Abstract
Objective
Reproducibility problems are a known limitation of radiomics. The segmentation of the target lesion plays a critical role in texture analysis variability. This study’s aim was to compare the interobserver reliability of manual 2D vs. 3D lung lesion segmentation with and without pre-definition of the volume using a threshold of − 50 HU.
Methods
Seventy-five patients with histopathologically proven lung lesions (15 patients each with adenocarcinoma, squamous cell carcinoma, small cell lung cancer, carcinoid, and organizing pneumonia) who underwent an unenhanced CT scan of the chest were included. Three radiologists independently segmented each lesion manually in 3D and 2D with and without pre-segmentation volume definition by a HU threshold, and shape parameters and original, Laplacian of Gaussian–filtered, and wavelet-based texture features were derived. To assess interobserver reliability and identify the most robust texture features, intraclass correlation coefficients (ICCs) for different segmentation settings were calculated.
Results
Shape parameters had high reliability (64–79% had excellent and good ICCs). Texture features had weak reliability levels, with the highest ICCs (38% excellent or good) found for original features in 3D segmentation without the use of a HU threshold. A small proportion (4.3–11.5%) of texture features had excellent or good ICC values at all segmentation settings.
Conclusion
Interobserver reliability of texture features from CT scans of a heterogeneous collection of manually segmented lung lesions was low with a small proportion of features demonstrating high reliability independent of the segmentation settings. These results indicate a limited applicability of texture analysis and the need to define robust texture features in patients with lung lesions.
Key Points
• Our study showed a low reproducibility of texture features when 3 radiologists independently segmented lung lesions in CT images, which highlights a serious limitation of texture analysis.
• Interobserver reliability of texture features was low regardless of whether the lesion was segmented in 2D and 3D with or without a HU threshold.
• In contrast to texture features, shape parameters showed a high interobserver reliability when lesions were segmented in 2D vs. 3D with and without a HU threshold of − 50.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiomics, which involves the extraction of a large number of quantitative features from radiology images, may provide additional information on the lesion composition [1], can potentially predict survival in patients with colorectal cancer, high-grade gliomas, and renal cell carcinomas [2,3,4], and has prognostic power in head-and-neck cancer [5]. In the lung, radiomics has the potential to differentiate between benign and malignant lung nodules [6], to predict the prognosis for non–small cell lung cancer [5, 7], and to distinguish between different types of lung cancer [7]. Nevertheless, to obtain a final diagnosis, patients in a clinical setting routinely undergo percutaneous or transbronchial tissue sampling, which carries the risk of severe complications [8,9,10,11]. Concerns about the reproducibility of radiomics, especially texture features, constitute one of the main obstacles to the clinical acceptance and implementation of CT texture analysis (CTTA) [12]. The segmentation of the lesion of interest is considered a substantial source of inadequate reproducibility [13]. Manual segmentation is the reference standard in most trials when it is performed by experts; however, lesion delineation is prone to variability. Whether the lung lesion should be segmented in three dimensions (3D) or only in the maximum diameter in two dimensions (2D) remains controversial. The application of a Hounsfield-unit (HU) threshold in order to restrict the volume before segmentation of pulmonary lesions may also influence CTTA results. A − 50 HU threshold for CTTA in the lung has been proposed as a means to prevent the potential segmentation of extra-lesional pulmonary tissue [14, 15]. However, sub-solid parts of lung lesions, as previously reported characteristic of adenocarcinomas [16], may also be excluded when using such a threshold. Since reliable reproducibility is a pre-requisite for the applicability of CTTA and the optimum segmentation settings for pulmonary lesions remain controversial, the aim of our study was to compare the interobserver reliability of CTTA parameters derived with manual 2D vs. 3D lung lesion segmentation, with and without pre-definition of the volume using a threshold of − 50 HU.
Materials and methods
Patient population
The Institutional Ethics Committee approved this retrospective, single-institution study.
A search in the institution’s data system yielded 1049 patients who underwent CT-guided biopsy of the lung at our institution between January 2013 and December 2018 for whom both a pre-interventional planning CT examination without contrast administration and a corresponding histopathologic diagnosis were available. To represent the heterogeneity of suspicious lung lesions encountered in routine clinical thoracic imaging, we selected patients with the histopathologic diagnosis of adenocarcinoma, squamous cell carcinoma, carcinoid, small cell lung cancer, or organizing pneumonia. Overall, 75 patients (47 males, 28 females) were included. Because our data search retrieved only 15 patients with the diagnosis of carcinoid, we included 15 consecutive patients each with adenocarcinoma, squamous cell carcinoma, small cell lung cancer, and organizing pneumonia in chronological order starting with the earliest to maintain homogeneity of group size.
