Abstract
Objectives
Quantitative MRI techniques, such as diffusion microstructure imaging (DMI), are increasingly applied for advanced tissue characterization. We determined its value in rotator cuff (RC) muscle imaging by studying the association of DMI parameters to isometric strength and fat fraction (FF).
Methods
Healthy individuals prospectively underwent 3T-MRI of the shoulder using DMI and chemical shift encoding-based water-fat imaging. RC muscles were segmented and quantitative MRI metrics (V-ISO, free fluid; V-intra, compartment inside of muscle fibers; V-extra, compartment outside of muscle fibers, and FF) were extracted. Isometric shoulder strength was quantified using specific clinical tests. Sex-related differences were assessed with Student’s t. Association of DMI-metrics, FF, and strength was tested. A factorial two-way ANOVA was performed to compare the main effects of sex and external/internal strength-ratio and their interaction effects on quantitative imaging parameters ratios of infraspinatus/subscapularis.
Results
Among 22 participants (mean age: 26.7 ± 3.1 years, 50% female, mean BMI: 22.6 ± 1.9 kg/m2), FF of the individual RC muscles did not correlate with strength or DMI parameters (all p > 0.05). Subjects with higher V-intra (r = 0.57 to 0.87, p < 0.01) and lower V-ISO (r = −0.6 to −0.88, p < 0.01) had higher internal and external rotation strength. Moreover, V-intra was higher and V-ISO was lower in all RC muscles in males compared to female subjects (all p < 0.01). There was a sex-independent association of external/internal strength-ratio with the ratio of V-extra of infraspinatus/subscapularis (p = 0.02).
Conclusions
Quantitative DMI parameters may provide incremental information about muscular function and microstructure in young athletes and may serve as a potential biomarker.
Key Points
• Diffusion microstructure imaging was successfully applied to non-invasively assess the microstructure of rotator cuff muscles in healthy volunteers.
• Sex-related differences in the microstructural composition of the rotator cuff were observed.
• Muscular microstructural metrics correlated with rotator cuff strength and may serve as an imaging biomarker of muscular integrity and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantitative MRI techniques are increasingly applied to non-invasively assess the structural integrity of muscles. Chemical shift encoding-based water-fat MRI allows the evaluation of the degree of muscle fatty infiltration (fat fraction, FF) and may play a role in the context of diagnosis and management of degenerative diseases, including planning and outcome assessment of surgical therapies [1]. More recently, several quantitative MRI techniques have been evaluated for trauma and therapy monitoring of muscle injuries in athletes. T2 relaxation time measurements have been applied for the assessment of edema development in delayed-onset muscle soreness (DOMS) [2, 3]. Intravoxel incoherent motion (IVIM) MRI has been used for measurement of microvascular muscle perfusion and visualization of muscle activation in walking and running [4].
Due to the highly organized macro- and microstructure, a directional diffusivity similar to that of neuronal axons is found in muscle [5]. As the sizes of myofibrils (1–2 μm) and neuronal axons (0.01 to 10 μm) are in a similar range [6, 7], diffusion-based MRI (dMRI) techniques such as diffusion tensor imaging (DTI) may provide additional information on structural and microstructural alterations of skeletal muscle before they become visible on morphologic MRI [8,9,10]. DTI metrics provide insight into the microstructure as they reveal subvoxel information in a mesoscopic approach [11, 12]. Elevated diffusivity was described in inflamed muscle, and reduced diffusivity and less anisotropic diffusion were found in muscles with fatty infiltration [13, 14]. Moreover, DTI has been applied to assess muscle tears in athletes [15, 16].
Recently, advanced multicompartment techniques like diffusion microstructure imaging (DMI) have provided substantial additional value in neuroimaging and allowed for even more specific insights into the microstructural integrity of tissue compared to DTI [17,18,19,20]. Rather than only providing information on the directionality and orientation of the diffusivity within a voxel as in DTI, DMI approximates the distribution of three microstructural compartments per voxel.
