assessed regarding localisation: basal, midventricular, apical, or focal. Additionally, the presence of pleural effusion (> 20 mm) and pericardial effusion (> 5 mm), right ventricular involvement with visual RWMA [10], and ventricular thrombus was assessed and classified as “present” or “not present”. Based on expert knowledge, the PE2RT score was built by calculating the sum of these typical TTS complications (one point for each). The calculated PE2RT score ranged from 0 (no complication) to 4 (all defined complications present).
Outcome assessment
Follow-up data were collected by reviewing in-house and outpatient medical records. MACE was defined as a composite of cardiovascular death, re-hospitalisation due to acute myocardial injury (e.g., positive troponin, electrocardiogram abnormalities, angina pectoris), new onset of arrhythmia, or re-hospitalisation due to chronic heart failure symptoms. Cardiovascular death was defined as death directly related to heart failure, myocardial infarction, arrhythmia, or cardiovascular disease. Other causes of death were classified as non-cardiovascular or unknown. The primary endpoint was the occurrence of MACE up to 3 years after discharge.
Statistical analysis
Statistical analysis was performed using Prism (version 8.4.3; GraphPad Software Inc.) and SPSS Statistics (version 26; SPSS Inc., IBM). Normality assumptions were assessed by visual inspection of the data distribution supplemented by the Shapiro-Wilk test. For continuous variables, data are presented as means ± standard deviation or median with interquartile range (IQR) as appropriate. Categorical variables were presented as absolute frequencies with percentages in parentheses. Group comparisons between patients with and without MACE were performed using Student’s t-test or Mann-Whitney’s U-test for continuous variables, and chi-squared test or Fisher’s exact test for dichotomous variables. Univariable Cox regression analysis was applied to test the association of different clinical and imaging parameters regarding MACE. After a forward selection of relevant covariates with p < 0.05 in univariable analysis, covariates were added in a multivariable model to further fit the impact of variables. Kaplan-Meier analysis stratified by PE2RT score (< 2 vs. ≥ 2) was performed, and a log-rank test was used to compare survival curves. The level of statistical significance was set to p < 0.05.
Results
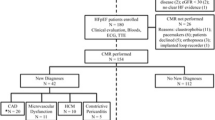
One-hundred-sixty patients referred for CMR with clinical suspicion of TTS were identified. Patients with unconfirmed TTS diagnosis were excluded (n = 81). Patients with a diagnosis of TTS (n = 79) were included (mean age, 68 ± 14 years; 72 women, 91%) for analysis. A study flow diagram is presented in Fig. 1.
Clinical characteristics
The demographic and clinical data of the study population at the time of CMR are shown in Table 1. The most common symptoms on admission were chest pain (49/79, 62%), electrocardiogram abnormalities (47/79, 59%; ST-segment elevation/depression and/or T wave changes), and elevated troponin levels (75/79, 95%). In most patients (53/79, 67%) a preceding trigger event was identified: physical stress in 32/79 patients (41%) or emotional stress in 21/79 patients (27%) (Fig. 2). Coronary angiography was performed in 77/79 patients (97%) at the time of admission. Coronary heart disease was diagnosed in 31/77 of these patients (40%), and 5/77 of these patients (6%) needed coronary intervention due to coronary obstruction. However, the ventriculography and/or the clinical setting indicated co-existing TTS. Thus, these patients were additionally referred for CMR. All included patients with co-existing non-obstructive and obstructive coronary heart disease had CMR findings typical for TTS (circumferential RWMA extending beyond the territory of the involved coronary artery). Twenty-nine out of 79 patients (37%) were admitted to intermediate care or intensive care unit, with a median stay of 2 days (IQR 1–6). Six out of 79 patients (8%) developed cardiogenic shock during their stay.
CMR characteristics
The CMR characteristics of the study population are given in Table 1. CMR was performed a median of 4 days (IQR 2–6) after symptom onset. TTS patients had reduced LV ejection fraction (46 ± 13%). Forty-seven out of 79 patients (59%) had an LV ejection fraction below 50%. The apical TTS type was most frequently observed (60/79, 76%). According to the 17-segment model, a median of 9 segments (IQR, 5–11) were hypokinetic or akinetic. The majority of patients showed visual myocardial oedema corresponding to the RWMA (74/79, 94%). Mapping parameters were available in 37/79 patients (47%). Based on the local reference value for inflammatory cardiomyopathy, global T1 relaxation times were elevated in all patients (37/37, 100%), and global T2 relaxation times in 36/37 patients (97%). LGE lesions were present in 11/79 patients (14%; 5/79 [6%] with ischemic, and 6/79 [8%] with non-ischemic distribution) and were unrelated to RWMA and myocardial oedema. Pericardial effusion (34/79, 43%) and pleural effusion (25/79, 32%) were the most frequently associated complications. RV involvement was seen in 17/79 patients (22%) and intraventricular thrombus in 7/79 patients (9%). The PE2RT score was 0 points in 30/79 patients (38%), 1 point in 28/79 patients (35%), 2 points in 12/79 patients (15%), 3 points in 6/79 patients (8%), and 4 points in 3/79 patients (4%). Representative examples of imaging-based complications are shown in Fig. 3.