CT imaging
All CT-guided biopsies were performed on the same 128-multislice-CT scanner (Somatom Definition AS, Siemens Healthineers). A CT scan without contrast (craniocaudal scanning direction; tube voltage: 100 kV; tube current: 100 mAs; collimation: 128 × 0.6 mm; pitch: 1.2; section thickness: 3 mm; increment: 3.0 mm; reconstruction: standard kernel using iterative reconstruction) was carried out to determine the exact position of the target lesion and to plan the access route for the biopsy needle for each patient. These pre-procedure CT scans were used for CTTA of all lesions.
CT texture analysis (CTTA)
Image segmentation was performed with the open-source 3D Slicer software (version 4.11.0–2019-03–24), and radiomic feature extraction was done with SlicerRadiomics, a freeware extension to 3D Slicer. CT images were extracted from the institution’s PACS in DICOM format and transferred to 3D Slicer. Lesion segmentations were performed manually and independently by three radiologists (G.A., M.J., and A.K., with 4–8 years of experience in chest CT image interpretation), who were blinded to the histopathologic diagnosis.
For lesion segmentation, the borders of the target lung nodules and masses were manually drawn in the CT lung window setting (center, − 600 HU; width, 1200 HU) in pre-interventional, non-contrast-enhanced CT images. Lesions were segmented in 3D slice by slice in axial slice orientation and in 2D in the largest diameter in axial slice orientation, including perilesional ground-glass opacities when present. Bones and soft tissue not considered part of the target lesion were excluded. Additional analyses of the 3D and 2D segmentations were performed with an attenuation threshold that excluded density values below − 50 HU to avoid accidentally segmenting air, as previously described elsewhere [17].
Texture analysis included first-order features (18 features describing gray-level values), gray-level co-occurrence matrix (GLCM) features (24 features relating the frequencies of pairs of pixels or voxels with certain values and a specified spatial relationship), gray-level dependence matrix (GLDM) features (14 features based on the number of connected pixels or voxels within a distance dependent on the center), gray-level run length matrix (GLRLM) features (16 features based on the length in number of consecutive pixels or voxels having the same gray-level value), gray-level size zone matrix (GLSZM) features (16 features, which quantify gray-level zones defined by the number of pixels or voxels with the same gray-level value), and neighboring gray tone difference matrix (NGTDM) features (5 features describing the difference between a gray-level value and the mean of its neighbors within a certain distance) [12]. Additionally 14 parameters representing shape characteristics of the segmented lesion were analyzed.
A Laplacian of Gaussian (LoG) filter was applied to produce fine-to-coarse texture features with filter values of 0.5, 1.5, and 2.5, as previously described in CTTA of the lung [14, 17, 18].
In addition, 744 wavelet-based texture features with four different band combinations (low-low, high-high, high-low, low–high) were derived.
Overall, 1130 radiomic features were included in the analysis.
Statistical analysis
Descriptive statistics are presented as absolute and relative frequencies for categorical data and as means and standard deviations (SD) or medians and ranges for continuous data. Agreement in the segmented lesions between different raters was evaluated by an intraclass correlation coefficient (ICC) for each of the 1130 radiomic features separately. The ICC was assessed by a two-way random model (ICC (2,1) concept). ICC values were defined as poor (< 0.50), moderate (0.50–0.75), good (0.75–0.90), or excellent (> 0.90), according to Koo and Li [19]. The statistical analyses were performed with the SAS software (version 9.4; SAS Institute, Inc.).
Results
Detailed information on patients’ demographics and the segmented lung lesions is provided in Table 1. Two patients with organizing pneumonia and three patients with carcinoids were excluded from the analysis when applying a threshold of − 50 HU, because there remained no computable lesion above this density.
