Abstract
Objectives
To investigate the diagnostic value of functional MRI to assess renal interstitial fibrosis in patients with chronic kidney disease (CKD).
Methods
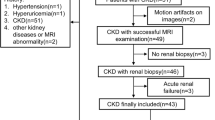
We prospectively recruited 80 CKD patients who underwent renal biopsies and 16 healthy volunteers to undergo multiparametric functional MRI examinations. The Oxford MEST-C classification was used to score the interstitial fibrosis. The diagnostic performance of functional MRI to discriminate interstitial fibrosis was evaluated by calculating the area under the receiver operating characteristic (ROC) curves.
Results
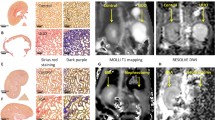
IgA nephropathy (60%) accounted for the majority of pathologic type in the CKD patients. Apparent diffusion coefficient (ADC) from diffusion-weighted imaging (DWI) was correlated with interstitial fibrosis (rho = −0.73). Decreased renal blood flow (RBF) derived from arterial spin labeling (rho = −0.78) and decreased perfusion fraction (f) derived from DWI (rho = −0.70) were accompanied by increased interstitial fibrosis. The T1 value from T1 mapping correlated with interstitial fibrosis (rho = 0.67) (all p < 0.01). The areas under the ROC curve for the discrimination of ≤ 25% vs. > 25% and ≤ 50% vs. > 50% interstitial fibrosis were 0.87 (95% confidence interval, 0.78 to 0.94) and 0.93 (0.86 to 0.98) by ADC, 0.84 (0.74 to 0.91) and 0.94 (0.86 to 0.98) by f, 0.93 (0.85 to 0.98) and 0.90 (0.82 to 0.96) by RBF, and 0.91 (0.83 to 0.96) and 0.77 (0.66 to 0.85) by T1, respectively.
Conclusions
Functional MRI parameters were strongly correlated with the interstitial fibrosis of CKD. Therefore, it might a powerful tool to assess interstitial fibrosis of CKD noninvasively.
Key Points
• In CKD patients, the renal cortical ADC value decreased and T1 value increased significantly compared with healthy volunteers.
• Functional MRI revealed significantly decreased renal perfusion in CKD patients compared with healthy volunteers.
• The renal cortical ADC, f, RBF, and T1 values were strongly correlated with the interstitial fibrosis of CKD.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ASL:
-
Arterial spin labeling
- AUC:
-
Area under the curve
- CKD:
-
Chronic kidney disease
- DWI:
-
Diffusion-weighted imaging
- IF:
-
Interstitial fibrosis
- IVIM:
-
Intravoxel incoherent motion
- pCASL:
-
Pseudo-continuous arterial spin labeling
- RBF:
-
Renal blood flow
- ROC:
-
Receiver operating characteristic
- ROIs:
-
Regions of interest
References
Levey AS, Atkins R, Coresh J et al (2007) Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72:247–259
Eddy AA (2011) (2014) Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl 4:2–8
Luciano RL, Moeckel GW (2019) Update on the native kidney biopsy: Core Curriculum 2019. Am J Kidney Dis 73:404–415
Cheng O, Thuillier R, Sampson E et al (2006) Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant 6:2292–2306
Papasotiriou M, Genovese F, Klinkhammer BM et al (2015) Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol Dial Transplant 30:1112–1121
Morrell GR, Zhang JL, Lee VS (2017) Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol 28:2564–2570
Cutajar M, Thomas DL, Hales PW, Banks T, Clark CA, Gordon I (2014) Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: quantification and reproducibility. Eur Radiol 24:1300–1308
Wang W, Yu Y, Wen J et al (2019) Combination of functional magnetic resonance imaging and histopathologic analysis to evaluate interstitial fibrosis in kidney allografts. Clin J Am Soc Nephrol 14:1372–1380
Lu F, Yang J, Yang S et al (2021) Use of three-dimensional arterial spin labeling to evaluate renal perfusion in patients with chronic kidney disease. J Magn Reson Imaging 54:1152–1163
Thoeny HC, Zumstein D, Simon-Zoula S et al (2006) Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology 241:812–821
Thoeny HC, De Keyzer F, Oyen RH, Peeters RR (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M (1999) Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 9:832–837
Mao W, Zhou J, Zeng M et al (2018) Chronic kidney disease: pathological and functional evaluation with intravoxel incoherent motion diffusion-weighted imaging. J Magn Reson Imaging 47:1251–1259
Cox EF, Buchanan CE, Bradley CR et al (2017) Multiparametric renal magnetic resonance imaging: validation, interventions, and alterations in chronic kidney disease. Front Physiol 8:696
