Abstract
Objectives
Cardiovascular disease (CVD), lung cancer (LC), and respiratory diseases are main causes of death in smokers and former smokers undergoing low-dose computed tomography (LDCT) for LC screening. We assessed whether quantification of pulmonary emphysematous changes at baseline LDCT has a predictive value concerning long-term mortality.
Methods
In this longitudinal study, we assessed pulmonary emphysematous changes with densitometry (volume corrected relative area below − 950 Hounsfield units) and coronary artery calcifications (CAC) with a 0–3 visual scale in baseline LDCT of 524 participants in the ITALUNG trial and analyzed their association with mortality after 13.6 years of follow-up using conventional statistics and a machine learning approach.
Results
Pulmonary emphysematous changes were present in 32.3% of subjects and were mild (6% ≤ RA950 ≤ 9%) in 14.9% and moderate-severe (RA950 > 9%) in 17.4%. CAC were present in 67% of subjects (mild in 34.7%, moderate-severe in 32.2%). In the follow-up, 81 (15.4%) subjects died (20 of LC, 28 of other cancers, 15 of CVD, 4 of respiratory disease, and 14 of other conditions). After adjusting for age, sex, smoking history, and CAC, moderate-severe emphysema was significantly associated with overall (OR 2.22; 95CI 1.34–3.70) and CVD (OR 3.66; 95CI 1.21–11.04) mortality. Machine learning showed that RA950 was the best single feature predictive of overall and CVD mortality.
Conclusions
Moderate-severe pulmonary emphysematous changes are an independent predictor of long-term overall and CVD mortality in subjects participating in LC screening and should be incorporated in the post-test calculation of the individual mortality risk profile.
Key Points
• Densitometry allows quantification of pulmonary emphysematous changes in low-dose CT examinations for lung cancer screening.
• Emphysematous lung density changes are an independent predictor of long-term overall and cardio-vascular disease mortality in smokers and former smokers undergoing screening.
• Emphysematous changes quantification should be included in the post-test calculation of the individual mortality risk profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardio-vascular disease (CVD), lung cancer (LC), and respiratory diseases are the three main causes of death in smokers and former smokers undergoing low-dose computed tomography (LDCT) for LC screening [1,2,3,4]. The latter is currently recommended in the USA for subjects 50–80 years of age with a smoking history of at least 20 pack-years or who have quit in the past 15 years [5]. Notably, LDCT also enables visualization and assessment of smoking-related biomarkers of co-morbidities, in particular coronary artery calcifications (CAC) and pulmonary emphysematous changes [6]. Although the correlation between emphysematous changes on CT and clinical or functional evidence of airflow obstruction qualifying for chronic obstructive lung disease (COPD) is weak [7], there is growing interest in incorporating post-LDCT information concerning potential biomarkers of smoking-related co-morbidities as CAC and emphysematous changes for the definition of the individual risk profile [8,9,10]. This might improve LC risk stratification, adapt screening LDCT intervention (starting age, frequency of LDCT rounds, and stopping age), and predict individual mortality outcome for smoking-related competing causes [11], according to a personalized medicine approach which maximizes the benefit and minimizes the harms of LC screening.
Assessment of pulmonary emphysematous changes in LDCT examinations for LC screening can be performed visually [12, 13] or with densitometry [11, 14, 15]. Lung densitometry is less affected by inter-observer variability [16, 17] and better allows stratification for emphysema severity [18].
In this study, we assessed the association between quantification of emphysematous changes at baseline LDCT and mortality after 13 years since randomization in subjects recruited in the ITALUNG randomized clinical trial [2].