For the quantitative assessment of rotator cuff (RC) muscles, advanced quantitative MR imaging techniques are of major interest [1, 12, 21,22,23]. Quantitative muscle MR measurements may be important for treatment decisions with respect to the operative treatment technique [1, 21]. In young, active patients, decentering of the shoulder often occurs due to weakness of the external rotators. These patients require strengthening of the RC to address external rotation strength imbalances for injury prevention [24,25,26]. MRI may help to detect and monitor such insufficiencies and contribute to evaluate physical therapy success and decision-making on return-to-play in athletes.
Therefore, the purpose of this study was (i) to demonstrate the feasibility of DMI of RC muscles and provide an initial estimate of normal values, (ii) to assess the association with MR imaging-based FF measurements, and (iii) to correlate DMI with isometric strength measurements in healthy volunteers.
Material and methods
Study participants
This prospective study was approved by the local Institutional Review Board (EK:1446/21). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study. Exclusion criteria were age < 18 years, previous shoulder surgery, and neurologic, or muscular disorders.
Magnetic resonance imaging
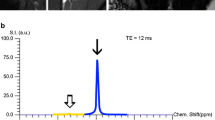
MR imaging of the right shoulder was performed at a 3.0 T MR scanner (Magnetom Vida, Siemens Healtineers) using a dedicated 16-channel shoulder coil (Shoulder Shape 16, Siemens Healtineers). The arm was positioned in neutral zero position. For chemical shift encoding-based water-fat separation of the RC, a transverse-prescribed two-point T1w Dixon sequence was acquired with the following parameters: repetition time (TR) = 4.2 ms, echo times (TE) = 1.23 and 2.46 ms, flip angle = 5°, GRAPPA factor = 2, voxel size = 0.7 × 0.7 × 3.0 mm3, phase encoding direction = A/P, consecutive slices = 48, scan time = 54 s. The dMRI sequence was adapted from the previously published brain-specific sequence [19] to address the specific requirements for dMRI in the musculature [27,28,29] resulting in the following parameters: transverse orientation, 23 consecutive slices, voxel size = 2.0 × 2.0 × 4 mm3, TR = 4500 ms, TE = 90 ms, bandwidth = 2222 Hz/Px, GRAPPA factor = 2, 12 diffusion-encoding gradient directions, b-factors = 600 and 1000 s/mm2; scan time = 5:35 min.
Semiquantitative MR analysis
MR images were transferred on Picture Archiving Communication System workstations (Deep Unity, Dedalus HealthCare) and evaluated semiquantitatively by 2 radiologists in consensus with 3 and 7 years of experience in musculoskeletal imaging, respectively. The in-phase Dixon images were used for semiquantitative evaluation of RC muscle atrophy and fatty infiltration. Goutallier score was employed for assessment of fatty infiltration [30]. The Cofield score was used for semiquantitative evaluation of muscle atrophy [31]. Additionally, the presence of partial or complete RC tear was excluded on standard clinical proton density-weighted fat-saturated sequences which were assessed in transverse, coronal, and sagittal orientation.
Quantitative MRI parameter calculation
To investigate the fat-water composition of the RC muscles, the fat fraction (FF) was derived from the Dixon sequence as the ratio of the fat signal over the sum of fat and water signals. The DMI parameters of the RC muscles were calculated within our in-house post-processing platform NORA (www.nora-imaging.org). Pre-processing of diffusion-weighted images included a denoising step [32] followed by correction of the Gibbs-ringing artifacts [33] and upsampling to an isotropic resolution of 2.0 mm3. Microstructural diffusion metrics were estimated using a Bayesian approach [34] and three volume fractions were determined adapted from the brain’s white matter standard model: (1) the free water fraction (V-ISO) in that molecules randomly move at the distance of their diffusion length (in the range of a tenth of micrometers); (2) the volume fraction within muscular fibers with almost one-dimensional molecule diffusion due to tight membrane borders of the sarcolemma (V-intra); (3) the volume fraction outside of muscular fibers (V-extra) is characterized by an intermediate constraint to molecule diffusion representing intramuscular connective tissues such as endo- and perimysium.