Gillis KA, McComb C, Patel RK et al (2016) Non-contrast renal magnetic resonance imaging to assess perfusion and corticomedullary differentiation in health and chronic kidney disease. Nephron 133:183–192
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Robson PM, Madhuranthakam AJ, Dai W, Pedrosa I, Rofsky NM, Alsop DC (2009) Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med 61:1374–1387
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83:756–762
Trimarchi H, Barratt J, Cattran DC et al (2017) Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 91:1014–1021
Peperhove M, Vo CV, Jang MS et al (2018) Assessment of acute kidney injury with T1 mapping MRI following solid organ transplantation. Eur Radiol 28:44–50
Ren T, Wen CL, Chen LH et al (2016) Evaluation of renal allografts function early after transplantation using intravoxel incoherent motion and arterial spin labeling MRI. Magn Reson Imaging 34:908–914
Graham-Brown MP, Singh A, Wormleighton J et al (2019) Association between native T1 mapping of the kidney and renal fibrosis in patients with IgA nephropathy. BMC Nephrol 20:256
Zhao J, Wang ZJ, Liu M et al (2014) Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol 69:1117–1122
Chin CL, Wehrli FW, Hwang SN, Takahashi M, Hackney DB (2002) Biexponential diffusion attenuation in the rat spinal cord: computer simulations based on anatomic images of axonal architecture. Magn Reson Med 47:455–460
Luciani A, Vignaud A, Cavet M et al (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology 249:891–899
Cai XR, Yu J, Zhou QC, Du B, Feng YZ, Liu XL (2016) Use of intravoxel incoherent motion MRI to assess renal fibrosis in a rat model of unilateral ureteral obstruction. J Magn Reson Imaging 44:698–706
Afsar B, Afsar RE, Dagel T et al (2018) Capillary rarefaction from the kidney point of view. Clin Kidney J 11:295–301
Ehling J, Babickova J, Gremse F et al (2016) Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27:520–532
Schmidbauer M, Rong S, Gutberlet M et al (2021) Diffusion-weighted imaging and mapping of T1 and T2 relaxation time for evaluation of chronic renal allograft rejection in a translational mouse model. J Clin Med 10:4318
O'Connor JP, Jackson A, Buonaccorsi GA et al (2007) Organ-specific effects of oxygen and carbogen gas inhalation on tissue longitudinal relaxation times. Magn Reson Med 58:490–496
Jones RA, Ries M, Moonen CT, Grenier N (2002) Imaging the changes in renal T1 induced by the inhalation of pure oxygen: a feasibility study. Magn Reson Med 47:728–735
Wu J, Shi Z, Zhang Y et al (2021) Native T1 mapping in assessing kidney fibrosis for patients with chronic glomerulonephritis. Front Med (Lausanne) 8:772326
Hall ME, Rocco MV, Morgan TM et al (2016) Beta-blocker use is associated with higher renal tissue oxygenation in hypertensive patients suspected of renal artery stenosis. Cardiorenal Medicine 6:261–268
Pruijm M, Milani B, Burnier M (2016) Blood oxygenation level-dependent MRI to assess renal oxygenation in renal diseases: progresses and challenges. Front Physiol 7:667
Funding
This work was supported by the Science and Technology Guided Project of Fujian Province (grant number: 2019D025); National Natural Science Foundation of China (grant number: 82171897); Shanghai Science and Technology Committee (grant number: 19411965500); Shanghai Municipal Key Clinical Specialty (grant number: shslczdzk03202); Clinical Research Plan of SHDC (grant number: SHDC2020CR1029B); Clinical Research Project of Zhongshan Hospital, Fudan University (grant number: 2020ZSLC61); and “Science and Technology Innovation Action Plan” Star Project/Star Cultivation (Sailing Special Project) (grant number: 22YF1443700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jianjun Zhou.
Conflict of interest
Authors Caixia Fu, Bernd Kuehn, Thomas Benkert, Robert Grimm, Dominik Nickel are employees of Siemens. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic study/observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, W., Ding, X., Ding, Y. et al. Evaluation of interstitial fibrosis in chronic kidney disease by multiparametric functional MRI and histopathologic analysis. Eur Radiol 33, 4138–4147 (2023). https://doi.org/10.1007/s00330-022-09329-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09329-7