Figure composition shows the combined role of cardiac magnetic resonance for establishing the diagnosis of takotsubo syndrome and assessment of associated complications. Proposed PE2RT complications are illustrated: Pericardial effusion, pleural effusion, right ventricular (RV) involvement, ventricular thrombus (score calculated from the sum of complications, ranging from 0 to 4). 2CV, two-chamber view; 4CV, four-chamber view; SAX: short-axis view; STIR: short-tau inversion recovery; LGE, late gadolinium enhancement; EGE, early gadolinium enhancement. The figure contains modified free medical images from Servier Medical Art (https://smart.servier.com) under a CC BY 3.0 license
Outcome
The median duration of follow-up was 13.3 months (IQR, 0.4–36.0). During follow-up, MACE occurred in 14/79 patients (18%). All events are summarised in Table 2. The median time between CMR and MACE was 10.2 months (IQR, 2.1–32.5). Events included re-hospitalisation due to acute symptoms (9/79, 11%) or due to chronic heart failure symptoms (4/79, 5%). Six out of 79 patients (8%) had new-onset arrhythmia (ventricular arrhythmia: 3/6 [50%], supraventricular arrhythmia: 2/6 [33%], third-degree atrioventricular block: 1/6 [17%]; see Table 2). Three of these patients subsequently underwent implantable cardioverter defibrillator (2/6, 33%) or pacemaker implantation (1/6, 17%). Two of 79 patients (3%) were diagnosed with a recurrence of TTS. Four of 79 patients (5%) passed away within the follow-up timeframe (one patient [1%] due to cardiac complication [cardiac arrest], two patients [3%] due to septic multiorgan failure, one patient [1%] had an uncertain cause of death). One patient (1%) experienced a stroke during the observational period.
There were no significant differences in clinical and laboratory parameters between patients with and without MACE, except that of diabetes mellitus (Table 1). In patients with MACE, a physical trigger was more frequently observed (11/14, 79% vs 21/65, 32%; p = 0.001). On CMR, patients with MACE had lower LV ejection fractions (40 ± 15% vs 48 ± 12%; p = 0.03), higher heart rates (82 ± 19 bpm vs 70 ± 13 bpm, p = 0.004), and lower T2 signal intensity ratios (1.8 ± 0.3 vs 2.2 ± 0.5, p = 0.01). No significant differences were found in T1 and T2 relaxation times or ECV values (Table 1). Patients with MACE were more likely to present previously defined complications on CMR: pericardial effusion (12/14, 86% vs 22/65, 33%; p < 0.001), pleural effusion (10/14, 71% vs 15/65, 23%; p < 0.001), ventricular thrombus (4/14, 29% vs 3/65, 5%, p = 0.004), and RV involvement (8/14, 57% vs 9/65, 14%; p < 0.001). A representative clinical example is shown in Fig. 4. Accordingly, the PE2RT score was higher in patients with MACE (median [IQR], 2 [2,3] vs 1 [0–1]; p < 0.001). MACE did not occur in patients who did not have any of the PE2RT features (0/30, 0%).