Volume of interest (VOI)–based analysis
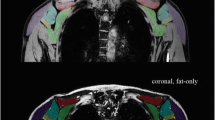
Supraspinatus (SSP), subscapularis (SSC), and infraspinatus (ISP) were manually segmented on the opposed-phase Dixon sequence along the muscular borders by one trained radiologist (3 years of experience in musculoskeletal radiology, Fig. 1). Tendinous structures and the investing fascia were carefully excluded from segmentation. VOIs were subsequently overlaid on FF maps and on the b = 0 images to approve correct alignment. DMI-derived parameters and FF were extracted from the VOIs.
Isometric strength measurements
Shoulder abduction, internal rotation, and external rotation isometric strengths were measured (in kilogram) bilaterally by using an IsoForceControl Evo2 dynamometer (MDS Medical Device Solutions) with 33 measurements per second for a 5-s period. The dynamometer cuff was positioned just proximal to the wrist. For SSP function, strength testing of abduction was assessed with the participant in a seated position and the arm at 90° of elevation in the scapular plane, and the forearm in pronation (Jobe test position). In addition, a strength measurement with the patient in a standing position and the arm in 0° abduction was obtained (Starter test position). For ISP and SSC analyses, measurements of internal and external rotation strength were performed at 0° of abduction, maintaining the elbow in 90° flexion and the wrist at neutral position. The mean and maximum value within the 5-s period of the second measurement of each shoulder was used for data analysis. The ratios of the mean and maximum strength of external/internal rotation (ER/IR) were calculated [25].
Statistical analysis
Shapiro–Wilk test was used to assess the normal distribution of data. Sex-related differences in DMI-parameters, FF, and strength between male and female participants were assessed with Student’s t. Pearson’s and partial Spearman’s (controlling for sex) correlation were employed to relate DMI-metrics, FF, and strength. To investigate microstructural correlates of muscular dysbalance in the RC, we split our cohort with an ER/IR strength ratio cutoff of 0.95 [25, 35,36,37]. Similar to the ER/IR strength ratio, we calculated a corresponding ratio of the quantitative MRI parameters of ISP/SCC. A factorial two-way ANOVA was performed to compare the main effects of sex and ER/IR strength ratio and their interaction effects on quantitative parameters ISP/SSC ratios. Continuous parametric variables are reported as median and range or mean and SD, and values with an α-level of 0.05 were considered statistically significant. Since the analyses were mainly explorative, p-values were not adjusted for multiple testing.
All statistical analyses were performed using R statistics (R-3.5.3 – R Core Team, https://www.R-project.org).
Results
Study participants
A total of 22 healthy volunteers (mean age 26.7 ± 3.1 years; 11 male, 11 female) were included in this study. There was a statistically significant difference for male and female participants in BMI (23.6 ± 1.8 kg/m2 vs. 21.6 ± 1.5 kg/m2; p = 0.01) but not in age (26.3 ± 1.8 years vs. 27.1 ± 3.5 years; p = 0.55).
Strength measurements
Strength measurements revealed statistically significant higher values for mean and maximum strength for the Jobe test, starter test, internal rotation, and external rotation in males (all p = < 0.001; Table 1). Ipsilateral and contralateral strength and ER/IR strength ratios were not different (all p > 0.05). Ipsilateral and contralateral strength correlated significantly (all p < 0.001).
Semiquantitative RC assessment
None of the participants showed muscle atrophy (all Cofield grade 0). Regarding fatty infiltration of the RC muscles, n = 8 (5 female) showed Goutallier grade 1. All other participants (n = 14) had a Goutallier grade of 0. All volunteers had no RC tear.
Quantitative MR imaging
Quantitative image parameters were successfully calculated in all subjects (an exemplary case is depicted in Fig. 2). Mean FF for the SSP was 18.5% ± 19.8%, for the ISP 5.8% ± 10.7%, and for the SSC 5.7% ± 11.3%. Although FF was lower in males as compared to females for all analyzed RC muscles, the difference was not statistically significant (mean of all muscles was 6.3% ± 10.3% in males vs. 12.5% ± 18.6% in females, p > 0.05; see Table 1).