Materials and methods
Participants’ selection and characterization
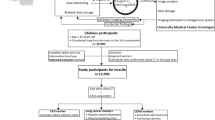
The ITALUNG trial (clinical trial registration, number = NCT02777996) was approved by the Local Ethics Committee of the three participating institutions in the Tuscany region of Italy (approval number 29–30 of 30 September 2003; number 23 of 27 October 2003; and number 00028543 of 13 May 2004), and each participating subject provided written informed consent. Protocols, LDCT results, and mortality were previously reported [2, 19, 20]. Eligible were 55–69 years aged subjects with a smoking history ≥ 20 pack-years and who were current smokers or have quitted in the last 10 years. Overall, 1613 subjects were randomized to receive 4 annual LDCT and 1593 to usual care. Baseline LDCT was obtained between 2004 and 2006 in 1406 subjects of the active arm. In ITALUNG, we employed 8 different spiral CT scanners from two vendors over the 2004–2012 period of LDCT active screening [21]. CT scanner technology affects the quantification of emphysematous changes because it defines the range of acquisition parameters among which slice collimation and image reconstruction filtering are the most important [17, 22]. In 536 subjects recruited in two (Florence and Pisa) screening centers of ITALUNG, the baseline LDCT examination was obtained using two identical 4 rows of detector CT scanners (Somatom Volume Zoom, Siemens Healthcare) and the same LDCT examination protocol. This baseline LDCT constituted the basis for the present investigation.
LDCT and its evaluation
The scanners were calibrated on air daily. The acquisition technique for LC screening included 1-mm collimation slices from the apex to the base, beam pitch 1.75 × 4, reconstruction 1.25 mm, 140 kV, and 40 mAs, during one breath-hold obtained at end-inspiration.
Before the assessment, the quality of LDCT examination images was judged as adequate or inadequate due to artifacts due to motion, metallic stent placement, etc.
Emphysema
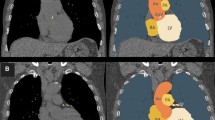
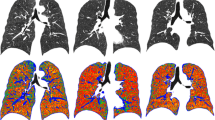
We performed a quantitative assessment of lung emphysematous changes with densitometry in images reconstructed with the smooth (b31f Siemens) kernel. For such a purpose, we used the Pulmo (Siemens, Healthcare) software in the Florence cohort and the SYNAPSE 3D (Fujifilm) software in the Pisa cohort, according to an established procedure [17]. The presence of emphysematous changes was defined using the 6.0% threshold of the relative area of the lung with density values below 950 Hounsfield units (RA950) [18] normalized to the lung volume at acquisition. Subjects with emphysematous changes were then arbitrarily divided into those with mild (6% ≤ RA950 ≤ 9%) and those with moderate-severe (RA950 > 9%) emphysema.
CAC
CAC visualized in LDCT for LC screening are established predictors of overall and CVD mortality in the screened population [6, 23, 24]. Hence, they must be assessed in order to control for this possible confounding variable. Accordingly, in the present study, we evaluated CAC with a visual semi-quantitative scoring system which is fast, reproducible, and performed as well as the Agatston score in predicting CV events and mortality [23] and whose results in the whole ITALUNG cohort were previously reported [25]. By evaluating the entire coronary arterial vessels, it distinguishes 4 scores: 0 = absent, 1 = mild (isolated flecks of CAC within a segment), 2 = moderate (any degree between mild and severe), and 3 = severe (continuous CAC within a segment). Here, we considered moderate and severe CAC altogether, since they significantly predict CVD mortality as opposed to absent or mild CAC [25].
Assessment of both pulmonary emphysema and CAC was possible in baseline LDCT of 524 subjects (267 subjects of the Pisa cohort and 257 subjects of the Florence cohort), whereas 12 subjects were excluded because of technically inadequate baseline LDCT. The characteristics of the 524 subjects who were finally enrolled are reported in Table 1. Notably, their age, gender, smoking history, and CAC severity were similar to those in the remainder of 840 subjects undergoing baseline LDCT in ITALUNG for whom the CAC evaluation was available (Table 1).