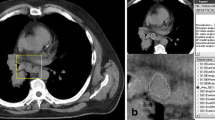
Figure 1 shows an example of a lung lesion from a CT scan of a patient with organizing pneumonia segmented with and without a density threshold of − 50 HU in maximum axial diameter. Additional figures demonstrating segmentations of adenocarcinoma, squamous cell carcinoma, carcinoid, and small cell lung cancer are provided as electronic supplementary material. The ICCs of all 1130 extracted radiomic features in 3D and 2D segmentation without and with a density threshold of − 50 HU were distributed over a wide range (Fig. 2).
a–c Non-contrast-enhanced CT image of a 50-year-old male patient with histologically proven organizing pneumonia without segmentation (a), with green-colored segmentation without a HU threshold (b) and with red-colored segmentation with a threshold of − 50 HU (c). Example is shown in maximum diameter only
Excellent or good ICC values at all four different segmentation settings were found for 8 (57.1%) shape parameters, 4 (4.3%) original features, 32 (11.5%) LoG-filtered texture features, and 50 (6.7%) wavelet-based texture features (Table 2).
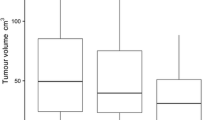
The majority (8/14) of shape parameters had excellent or good ICC values at all segmentation settings. ICC values were highest for 2D segmentations without a HU threshold (79% excellent or good). For shape parameters derived after 2D segmentation with a HU threshold as well as those derived after 3D segmentation without a HU threshold, 71% of ICC values were excellent or good. For shape parameters segmented in 3D with a HU threshold, 64% of ICC values were excellent or good. Details of interobserver reliability for shape parameters are shown in Table 3 and Fig. 3.
ICCs of 93 original features (first order, GLCM, GLDM, GLRLM, GLSZM, NGTDM) were calculated in 2D and 3D with and without a HU threshold of − 50. Higher ICC values were observed when segmentation was performed without a HU threshold, both for 3D (38% excellent or good) and 2D segmentation (33% excellent or good), as opposed to with a HU threshold. Original features derived from segmentations performed with a HU threshold had remarkably low ICC values (3D: 11% excellent or good; 2D: 8% excellent or good; Table 3 and Fig. 3).
LoG filters of 0.5, 1.5, and 2.5 were applied on original features, resulting in a total of 279 LoG-filtered parameters that were analyzed for interobserver reliability. ICC values for these parameters were higher when segmentations were performed without a HU threshold as opposed to with a HU threshold of − 50 for both 3D segmentation (33% excellent or good vs. 31% excellenct or good) and 2D segmentation (34% excellent or good vs. 20% excellent or good).
Details regarding ICCs for original and LoG-filtered features are presented in Table 3 and Fig. 3.
A total of 744 wavelet-based texture parameters with four different band combinations were derived. Among wavelet-based texture parameters derived from 3D segmentations, ICC values did not differ substantially between parameters derived after application of a HU threshold (25.0% excellent or good) and those derived without a HU threshold (24.6% excellent or good). For wavelet-based texture parameters derived from 2D segmentations, however, the application of a HU threshold yielded even lower ICC values (16.4% excellent or good) than did omission of the HU threshold (20.6% excellent or good). Detailed results on the interobserver reliability of wavelet-based texture parameters are given in Table 3.
Discussion
This study explored the influence of 2D vs. 3D manual segmentation with and without the application of a HU threshold of − 50 on the reliability of CTTA in different types of histologically proven pulmonary lesions. Overall, the reliability of texture analysis for original, LoG-filtered, and wavelet-based texture features was rather low, with the percentages of these features demonstrating excellent or good ICCs ranging from just 8 to 38%. The highest proportion of excellent and good ICC values was found when segmentation was performed in 3D without a HU threshold, whereas the lowest proportion of excellent and good ICC values was observed for 2D segmentation with a HU threshold.
Radiomics is a promising approach for non-invasive tissue characterization of lesions based on image analysis [1]. Several studies, however, have identified the lesion segmentation process as a relevant source of variability of texture analysis [20]. Tumor type and tumor site have been shown to influence interobserver variability in lesion delineation and therefore radiomics analysis [21]. In renal masses, investigation of intra- and interobserver variability showed greater reliability of segmentation in contrast-enhanced CT vs. non-enhanced CT [12], and texture analysis differed by the phase of the contrast injection protocol [22]. For PET/CT in non–small cell lung cancer (NSCLC), radiomic features had high test–retest and interobserver stability [23] similar to the more commonly used PET parameters SUVmax, SUVmean, and SUVpeak, and the performance of radiomic features depended more on the delineation method than on the applied reconstruction method [24].