Detailed descriptives of the microstructural DMI parameters V-intra, V-extra, and V-ISO are displayed in Table 1. V-intra was significantly higher in all RC muscles of male compared to female subjects (in contrast, V-ISO was significantly lower in the respective RC muscles of male participants compared to females). No statistically significant sex difference was found for V-extra (all p > 0.05). Please see Fig. 3 for some exemplary cases.
Quantitative DMI parameters of the SSC and ISP in an exemplary male (left column) and female participant (right column). V-intra (A, B) was higher and V-ISO (A’, B’) was lower in all RC muscles in male (left column) compared to female subjects (right columns). Calculated V-intra (A, B) and V-ISO (A’, B’) color maps overlaid onto the water image of T1w Dixon. Cold color indicates low, and hot color indicates high values
Association of FF and DMI parameters with strength
Mean FF of the individual RC muscles did not correlate with strength or DMI parameters (all p > 0.05). A summary of the associations of DMI and FF parameters with strength is shown in Fig. 4. For SSC and ISP, higher V-intra was significantly correlated with higher maximum strength (p < .001 and p = 0.008). Further, there was a statistical significance for the correlation of lower V-ISO with a higher maximum strength of the SSC (p < 0.001), ISP (p = 0.003), and SSP (p = 0.04).
Controlling for sex, we observed significant associations of SSC V-intra and maximum strength (rho 0.52, p = 0.01) and SSC V-ISO and maximum strength (rho 0.65, p = 0.003) as well as SSP V-extra and mean strength (rho 0.46, p = 0.04).
Relationship of sex, ER/IR strength ratio, and quantitative MRI parameters
For the V-extra ISP/SSC ratio, the main effect for ER/IR strength ratio yielded an F ratio F(1, 16) = 6.59, p = 0.02, indicating a significant difference between the subgroup with an ER/IR ratio > 0.95 vs. the subgroup with a ratio < 0.95. The main effect of sex yielded an F ratio F(1, 16) = 0.11, p = 0.74, indicating that the effect of sex was not significant. The interaction of sex and the ER/IR strength ratio was not significant (p = 0.66). No significant ER/IR strength ratio differences were observed for V-intra, V-ISO, or FF.
Discussion
In this study, we have used specifically adapted, advanced quantitative MRI techniques to assess the correlation between fat-water and microstructural composition of RC muscles and their associations with muscle strength in young healthy individuals. We found significant correlations between DMI parameters and the mean and maximum strength of the respective RC muscles with the strongest effects in V-intra and V-ISO. Further, there were significant sex differences in the DMI parameters V-intra and V-ISO. In contrast, the mean FF of the individual RC muscles did not correlate with individual strength. Therefore, our results indicate that multicompartmental DMI measurements could provide more detailed information about RC muscle composition and function than FF in young healthy individuals.
Preoperative characterization of RC muscle quality is crucial for surgical treatment planning and postoperative outcome. In addition, muscle quality is important for proper function in healthy individuals and athletes. MR imaging may help to detect muscle weakness leading to decentering of the shoulder and may potentially be able to monitor the effects of physical therapy. Increasingly applied quantitative MRI techniques may overcome the current problem of high interobserver variability in classic qualitative MRI [38, 39]. Previous studies showed correlations of RC muscle FF with the semi-quantitative Goutallier score [30, 40]. Further, Karampinos et al showed significant inverse correlations of RC FF with isometric strength measurements 10 years after RC repair in patients with a mean age of 72 years [40]. A similar finding was reported by Davis et al in a mixed study population of patients with and without full-thickness RC tears and a combined mean age of 62.2 years [41]. In contrast, no significant correlation between RC muscle FF and strength was found in the present study. This may be due to the notably younger study population (mean age 26.7 years) without relevant fatty infiltration. Most interestingly, despite the lack of correlations with FF, we observed significant correlations of strength with DMI parameters.