Follow-up
The 524 subjects (and the remainder of the ITALUNG active group) were followed up until 31 December 2018 with a median follow-up time from the date of randomization of 13.6 years (Q1–Q3: 12.9–13.9 years). The incidence of LC in the cohort was established by a link to the tumor registry of the Tuscany region. Follow-up for vital status and cause of death was performed through the linkage with the regional mortality registry and included a check for residential status. Causes of death were coded according to the International Classification of Disease (ICD) version IX for subjects deceased until 2009 and version X for subjects deceased from 2010 onwards considering LC (ICD-9 code 162 and ICD-10 code C33-C34), other cancers (ICD-9 codes 140–239 and ICD-10 codes C00-D48), CVD (ICD-9 codes 390–459 and ICD-10 codes I00-I99), and respiratory illness (ICD-9 codes 460–519 and ICD-10 codes J00-J99).
Statistical analyses
Person-years at risk were calculated from the date of randomization either to the date of death or the date of censoring (migration or end of follow-up). Comparisons of frequency distribution between categories were performed using the chi-square test. Adjusted mortality ratio and confidence interval were estimated using the Cox model. All data analyses were conducted using Stata software, version 16.1.
Machine learning analysis
Based on the results of conventional statistics (see below), we used machine learning techniques to evaluate the contribution to the prediction of the overall, LC, and CVD mortality of the following features: age, gender, smoking status, pack-years, CAC visual score, and RA950. To this aim, we trained, validated, and tested the eXtreme Gradient Boosting (XGBoost) model (with default parameters) [26] that is a tree-based machine learning model widely used to achieve cutting-edge performance on various recent machine learning challenges. We adopted a repeated (ten times) stratified tenfold cross-validation strategy for LC and CVD mortality prediction. For the overall mortality prediction, given the larger number of subjects in each class (i.e., 81 subjects had died as of December 31, 2018, vs. 443 who did not), we performed a repeated (ten times) stratified nested 10–fivefold (i.e., outer tenfold and inner fivefold) cross-validation, to estimate the unbiased generalization performance of the model along with performing, at the same time, hyperparameter optimization [27]. The hyperparameter space was defined as follows: the step size shrinkage used in the update to prevent overfitting, i.e., eta ∈ (0.5, 0.7, 0.9), the minimum loss reduction required to make a further partition on a leaf of the tree, i.e., gamma ∈ (10, 100, 1000), and the maximum depth of a tree, i.e., max_depth ∈ (2, 3, 4, 5). The performance was measured through the area under the receiver operating characteristic curve (AUROC), and we computed the average AUROC over the ten repetitions. Then, we interpreted predictions by using the Shapley additive explanations (SHAP) approach [28]. By assigning each feature an importance value for each sample, SHAP is a powerful explainable artificial intelligence (XAI) framework for evaluating predictions based on traditional Shapley values from game theory [29]. This method may explain the model locally (on a single sample) and globally. All machine learning analyses were performed using in-house Python code (Version 3.7.0) on an M1 MacBook Air (macOS Monterey version 12.6).
Results
Pulmonary emphysematous changes were present in 169 (32.3%) of the 524 subjects enrolled in the study and were mild in 14.9% and moderate-severe in 17.4% (Table 1). The majority (351/524; 67%) of subjects showed some degree of CAC which were mild in 34.7% and moderate or severe in 32.2%. Notably, age, gender, smoking history, and CAC severity in the 524 subjects were similar to those in the remainder 840 subjects recruited in ITALUNG for whom CAC could be visually assessed (see Table 1). The presence of emphysematous changes (any degree) was correlated with male sex, age, and status of former smokers, whereas the presence of CAC (any degree) was correlated with male sex, age, and pack-years (Table 2). There was no correlation between the presence or severity of pulmonary emphysematous changes and CAC (Table 2).
No difference in LC incidence was observed in association with pulmonary emphysema or CAC (Supplementary E-Table 1).