Studies on the reliability of CTTA of pulmonary lesions in different segmentation settings are scarce. In patients with NSCLC, feature reliability varied between manual and different semi-automatic segmentation approaches [25]. Representations of pulmonary tumor heterogeneity and reproducibility in 3D segmentation were previously described as superior compared to those in 2D segmentation [26, 27]. Maximum-diameter-only 2D segmentations could provide the benefit of reducing potential motion and breathing artifacts that might be more pronounced when more CT slices are segmented in three dimensions. On the other hand, 3D segmentation includes the whole tumor and may better depict the average tissue properties for lesion classification in CTTA. A potential drawback of 3D segmentation, however, is that a higher number of segmented image slices could lead to inclusion of a larger quantity of potentially non-tumorous tissue that may distort the results. In the present study, the largest differences in ICC values between 3D and 2D segmentations were found for LoG-filtered texture features derived after use of a HU threshold (3D: 31% excellent or good vs. 2D: 20% excellent or good ICC values) and for wavelet-based parameters derived after pre-segmentation use of a HU threshold (3D: 25.0% excellent or good vs. 2D: 16.4% excellent or good ICC values). For all other combinations of segmentation settings, the differences in ICC values between 3D and 2D were smaller, but nevertheless, 3D segmentations always resulted in higher ICCs than did 2D segmentations.
The most pronounced differences in ICC values were observed between original texture features derived after 3D or 2D segmentation without a HU threshold (38% and 33% excellent or good ICC values, respectively) and those derived after 3D or 2D segmentation with a HU threshold of − 50 (11% and 8% excellent or good ICC values, respectively). Except for wavelet-based features derived from 3D segmentation, which showed minimally higher ICC values with than without application of a HU threshold (25% excellent or good vs. 24.6% excellent or good), all other parameters derived from 3D and 2D segmentations showed a higher proportion of excellent and good ICC values when segmentation was performed without a HU threshold. Although it has been postulated that applying a − 50 HU threshold for CTTA could prevent the potential segmentation of extra-lesional pulmonary tissue [14, 15], it remains unclear whether a HU threshold excludes not only negligible extra-lesional parenchymal changes but also characteristic sub-solid parts of lung lesions, as previously reported for adenocarcinomas [16]. Moreover, the optimum value of the HU threshold that should be applied remains uncertain. In our study cohort, where one fifth of lung lesions (14 out of 75, Table 1) had associated ground-glass opacities, the results favor segmentation without a HU threshold, especially when original texture features are to be analyzed. Notably the overall reliability of texture features was lower than previously reported [12, 21]. This could be due to the selection of lung lesions in our study. In order to represent a realistic scenario encountered in clinical practice, we included various types of lung lesions with heterogeneous appearances. This could have led to a substantial variability of tumor delineation from atelectatic lung tissue, large vessels, and mediastinal structures. When using a − 50 HU threshold, two patients with organizing pneumonia and three patients with carcinoids were excluded from our analysis, because there was no computable lesion with a density above this threshold—an indication of the limitations of using a negative density threshold for the segmentation of sub-solid pulmonary lesions.
Shape parameters had excellent-to-good ICCs in over half of all cases. The highest ICCs for shape parameters were found with 2D segmentation without a HU threshold, the lowest with 3D segmentation with a HU threshold. These results suggest a robust interobserver agreement of lesion delineation for 2D segmentations. An explanation for the lower ICCs in 3D segmentation could be that interobserver variation in lesion delineation increases in 3D due to the higher number of slices on which such delineation must be performed.
We were able to identify a small proportion of texture features (Table 2) that had very high ICC values independent of the segmentation settings. Further investigations may be warranted to determine whether these robust texture features can reliably offer support for the characterization of the lesion of interest.
The foremost limitation of the present study was the small number of patients included. Nevertheless, this is the largest study to date investigating the influence of different segmentation settings on the reliability of radiomics of various lung lesions in a clinical setting.
It was previously shown that many radiomic features are non-reproducible and redundant with different CT acquisition parameters and scanners [28]. Therefore, the present study included only patients who were scanned with the same acquisition parameters and on the same CT scanner. Consequently the results of our trial cannot be generalized for different CT scanner types and image acquisition parameters. To test the robustness of radiomics and advance progress toward routine applicability of CTTA, it would have been helpful to be able to assess the influence of different scanner types and image acquisition parameters in our study.