Previously, advanced dMRI techniques have been assessed for potential applications in shoulder imaging. In a case series of two patients after RC tendon repair, Fieremans et al found myofiber size reduction in SSP and ISP using time-dependent DTI measurements and a random permeable barrier model [27]. Moreover, age-associated changes and variances of DTI parameters among visually intact RC have been shown in a cohort of 12 females (mean age 44.3 years) and 28 males (mean age 39.4 years) [12]. The same study reported a low but significant correlation of DTI parameters with FF. In contrast to this, we found no significant correlation between DMI parameters and RC FF. In combination with the fact that we nevertheless found correlations between DMI parameters and RC strength, this suggests that DMI parameters may provide additional, FF-independent information on muscle quality. However, in our cohort of young individuals with a narrow BMI range, most correlations of DMI parameters and RC strength attenuated when controlling for sex. Therefore, larger studies are required to assess influence factors such as sex, age, BMI, and metabolic factors as well as morphological alterations of the RC on DMI parameters. Of note, DMI parameters showed a significant sex difference. Most likely the lacking sex difference of the FF may become evident in a larger cohort, while the difference was already evident for DMI in the analyzed cohort with n = 11 subjects of each sex. This finding underlines that DMI may be more sensitive than FF to interpersonal differences in muscle composition.
In neuroimaging, multicompartment dMRI-based approaches were found to offer more specific metrics and to be more sensitive to pathological tissue alterations than single-compartment DTI [18, 42]. Similar assumptions are reasonable for musculoskeletal radiology. Karampinos and colleagues assumed two main compartments contributing to the dMRI signal, i.e., the intracellular (within the muscle fiber) and the extracellular space (collagenous intramuscular connective tissues consisting of endomysium and perimysium) [43]. Tan et al. assessed the fiber diameter in patients with muscle denervation at the shoulder based on the diffusion signal and observed a more robust depiction of the microstructural changes in comparison to DTI [28]. Our DMI approach further considers an additional free fluid compartment (V-ISO). In a previous study, our DMI approach was reported to be highly sensitive to discrete shifts of microstructural compartments otherwise not detectable on conventional MRI [19]. DMI was also more sensitive in detecting pathological changes in brain tissue in the context of neurodegeneration than DTI [18].
Comparable to neuronal axons, the myofiber is a complex, but highly organized structure that is composed of myofibrils. Myofiber hypertrophy is a well-known adaptation of skeletal muscle to repetitive exercise to increase muscle strength and performance [44,45,46]. In line with this, we here report that higher V-intra and lower V-ISO — which corresponds to a larger proportion of myofibrils per voxel — were associated with higher mean and maximum muscle strength. Therefore, DMI as a novel imaging tool may serve as an imaging biomarker on microstructural integrity or changes in the myofiber fraction before being detectable on conventional MRI. Interestingly, the V-extra ISP/SSC ratio differed significantly between the groups with and without RC external rotation imbalance. DMI may therefore be a valuable tool for the assessment of RC imbalance and non-invasive monitoring of therapy outcomes in athletes with external rotation weakness.
The value of DMI in monitoring physical therapy, as well as evaluating pathological conditions such as trauma, DOMS, or muscle tears, has yet to be evaluated in further prospective studies with larger sample sizes of different age groups. We believe that DMI could serve as an additional non-invasive method to detect early degenerative changes of the RC muscles in elderly patients.
Our study has several limitations. First, our results are limited by the relatively small sample size and the shoulder as the only region investigated, therefore requiring confirmation in larger longitudinal cohort studies. In addition, further adaptation of the DMI model to muscle tissue is needed as the employed Bayesian approach was primarily developed for brain tissue. For this reason, a publicly accessible toolbox is not yet available.
Our MR-based results of FF and DMI measurements were not validated by histopathological correlation which is still considered the gold standard for quantification of fat content and muscle quality assessment. However, previous studies have demonstrated the validity and reproducibility of a standardized MRI-based skeletal muscle FF quantification with good concordance to histology [47].