Representative cardiac magnetic resonance (CMR) imaging examples show two patients with typical diagnostic features of takotsubo syndrome (apical type). Regional wall motion abnormalities (apical ballooning) are visible on cine images in two- and four-chamber views (2CV, 4CV; white arrows) with corresponding myocardial oedema on T2-weighted short-tau inversion recovery images (T2 short-tau inversion recovery [STIR], black arrows) in the absence of late gadolinium enhancement (LGE). Images in the upper row show a 68-year-old female patient without major adverse cardiac event during follow-up (PE2RT score: 0; only slight pericardial fluid is visible on systolic cine images at the basal lateral wall, measuring < 5 mm on cine images at diastolic phase). Images in the lower row show a 70-year-old female patient with the occurrence of a major adverse cardiac event during follow-up (PE2RT score: 3; moderate circumferential pericardial effusion > 5 mm [white arrowheads], bilateral pleural effusions > 20 mm [orange asterisks], and regional wall motion abnormalities involving the right ventricle [yellow arrows])
In univariable Cox regression analyses, an association was found between the occurrence of MACE and physical TTS trigger, diabetes mellitus, heart rate, LV ejection fraction, T2 signal intensity ratio, pleural effusion, pericardial effusion, ventricular thrombus, and RV involvement (Table 3). Furthermore, an association was observed between MACE and the PE2RT score (HR per PE2RT point, 2.44; 95%CI: 1.62–3.68; p < 0.001; HR for PE2RT score ≥ 2, 15.68; 95%CI: 3.51–70.10; p < 0.001). In multivariable Cox regression analysis, the presence of 2 or more PE2RT features was an independent predictor for MACE (HR for PE2RT score ≥ 2, 7.98; 95%CI: 1.34–47.40; p = 0.02; see Table 4). Kaplan-Meier analysis showed that the presence of 2 or more PE2RT features was associated with the incidence of MACE (log-rank p < 0.001) (see Fig. 5).
Kaplan-Meier curve analysis shows the difference in cumulative major adverse cardiac events (MACE) after patients were stratified according to the presence of two or more PE2RT features (pericardial effusion, pleural effusion, right ventricular involvement, ventricular thrombus) on cardiac magnetic resonance
Discussion
In this study, we demonstrated that easy-to-identify imaging markers on CMR summarised as PE2RT score (pericardial effusion, pleural effusion, RV involvement, ventricular thrombus) are frequently observed in TTS and are associated with MACE. At least one of these complications was observed in 62% of patients, and at least two of these complications were present in 27% of patients. A PE2RT score of ≥ 2 was associated with MACE and was identified as an independent predictor in multivariable analysis. Our results demonstrate that a simple CMR assessment of concomitant complications may allow risk stratification of TTS.
In the past, TTS was believed to be a primarily benign syndrome due to recovery of LV function over the short to medium term in the majority of patients. However, in the last decade, several large multicentre studies have shown that the short- and long-term prognosis among patients with TTS is less favourable than previously thought [1, 2]. In a large registry study by Ghadri et al, the long-term outcome of TTS was comparable to matched cohorts of myocardial infarction or unstable angina [23]. In our study, the incidence of MACE was approximately 18% and all-cause mortality was about 5% during a median follow-up of 13.3 months. The event rates in our study are comparable to the study by Citro et al, which used a similar composite of MACE (overall MACE: 16.6%, overall mortality: 7.4%, median follow-up: 26.5 months) [24].
Several studies also demonstrated that different clinical (e.g., sex, age, physical trigger, cardiogenic shock) and imaging characteristics (e.g., LV ejection fraction, RV involvement, and non-apical ballooning) were factors related to worse long-term prognosis [1, 7, 8, 10, 25]. However, CMR data was not included in these outcome studies. In a recent study that was focused on the prognostic value of different CMR strain parameters in TTS, the longitudinal strain was identified as a valuable prognostic parameter; however, long-term mortality was mainly determined by comorbidities [26].
Although CMR is becoming more and more important for the diagnostic work-up of patients with suspected TTS [1, 12], its long-term prognostic value has not been sufficiently elucidated so far [16]. Complications, such as pleural or pericardial effusion, RV involvement, and ventricular thrombus, can all be easily assessed with standard CMR cine images without the need for quantitative analysis or time-consuming post-processing [27]. The frequency of these complications in our study (pericardial effusion: 43%, pleural effusion: 32%, RV involvement: 22%, ventricular thrombus: 9%) tends to be broadly consistent with previous CMR studies [12, 26]. In a CMR study of 34 TTS patients by Haghi et al, RV involvement was found in 26% of patients and was associated with lower LV ejection fraction and pleural effusion. The authors suggested that pathophysiological mechanisms caused by LV and/or RV dysfunction were responsible for this coincidence (elevation of pulmonary venous pressure and systemic venous pressure). Moreover, our results demonstrate that TTS patients who experienced MACE were more likely to have pericardial effusions, pleural effusions, RV involvement, and ventricular thrombus on initial CMR. A combined score based on these complications (summarised as PE2RT) revealed an HR of 2.38 per score point for the occurrence of MACE during follow-up. A PE2RT score of ≥ 2 was associated with MACE and was identified as an independent predictor in multivariable analysis. These results are also in partial agreement with an echocardiographic study by Kagiyama et al that revealed a prognostic impact of RV involvement [10]. In a recent multicentre study, a clinical and echocardiographic-based score was developed for early risk stratification (in-hospital complications) of patients with TTS, which included male sex, history of neurologic disorder, RV involvement, and left ventricular ejection fraction [9]. Application of established prognostic scores, such as the CHA2DS2-VASc score and GRACE score (Global Registry of Acute Coronary Events), also allowed the prediction of mortality and MACE in patients with TTS [28, 29]. However, currently available risk scores for TTS often refer to early risk stratification, partly include parameters that may be more complex to measure and thus may differ between different clinical centres, and in particular, do not include CMR parameters.