In conclusion, our study demonstrated low interobserver reliability of CT-derived texture features in histopathologically heterogeneous pulmonary lesions, with overall higher reliability when segmentations were performed in 3D without the use of a HU threshold. A small proportion of texture features with very high interobserver reliability independent of segmentation settings were identified. These results indicate a limited applicability of CTTA and the need to define robust texture features for the characterization of various types of pulmonary lesions.
Abbreviations
- AC:
-
Adenocarcinomas
- CTTA:
-
Computed tomography texture analysis
- ICC:
-
Intraclass correlation coefficient
- LoG:
-
Laplacian of Gaussian
- OP:
-
Organizing pneumonia
- SCLC:
-
Small cell lung cancers
- SQCC:
-
Squamous cell carcinomas
References
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37:1483–1503
Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250:444–452
Lubner MG, Stabo N, Abel EJ, Del Rio AM, Pickhardt PJ (2016) CT textural analysis of large primary renal cell carcinomas: pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. AJR Am J Roentgenol 207:96–105
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim JH, Sohn CH (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Chen CH, Chang CK, Tu CY et al (2018) Radiomic features analysis in computed tomography images of lung nodule classification. PLoS One 13
Zhu X, Dong D, Chen Z et al (2018) Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur Radiol 28:2772–2778
Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT (2004) Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 15:479–483
vanSonnenberg E, Casola G, Ho M et al (1988) Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology 167:457–461
Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T (2002) Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest 121:1159–1164
Arslan S, Yilmaz A, Bayramgurler B, Uzman O, Nver E, Akkaya E 2002 CT-guided transthoracic fine needle aspiration of pulmonary lesions: accuracy and complications in 294 patients. Med Sci Monit 8:CR493–497
Kocak B, Durmaz ES, Kaya OK, Ates E, Kilickesmez O (2019) Reliability of single-slice-based 2D CT texture analysis of renal masses: influence of intra- and interobserver manual segmentation variability on radiomic feature reproducibility. AJR Am J Roentgenol 213:377–383
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22:796–802
Gevaert O, Xu J, Hoang CD et al (2012) Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data–methods and preliminary results. Radiology 264:387–396
Chae HD, Park CM, Park SJ, Lee SM, Kim KG, Goo JM (2014) Computerized texture analysis of persistent part-solid ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology 273:285–293
Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA (2010) Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 10:137–143
Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266:326–336
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Yang F, Simpson G, Young L, Ford J, Dogan N, Wang L (2020) Impact of contouring variability on oncological PET radiomics features in the lung. Sci Rep 10:369
Pavic M, Bogowicz M, Wurms X et al (2018) Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol 57:1070–1074
Nguyen K, Schieda N, James N, McInnes MDF, Wu M, Thornhill RE (2021) Effect of phase of enhancement on texture analysis in renal masses evaluated with non-contrast-enhanced, corticomedullary, and nephrographic phase-enhanced CT images. Eur Radiol 31:1676–1686
Leijenaar RT, Carvalho S, Velazquez ER et al (2013) Stability of FDG-PET Radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol 52:1391–1397
van Velden FH, Kramer GM, Frings V et al (2016) Repeatability of radiomic features in non-small-cell lung cancer [(18)F]FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol 18:788–795
Owens CA, Peterson CB, Tang C et al (2018) Lung tumor segmentation methods: impact on the uncertainty of radiomics features for non-small cell lung cancer. PLoS One 13
Zhao B, Tan Y, Tsai WY et al (2016) Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep 6:23428
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 82:342–348
Berenguer R, Pastor-Juan MDR, Canales-Vazquez J et al (2018) Radiomics of CT features may be nonreproducible and redundant: influence of CT acquisition parameters. Radiology 288:407–415
Acknowledgements
The authors are grateful to Ada Muellner for editing the manuscript.
Funding
Open access funding provided by Medical University of Graz. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dr. Michael Fuchsjaeger.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of the retrospective study design.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective.
• Performed at one institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adelsmayr, G., Janisch, M., Kaufmann-Bühler, AK. et al. CT texture analysis reliability in pulmonary lesions: the influence of 3D vs. 2D lesion segmentation and volume definition by a Hounsfield-unit threshold. Eur Radiol 33, 3064–3071 (2023). https://doi.org/10.1007/s00330-023-09500-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09500-8