In this proof-of-concept study, we successfully applied DMI to musculoskeletal imaging. In conclusion, DMI allows for a non-invasive quantitative approximation of muscle microstructure in vivo and may provide further insight into muscular integrity, function, and imbalances in healthy individuals and young athletes.
Abbreviations
- BMI:
-
Body mass index
- DMI:
-
Diffusion microstructure imaging
- dMRI:
-
Diffusion MRI
- DTI:
-
Diffusion tensor imaging
- ER:
-
External rotation
- FF:
-
Fat fraction
- IR:
-
Internal rotation
- ISP:
-
Infraspinatus
- RC:
-
Rotator cuff
- SSC:
-
Subscapularis
- SSP:
-
Supraspinatus
- V-extra:
-
Compartment outside of muscle fibers
- V-intra:
-
Compartment inside of muscle fibers
- V-ISO:
-
Free fluid compartment
References
Lansdown DA, Morrison C, Zaid MB et al (2019) Preoperative IDEAL (Iterative Decomposition of Echoes of Asymmetrical Length) magnetic resonance imaging rotator cuff muscle fat fractions are associated with rotator cuff repair outcomes. J Shoulder Elbow Surg 28:1936–1941. https://doi.org/10.1016/j.jse.2019.05.018
Heiss R, Hotfiel T, Kellermann M et al (2018) Effect of compression garments on the development of edema and soreness in delayed-onset muscle soreness (DOMS). J Sports Sci Med 17:392–401
Hotfiel T, Höger S, Nagel AM et al (2021) Multi-parametric analysis of below-knee compression garments on delayed-onset muscle soreness. Int J Environ Res Public Health 18:3798. https://doi.org/10.3390/ijerph18073798
Jungmann PM, Pfirrmann C, Federau C (2019) Characterization of lower limb muscle activation patterns during walking and running with intravoxel incoherent motion (IVIM) MR perfusion imaging. Magn Reson Imaging 63:12–20. https://doi.org/10.1016/j.mri.2019.07.016
Sinha S, Sinha U, Edgerton VR (2006) In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging 24:182–190. https://doi.org/10.1002/jmri.20593
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698. https://doi.org/10.1152/physrev.00031.2003
Perge JA, Niven JE, Mugnaini E et al (2012) Why do axons differ in caliber? J Neurosci 32:626–638. https://doi.org/10.1523/JNEUROSCI.4254-11.2012
Hooijmans MT, Damon BM, Froeling M et al (2015) Evaluation of skeletal muscle DTI in patients with duchenne muscular dystrophy. NMR Biomed 28:1589–1597. https://doi.org/10.1002/nbm.3427
Breckwoldt MO, Stock C, Xia A et al (2015) Diffusion tensor imaging adds diagnostic accuracy in magnetic resonance neurography. Invest Radiol 50:498–504. https://doi.org/10.1097/RLI.0000000000000156
Forsting J, Rohm M, Froeling M et al (2021) High inter-rater reliability of manual segmentation and volume-based tractography in healthy and dystrophic human calf muscle. Diagnostics (Basel) 11:1521. https://doi.org/10.3390/diagnostics11091521
Malis V, Sinha U, Csapo R et al (2019) Diffusion tensor imaging and diffusion modeling: application to monitoring changes in the medial gastrocnemius in disuse atrophy induced by unilateral limb suspension. J Magn Reson Imaging 49:1655–1664. https://doi.org/10.1002/jmri.26295
Kälin PS, Huber FA, Hamie QM et al (2019) Quantitative MRI of visually intact rotator cuff muscles by multiecho Dixon-based fat quantification and diffusion tensor imaging. J Magn Reson Imaging 49:109–117. https://doi.org/10.1002/jmri.26223
Williams SE, Heemskerk AM, Welch EB et al (2013) Quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J Magn Reson Imaging 38:1292–1297. https://doi.org/10.1002/jmri.24045
Chalela JA, Kidwell CS, Nentwich LM et al (2007) Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369:293–298. https://doi.org/10.1016/S0140-6736(07)60151-2
Biglands JD, Grainger AJ, Robinson P et al (2020) MRI in acute muscle tears in athletes: can quantitative T2 and DTI predict return to play better than visual assessment? Eur Radiol 30:6603–6613. https://doi.org/10.1007/s00330-020-06999-z