LGE is an established marker of worse outcomes in non-ischemic cardiomyopathies; however, no associations were found between the presence of LGE and the occurrence of MACE in our study. This can be explained by the fact that TTS is predominately characterised by the absence of LGE [12, 18], although slight or patchy LGE might be detected using quantitative approaches [12, 30]. Since a visual approach was used in this study, LGE lesions primarily reflect coexisting pathologies (myocardial scarring/fibrosis), presumably without any causal relationship to TTS abnormalities [30]. Using centre-specific reference values, elevated T1 and T2 relaxation times were observed in more than 97% of patients with TTS, indicating acute myocardial injury/oedema. However, no difference was found between T1 and T2 relaxation times and MACE in the subgroup of patients with available mapping parameters.
Focal myocardial oedema correlating with typical RWMA is a major diagnostic criterion for TTS, which was present in 94% of patients in our study. Only 6% of patients had a primarily diffuse pattern of myocardial oedema, which could not be detected visually, but with the T2 signal intensity ratio or with T2 mapping. Patients with MACE, however, had less pronounced signs of diffuse myocardial oedema with lower T2 signal intensity ratio values, indicating limited reversibility of myocardial injury. The absence or a lesser degree of myocardial oedema has been reported to be associated with prolonged recovery time or worse outcomes in non-ischemic cardiomyopathies [31, 32].
Our study has limitations. Due to its retrospective design, some clinical and imaging data (e.g., myocardial mapping) was not available for analysis in every patient. Due to the relatively low number of events, the multivariable analysis might be prone to overfitting. Therefore, information on the incremental diagnostic value of the score is limited. Furthermore, our sample size was too small to generate a score that is based on a training and validation cohort. Therefore, the regression models are limited in their generalizability, and this study should be considered hypothesis generating. The proposed complication score needs to be validated in larger TTS cohorts and compared with other clinical and imaging parameters, preferably in prospective studies.
In conclusion, our CMR study shows that scoring four easy-to-assess complications (pericardial effusion, pleural effusion, RV involvement, ventricular thrombus) might be useful for long-term risk stratification among patients with TTS. All complications are relatively common in TTS and should generally be included in any CMR report, as their presence may also influence patient management (e.g., percutaneous puncture, therapeutic anticoagulation, or close patient monitoring). The proposed PE2RT score can be visually assessed using standard diagnostic CMR images and does not require any additional advanced quantitative analysis like strain analysis or mapping analysis and can therefore be applied in any clinical centre.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CMR:
-
Cardiac magnetic resonance
- ECV:
-
Extracellular volume fraction
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- MACE:
-
Major adverse cardiovascular events
- RV:
-
Right ventricular
- RWMA:
-
Regional wall motion abnormalities
- STIR:
-
Short-tau inversion-recovery
- TTS:
-
Takotsubo syndrome
References
Ghadri J-R, Wittstein IS, Prasad A et al (2018) International Expert Consensus Document on Takotsubo Syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J 39(22):2047–2062
Templin C, Ghadri JR, Diekmann J et al (2015) Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 373(10):929–938
Wittstein IS, Thiemann DR, Lima JAC et al (2005) Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352(6):539–548
Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS (2007) Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 50(5):448–452
Stiermaier T, Moeller C, Oehler K et al (2016) Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 18(6):650–656
Murugiah K, Wang Y, Desai NR et al (2016) Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail 4(3):197–205
Uribarri A, Núñez-Gil IJ, Conty DA et al (2019) Short- and long-term prognosis of patients with takotsubo syndrome based on different triggers: importance of the physical nature. J Am Heart Assoc 8(24):e013701
Pelliccia F, Pasceri V, Patti G et al (2019) Long-term prognosis and outcome predictors in takotsubo syndrome: a systematic review and meta-regression study. JACC Heart Fail 7(2):143–154
Santoro F, Núñez Gil IJ, Stiermaier T et al (2019) Assessment of the German and Italian Stress Cardiomyopathy Score for risk stratification for in-hospital complications in patients with takotsubo syndrome. JAMA Cardiol 4(9):892–899
Kagiyama N, Okura H, Tamada T et al (2016) Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 17(2):210–216