In the follow-up period, 81 (15.4%) of 524 subjects died. Mortality and causes of death were similar in the remainder of the 840 subjects recruited in ITALUNG (Supplementary E-Table 2). Causes of death in the 81 subjects of the study group were LC in 20 subjects, other cancers in 28 subjects, CVD in 15 subjects, respiratory diseases in 4 subjects, and others in 14 subjects. The CVD mortality causes included ischemic heart disease (n = 8), atrial fibrillation (n = 2) intracerebral hemorrhage (n = 2), ruptured aneurysm of the abdominal aorta (n = 1), and other unspecified cardiovascular (n = 1) or cerebrovascular (n = 1). The respiratory mortality causes included interstitial pulmonary fibrosis (n = 2), COPD (n = 1), and unspecified respiratory failure (n = 1).
In the study group, the overall mortality rate ratios significantly increased with a moderate-severe degree of emphysematous changes [OR 2.22 (95%CI 1.34–3.70)] after adjusting for age, sex, smoking history, screening center, and CAC visual score. Moderate-severe degree of CAC was associated with an increased overall mortality, but the difference was not statistically significant [OR 1.51 (95%CI 0.94–2.43)] (Table 3). The adjusted CVD mortality rate ratios significantly increased with a moderate-severe degree of emphysematous changes [OR 3.66 (95%CI 1.21–11.04)] and with a moderate-severe degree of CAC [OR 3.18 (95%CI 0.99–10.16)], while the adjusted LC mortality did not (Table 3).
Machine learning analysis (Fig. 1) showed that RA950 was the best single feature for the prediction of overall and CVD mortality among age, gender, pack-years, smoking status, and CAC visual score, with AUROC values of 0.70 for overall and 0.73 for CVD mortality. For the prediction of LC mortality, the two most important features were pack-years and RA950, with AUROC = 0.61.
Discussion
In the present investigation, we quantitatively assessed pulmonary emphysematous changes in baseline LDCT of one sub-cohort of subjects recruited in the ITALUNG trial and analyzed 13 years after randomization of its potential impact on death and its causes, while controlling for demographic and smoking history variables, as well as CAC that are relevant predictors of overall and CVD mortality in the LC screening population [23,24,25]. Our data, as assessed with both conventional statistics and a machine learning approach, indicate that quantification of emphysematous changes at baseline LDCT independently predicts overall and CVD mortality in heavy smokers and former smokers undergoing LC screening.
Our sample was selected based on the type of CT scanner and acquisition protocol which substantially affect the quantitative assessment of diffuse lung changes [17, 18], but was representative of the entire group undergoing LDCT screening in ITALUNG. Also, the prevalence and distribution of pulmonary emphysematous changes [6, 14] and CAC [6, 23,24,25] in the sub-cohort were substantially consistent with previous data in subjects undergoing LC screening. So far, quantitative evaluation of pulmonary emphysematous changes in subjects undergoing screening LDCT has been correlated with the incidence of LC [15] and with the Pulmonary Functional Test (PFT) results [30, 31]. Moreover, emphysematous changes were significantly correlated with the risk of a next CV event [32], and the addition of mean lung density and RA950 lung percentage improved the prediction of LC incidence and especially of both COPD and CVD mortality in the National Lung Screening Trial (NLST) [11]. As a matter of fact, CVD is the first cause of death in patients with COPD exceeding respiratory failure and LC [33,34,35] consistently with the view that COPD is a powerful, independent risk factor for CV morbidity and mortality. The predictive value of emphysematous changes in baseline LDCT with respect to overall and CVD mortality in our study is in line with the above evidence. In general, since smoke is a risk factor for both COPD and vascular damage, one may assume that the correlation between emphysematous changes and CVD mortality could be mediated by CAC, and it is established that CAC are more frequent in subjects with COPD [36, 37]. However, our data indicate that the association between emphysematous changes and CVD mortality is independent from that between CAC and CVD mortality. This is not surprising because the mechanisms underlying the association of emphysematous changes or COPD with CVD are not entirely accounted for by those of the association between CAC and CVD. In fact, several additional mechanisms have been identified. Systemic inflammation in response to smoking