Giraudo C, Motyka S, Weber M et al (2018) Normalized STEAM-based diffusion tensor imaging provides a robust assessment of muscle tears in football players: preliminary results of a new approach to evaluate muscle injuries. Eur Radiol 28:2882–2889. https://doi.org/10.1007/s00330-017-5218-9
Rau A, Reisert M, Kellner E et al (2021) Increased interstitial fluid in periventricular and deep white matter hyperintensities in patients with suspected idiopathic normal pressure hydrocephalus. Sci Rep 11:19552. https://doi.org/10.1038/s41598-021-98054-0
Rau A, Jost WH, Demerath T, et al (2022) Diffusion microstructure imaging in progressive supranuclear palsy: reduced axonal volumes in the superior cerebellar peduncles, dentato-rubro-thalamic tracts, ventromedial thalami, and frontomesial white matter. Cereb Cortex bhac041. https://doi.org/10.1093/cercor/bhac041
Rau A, Schroeter N, Blazhenets G et al (2022) Widespread white matter oedema in subacute COVID-19 patients with neurological symptoms. Brain awac045. https://doi.org/10.1093/brain/awac045
Würtemberger U, Diebold M, Erny D et al (2022) Diffusion microstructure imaging to analyze perilesional T2 signal changes in brain metastases and glioblastomas. Cancers 14:1155. https://doi.org/10.3390/cancers14051155
Oh JH, Rhee SM, Park JH et al (2022) Quantitative magnetic resonance imaging assessment of the infraspinatus and teres minor in massive rotator cuff tear and its significance in clinical outcome after rotator cuff repair. J Shoulder Elbow Surg 31:56–62. https://doi.org/10.1016/j.jse.2021.06.001
Nguyen A, Ledoux J-B, Omoumi P et al (2017) Selective microvascular muscle perfusion imaging in the shoulder with intravoxel incoherent motion (IVIM). Magn Reson Imaging 35:91–97. https://doi.org/10.1016/j.mri.2016.08.005
Nguyen A, Ledoux J-B, Omoumi P et al (2016) Application of intravoxel incoherent motion perfusion imaging to shoulder muscles after a lift-off test of varying duration. NMR Biomed 29:66–73. https://doi.org/10.1002/nbm.3449
Andersson SH, Bahr R, Clarsen B, Myklebust G (2017) Preventing overuse shoulder injuries among throwing athletes: a cluster-randomised controlled trial in 660 elite handball players. Br J Sports Med 51:1073–1080. https://doi.org/10.1136/bjsports-2016-096226
Byram IR, Bushnell BD, Dugger K et al (2010) Preseason shoulder strength measurements in professional baseball pitchers: identifying players at risk for injury. Am J Sports Med 38:1375–1382. https://doi.org/10.1177/0363546509360404
Asker M, Waldén M, Källberg H et al (2020) Preseason clinical shoulder test results and shoulder injury rate in adolescent elite handball players: a prospective study. J Orthop Sports Phys Ther 50:67–74. https://doi.org/10.2519/jospt.2020.9044
Fieremans E, Lemberskiy G, Veraart J et al (2017) In vivo measurement of membrane permeability and myofiber size in human muscle using time-dependent diffusion tensor imaging and the random permeable barrier model. NMR Biomed 30. https://doi.org/10.1002/nbm.3612
Tan ET, Zochowski KC, Sneag DB (2022) Diffusion MRI fiber diameter for muscle denervation assessment. Quant Imaging Med Surg 12:80–94. https://doi.org/10.21037/qims-21-313
Oudeman J, Nederveen AJ, Strijkers GJ et al (2016) Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J Magn Reson Imaging 43:773–788. https://doi.org/10.1002/jmri.25016
Nardo L, Karampinos DC, Lansdown DA et al (2014) Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging 39:1178–1185. https://doi.org/10.1002/jmri.24278
Cofield RH (1982) Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet 154:667–672
Veraart J, Novikov DS, Christiaens D et al (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. https://doi.org/10.1016/j.neuroimage.2016.08.016
Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76:1574–1581. https://doi.org/10.1002/mrm.26054
Reisert M, Kellner E, Dhital B et al (2017) Disentangling micro from mesostructure by diffusion MRI: a Bayesian approach. Neuroimage 147:964–975. https://doi.org/10.1016/j.neuroimage.2016.09.058
Donatelli R, Ellenbecker TS, Ekedahl SR et al (2000) Assessment of shoulder strength in professional baseball pitchers. J Orthop Sports Phys Ther 30:544–551. https://doi.org/10.2519/jospt.2000.30.9.544
Magnusson SP, Gleim GW, Nicholas JA (1994) Shoulder weakness in professional baseball pitchers. Med Sci Sports Exerc 26:5–9
Sirota SC, Malanga GA, Eischen JJ, Laskowski ER (1997) An eccentric- and concentric-strength profile of shoulder external and internal rotator muscles in professional baseball pitchers. Am J Sports Med 25:59–64. https://doi.org/10.1177/036354659702500111
Spencer EE, Dunn WR, Wright RW et al (2008) Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med 36:99–103. https://doi.org/10.1177/0363546507307504
Slabaugh MA, Friel NA, Karas V et al (2012) Interobserver and intraobserver reliability of the Goutallier classification using magnetic resonance imaging: proposal of a simplified classification system to increase reliability. Am J Sports Med 40:1728–1734. https://doi.org/10.1177/0363546512452714
Karampinos DC, Holwein C, Buchmann S et al (2017) Proton density fat-fraction of rotator cuff muscles is associated with isometric strength 10 years after rotator cuff repair: a quantitative magnetic resonance imaging study of the shoulder. Am J Sports Med 45:1990–1999. https://doi.org/10.1177/0363546517703086
Davis DL, Almardawi R, Henn RF et al (2021) Correlation of quantitative versus semiquantitative measures of supraspinatus intramuscular fatty infiltration to shoulder range of motion and strength: a pilot study. Curr Probl Diagn Radiol 50:629–636. https://doi.org/10.1067/j.cpradiol.2020.06.007
Ogawa T, Hatano T, Kamagata K et al (2021) White matter and nigral alterations in multiple system atrophy-parkinsonian type. NPJ Parkinsons Dis 7:96. https://doi.org/10.1038/s41531-021-00236-0
Karampinos DC, King KF, Sutton BP, Georgiadis JG (2009) Myofiber ellipticity as an explanation for transverse asymmetry of skeletal muscle diffusion MRI in vivo signal. Ann Biomed Eng 37:2532–2546. https://doi.org/10.1007/s10439-009-9783-1
Shamim B, Camera DM, Whitfield J (2021) Myofibre hypertrophy in the absence of changes to satellite cell content following concurrent exercise training in young healthy men. Front Physiol 12:625044. https://doi.org/10.3389/fphys.2021.625044
Blocquiaux S, Gorski T, Van Roie E et al (2020) The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp Gerontol 133:110860. https://doi.org/10.1016/j.exger.2020.110860
Bamman MM, Roberts BM, Adams GR (2018) Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med 8:a029751. https://doi.org/10.1101/cshperspect.a029751
Smith AC, Parrish TB, Abbott R et al (2014) Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve 50:170–176. https://doi.org/10.1002/mus.24255
Acknowledgements
AR and MJ are supported by the Berta-Ottenstein-Programme for Clinician Scientists, Faculty of Medicine, University of Freiburg.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study has received funding by the German Society of Musculoskeletal Radiology (Deutsche Gesellschaft für muskuloskelettale Radiologie; DGMSR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Fabian Bamberg.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rau, A., Jungmann, P.M., Diallo, T.D. et al. Application of diffusion microstructure imaging in musculoskeletal radiology — translation from head to shoulders. Eur Radiol 33, 1565–1574 (2023). https://doi.org/10.1007/s00330-022-09202-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09202-7