Sörensson P, Ekenbäck C, Lundin M et al (2021) Early comprehensive cardiovascular magnetic resonance imaging in patients with myocardial infarction with nonobstructive coronary arteries. JACC Cardiovasc Imaging 14(9):1774–1783
Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P et al (2011) Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 306(3):277–286
Aikawa Y, Noguchi T, Morita Y et al (2019) Clinical impact of native T1 mapping for detecting myocardial impairment in takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 20(10):1147–1155
Dabir D, Luetkens J, Kuetting DLR et al (2019) Cardiac magnetic resonance including parametric mapping in acute takotsubo syndrome: preliminary findings. Eur J Radiol 113:217–224
Sharkey SW, Windenburg DC, Lesser JR et al (2010) Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 55(4):333–341
Jensch P-J, Stiermaier T, Eitel I (2021) Takotsubo Syndrome-Is There a Need for CMR? Curr Heart Fail Rep 18(4):200–210
Haghi D, Athanasiadis A, Papavassiliu T et al (2006) Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J 27(20):2433–2439
Ghadri J-R, Wittstein IS, Prasad A et al (2018) International Expert Consensus Document on Takotsubo Syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 39(22):2032–2046
Luetkens JA, Homsi R, Sprinkart AM et al (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17(2):154–161
Isaak A, Praktiknjo M, Jansen C et al (2020) Myocardial fibrosis and inflammation in liver cirrhosis: MRI study of the liver-heart axis. Radiology 297(1):51–61
Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B et al (2020) Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 22(1):87
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19(1):75
Ghadri JR, Kato K, Cammann VL et al (2018) Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol 72(8):874–882
Citro R, Radano I, Parodi G et al (2019) Long-term outcome in patients with Takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Fail 21(6):781–789
Di Vece D, Citro R, Cammann VL et al (2019) Outcomes associated with cardiogenic shock in takotsubo syndrome. Circulation 139(3):413–415
Stiermaier T, Busch K, Lange T et al (2020) Prognostic value of different CMR-based techniques to assess left ventricular myocardial strain in takotsubo syndrome. J Clin Med 9(12):3882
Ojha V, Khurana R, Ganga KP, Kumar S (2020) Advanced cardiac magnetic resonance imaging in takotsubo cardiomyopathy. Br J Radiol 93(1115):20200514
Parodi G, Scudiero F, Citro R et al (2017) Risk Stratification Using the CHA2DS2-VASc Score in Takotsubo Syndrome: Data From the Takotsubo Italian Network. J Am Heart Assoc 6(9):e006065
Scudiero F, Arcari L, Cacciotti L et al (2020) Prognostic relevance of GRACE risk score in Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 9(7):721–728
Kohan AA, Levy Yeyati E, de Stefano L et al (2014) Usefulness of MRI in takotsubo cardiomyopathy: a review of the literature. Cardiovasc Diagn Ther 4(2):138–146
Aquaro GD, Ghebru Habtemicael Y, Camastra G et al (2019) Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol 74(20):2439–2448
Friedrich MG (2010) Myocardial edema–a new clinical entity? Nat Rev Cardiol 7(5):292–296
Funding
Open Access funding enabled and organised by Projekt DEAL. A.I. was funded by the BONFOR Research Commission of the Medical Faculty Bonn (BONFOR-Forschungskommission der Medizinischen Fakultät Bonn) and by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) under Germany’s Excellence Strategy—EXC2151—390873048. The funders had no influence on study conceptualisation and design, collection and analysis of the data, manuscript preparation as well as the decision to publish.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Julian A. Luetkens.
Conflict of interest
C.C.P.—Activities not related to the present article: consultant for and has grants/grants pending with Guerbet; received payment for lectures including service on speakers bureaus from Philips Healthcare, Bayer Vital, and Guerbet. Activities related to the present article: disclosed no relevant relationships.
U.A. —Activities not related to the present article: received payment for lectures including service on speakers’ bureaus from Siemens Healthineers. Activities related to the present article: disclosed no relevant relationships.
J.A.L.—Activities not related to the present article: received payment for lectures including service on speakers’ bureaus from Philips Healthcare and Bayer Healthcare. Activities related to the present article: disclosed no relevant relationships.
All authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
L.W. (Institute of Medical Biometry, Informatics, and Epidemiology) kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isaak, A., Bratz, J., Kravchenko, D. et al. A novel and simple cardiac magnetic resonance score (PE2RT) predicts outcome in takotsubo syndrome. Eur Radiol 33, 5498–5508 (2023). https://doi.org/10.1007/s00330-023-09543-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09543-x