and air or professional pollution is implicated in both lung parenchymal disruption and airway remodelling and the genesis and evolution of atherosclerotic plaque [38]. In particular, in COPD patients with emphysema, also osteoporosis can contribute to a low-grade systemic inflammation. Osteoporosis is highly prevalent in these patients [39, 40] for the decreased physical activity and the frequently administered steroid therapy, and is associated with increased plasma levels of C-reactive protein, interleukin 6, and tumor necrosis factor alpha that further increase during exacerbations of lung disease. A second link between emphysematous changes and COPD and CVD can be represented by sustained or intermittent hypoxia due to gas exchange derangement creating a vicious circle of hypoxia and re-oxygenation. Overall, the relationship between pulmonary emphysematous changes and CVD mortality could reflect a dynamic multi-faceted interaction between lung and vessels with a possible role for inflammation, endothelial and elastin deficit, and hypoxia due to gas exchange derangements, while arterial calcium deposition can be considered a more advanced expression of arterial wall damage. Certainly, future researches are worthy on the interplays among pulmonary emphysema, COPD, CAC, and CVD risk and mortality in subjects with significant smoking history. Notably, in our study, the association between emphysematous changes and LC mortality was weak, but this could reflect the fact that screening typically detects and allows treatment and cure of early-stage LC.
Despite the above physio-pathological uncertainties, our data, confirming that emphysematous changes in baseline screening LDCT significantly and independently predict overall and CVD mortality [11], suggest that this biomarker of co-morbidity should be considered in post-test individual risk stratification [8,9,10].
We recognize the following limitations of our study. First, due to CT scanner heterogeneity, our data were obtained in a part only (37.2%, 524/1406), although representative, of the subjects who received baseline LDCT in the ITALUNG trial. Investigation in larger samples, as well as external validation of the machine learning algorithm in independent samples, is worthy. In particular, the low number of deaths due to respiratory diseases, which is in line with other studies [4, 41], did not allow us to assess the predictive value of emphysematous changes with respect to respiratory causes of death that have been observed in the NLST trial [11, 13]. Second, although measurement of airway thickness is feasible in LDCT for LC screening [11, 42], we did not perform it. This may have obscured the contribution of the full spectrum of the changes underlying COPD which can be assessed on CT to the mortality profile of our subjects. Third, we used two software for emphysema quantification which were applied to images acquired with the same CT scanners and protocols. However, in a study comparing the results of 8 software for emphysema assessment in 50 patients, the inter-software mean bias was within ± 0.46%, for RA950 [43]. Fourth, at the time of baseline screening in ITALUNG (2004–2006), no clear implications for CAC or emphysematous changes detected in LDCT examinations were established. Accordingly, although it was possible that visual evidence of moderate-severe CAC or emphysematous changes was sporadically contained in the screening test report provided to the subject and his/her general practitioner, in the present retrospective study, we could not assess the implications and consequences of such a report. Certainly, as demonstrated by our study, these biomarkers of smoking-related co-morbidities can be assessed on baseline LDCT, are no longer to be ignored, and deserve consideration in prospective LDCT screening cohorts. Fifth, visual assessment allows detailed assessment of the distribution and types of lung emphysematous changes, including centrilobular, panlobular, and paraseptal [18], which we did not analyze. The possibility that these additional features may better explain the mortality implications of the emphysematous changes in baseline LDCT of people undergoing LC screening might deserve additional studies.
Conclusions
Pulmonary emphysematous changes at baseline LDCT is independently associated with long-term overall and CVD death in subjects recruited for LC screening and should be incorporated in the post-test calculation of the individual mortality risk profile.
Data Availability
The data of this study are available on request at Professor Mario Mascalchi at mario.mascalchi@unifi.it.
Abbreviations
- AUROC:
-
Area under the receiver operating characteristic curve
- CAC:
-
Coronary artery calcifications
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- CVD:
-
Cardio-vascular disease
- ICD:
-
International Classification of Disease
- LC:
-
Lung cancer
- LDCT:
-
Low-dose computed tomography
- NLST:
-
National Lung Screening Trial
- OR:
-
Odds ratio
- PFT:
-
Pulmonary Functional Test
- RA950:
-
Relative area of the lung with density values below 950 Hounsfield units
- SHAP:
-
Shapley additive explanations
- XAI:
-
EXplainable Artificial Intelligence framework
References
National Lung Screening Trial Research Team, Aberle DR, Adams AM et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Paci E, Puliti D, Lopes Pegna A et al (2017) Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 72:825–831
de Koning HJ, van der Aalst CM, de Jong PA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503–513
Kaaks R, Christodoulou E, Motsch E et al (2022) Lung function impairment in the German Lung Cancer Screening Study (LUSI): prevalence, symptoms, and associations with lung cancer risk, tumor histology and all-cause mortality. Transl Lung Cancer Res. https://doi.org/10.21037/tlcr-22-63
US Preventive Services Task Force, Krist AH, Davidson KW et al (2021) Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA 325:962–970
Sverzellati N, Cademartiri F, Bravi F et al (2012) Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology 262:460–467
Regan EA, Lynch DA, Curran-Everett D et al (2015) Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 175:1539–1549
O’Dowd EL, Ten Haaf K (2019) Lung cancer screening: enhancing risk stratification and minimising harms by incorporating information from screening results. Thorax 74:825–827
Baldwin D, O’Dowd E, Ten Haaf K (2020) Targeted screening for lung cancer is here but who do we target and how? Thorax 75:617–618
Ten Haaf K, van der Aalst CM, de Koning HJ, Kaaks R, Tammemägi MC (2021) Personalising lung cancer screening: an overview of risk-stratification opportunities and challenges. Int J Cancer 149:250–263
Schreuder A, Jacobs C, Lessmann N et al (2021) Combining pulmonary and cardiac computed tomography biomarkers for disease-specific risk modelling in lung cancer screening. Eur Respir J. https://doi.org/10.1183/13993003.03386-2020
Wille AMW, Thomsen LH, Dirksen A et al (2014) Emphysema progression is visually detectable in low-dose CT in continuous but not in former smokers. Eur Radiol 24:2692–2699
Pinsky PF, Lynch DA, Gierada DS (2022) Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest 161:1092–1100
Camiciottoli G, Cavigli E, Grassi L et al (2009) Prevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trial. Eur Radiol 19:58–66
Labaki WW, Xia M, Murray S et al (2021) Quantitative emphysema on low-dose CT imaging of the chest and risk of lung cancer and airflow obstruction: an analysis of the National Lung Screening Trial. Chest 159:1812–1820
Cavigli E, Camiciottoli G, Diciotti S et al (2009) Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol 19:1686–1692
Mascalchi M, Camiciottoli G, Diciotti S (2017) Lung densitometry: why, how and when. J Thorac Dis 9:3319–3345
Lynch DA, Austin JH, Hogg JC et al (2015) CT-Definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner society. Radiology 277:192–205
Lopes Pegna A, Picozzi G, Mascalchi M et al (2009) Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 64:34–40
Lopes Pegna A, Picozzi G, Falaschi F et al (2013) Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 8:866–875
Mascalchi M, Mazzoni LN, Falchini M et al (2012) Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. Br J Radiol 85:1134–1139
Gierada DS, Bierhals AJ, Choong CK et al (2010) Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol 17:146–156
Chiles C, Duan F, Gladish GW et al (2015) Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology 276:82–90
Rasmussen T, Køber L, Abdulla J et al (2015) Coronary artery calcification detected in lung cancer screening predicts cardiovascular death. Scand Cardiovasc J 49:159–167
Mascalchi M, Puliti D, Romei C et al (2021) Moderate-severe coronary calcification predicts long-term cardiovascular death in CT lung cancer screening: the ITALUNG trial. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2021.110040
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system, in: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. Presented at the KDD ’16: The 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, ACM, San Francisco California USA, pp. 785–794. https://doi.org/10.1145/2939672.2939785
Varma S, Simon R (2006) Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics 7:91. https://doi.org/10.1186/1471-2105-7-91
Lundberg SM, Lee SI (2017) A unified approach to interpreting model predictions. arXiv:1705.07874
Shapley LS (2016) A value for n-person games. In Contributions to the Theory of Games (AM-28); Kuhn HW, Tucker AW, (Eds.) Princeton University Press: Princeton, NJ, USA, 2:307–318
Shaker SB, Dirksen A, Lo P, Skovgaard LT, de Bruijne M, Pedersen JH (2012) Factors influencing the decline in lung density in a Danish lung cancer screening cohort. Eur Respir J 40:1142–1148
Sørensen L, Nielsen M, Petersen J, Pedersen JH, Dirksen A, de Bruijne M (2020) Chronic obstructive pulmonary disease quantification using CT texture analysis and densitometry: results from the Danish Lung Cancer Screening Trial. AJR Am J Roentgenol 214:1269–1279
Takx RA, Vliegenthart R, Mohamed Hoesein FA et al (2015) Pulmonary function and CT biomarkers as risk factors for cardiovascular events in male lung cancer screening participants: the NELSON study. Eur Radiol 25:65–71
Janssens JP, Herrmann F, MacGee W, Michel JP (2001) Cause of death in older patients with anatomo-pathological evidence of chronic bronchitis or emphysema: a case-control study based on autopsy findings. J Am Geriatr Soc 49:571–576
Sin DD, Man SF (2005) Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2:8–11
Calverley PM, Anderson JA, Celli B et al (2010) Cardiovascular events in patients with COPD: TORCH study results. Thorax 65:719–725
Rasmussen T, Køber L, Pedersen JH et al (2013) Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Eur Heart J Cardiovasc Imaging 14:1159–1166
Williams MC, Murchison JT, Edwards LD et al (2014) Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax 69:718–723
Morgan AD, Zakeri R, Quint JK (2018) Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. https://doi.org/10.1177/1753465817750524
Camiciottoli G, Bigazzi F, Magni C et al (2016) Prevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 11:2229–2236
Ohara T, Hirai T, Muro S et al (2008) Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest 134:1244–1249
Pastorino U, Silva M, Sestini S et al (2019) Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 30:1162–1169
Xie X, Dijkstra AE, Vonk JM, Oudkerk M, Vliegenthart R, Groen HJ (2014) Chronic respiratory symptoms associated with airway wall thickening measured by thin-slice low-dose CT. AJR Am J Roentgenol 203:W383-390
Kirby M, Hatt C, Obuchowski N et al (2020) Inter- and intra-software reproducibility of computed tomography lung density measurements. Med Phys 47:2962–2969
Acknowledgements
The authors wish to thank Professor Rudolf Kaaks, Division of Cancer Epidemiology (C020), German Cancer Research Center (DKFZ), Heidelberg, Germany, for helpful suggestions with the paper structure and content.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mario Mascalchi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Donella Puliti, a senior statistician at ISPRO, performed the statistical analysis of the data for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Lopes-Pegna et al Lung Cancer Lung Cancer (2009) 64:34–40; Camiciottoli et al Eur Radiol (2009)19:58–66; Paci et al Thorax (2017) 72:825–831; Mascalchi et al Eur J Radiol (2021) https://doi.org/10.1016/j.ejrad.2021.110040.
Methodology
• prospective
• case–control study
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mascalchi, M., Romei, C., Marzi, C. et al. Pulmonary emphysema and coronary artery calcifications at baseline LDCT and long-term mortality in smokers and former smokers of the ITALUNG screening trial. Eur Radiol 33, 3115–3123 (2023). https://doi.org/10.1007/s00330-023-09504-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09504-4