Abstract
Objectives
To develop and validate a deep learning imaging signature (DLIS) for risk stratification in patients with multiforme (GBM), and to investigate the biological pathways and genetic alterations underlying the DLIS.
Methods
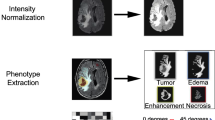
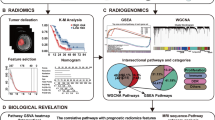
The DLIS was developed from multi-parametric MRI based on a training set (n = 600) and validated on an internal validation set (n = 164), an external test set 1 (n = 100), an external test set 2 (n = 161), and a public TCIA set (n = 88). A co-profiling framework based on a radiogenomics analysis dataset (n = 127) using multiscale high-dimensional data, including imaging, transcriptome, and genome, was established to uncover the biological pathways and genetic alterations underpinning the DLIS.
Results
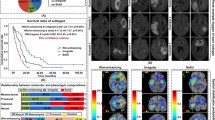
The DLIS was associated with survival (log-rank p < 0.001) and was an independent predictor (p < 0.001). The integrated nomogram incorporating the DLIS achieved improved C indices than the clinicomolecular nomogram (net reclassification improvement 0.39, p < 0.001). DLIS significantly correlated with core pathways of GBM (apoptosis and cell cycle-related P53 and RB pathways, and cell proliferation-related RTK pathway), as well as key genetic alterations (del_CDNK2A). The prognostic value of DLIS-correlated genes was externally confirmed on TCGA/CGGA sets (p < 0.01).
Conclusions
Our study offers a biologically interpretable deep learning predictor of survival outcomes in patients with GBM, which is crucial for better understanding GBM patient’s prognosis and guiding individualized treatment.
Key Points
• MRI-based deep learning imaging signature (DLIS) stratifies GBM into risk groups with distinct molecular characteristics.
• DLIS is associated with P53, RB, and RTK pathways and del_CDNK2A mutation.
• The prognostic value of DLIS-correlated pathway genes is externally demonstrated.






Similar content being viewed by others
Data availability
The RNA-seq and WES data has been deposited into Genome Sequence Archive (GSA) under accession code HRA000802 (https://ngdc.cncb.ac.cn/gsa-human/submit/hra/subHRA001168/finishedOverview), HRA000932 (https://ngdc.cncb.ac.cn/gsa-human/submit/hra/subHRA001358/finishedOverview), and HRA000972 (https://ngdc.cncb.ac.cn/gsa-human/submit/hra/subHRA001402/finishedOverview). The image and survival data from FAHZZU, NFHNFMU, HPH, TAHSYSU, and GGHGMC are not publicly available due to patient privacy constraints, but are available upon reasonable request from the corresponding authors (Zhenyu Zhang, Zhicheng Li, and Jingliang Cheng).
Abbreviations
- AI:
-
Artificial intelligence
- CAM:
-
Class activation map
- CGGA:
-
Chinese Glioma Genome Atlas
- CHT:
-
Chemotherapy
- CI:
-
Confidence interval
- CNN:
-
Convolutional neural network
- CNV:
-
Copy number variation
- DEG:
-
Differentially expressed gene
- DLIS:
-
Deep learning imaging signature
- FAHZZU:
-
First Affiliated Hospital of Zhengzhou University
- FDR:
-
False discovery rate
- GBM:
-
Glioblastoma multiforme
- GGHGMC:
-
Guangzhou General Hospital of Guangzhou Military Command
- GSVA:
-
Gene set variation analysis
- HPH:
-
Henan Provincial Hospital
- HR:
-
Hazard ratio
- IDH:
-
Isocitrate dehydrogenase
- KPS:
-
Karnofsky performance status
- NFHNFMU:
-
Nangfang Hospital of Nangfang Medical University
- NRI:
-
Net reclassification improvement
- OS:
-
Overall survival
- RB:
-
Retinoblastoma
- RNA-seq:
-
RNA sequencing
- RT:
-
Radiation therapy
- RTK:
-
Receptor tyrosine kinase
- SNV:
-
Single nucleotide variant
- TAHSYSU:
-
The 3rd Affiliated Hospital of Sun Yat-Sen University
- TCGA:
-
The Cancer Genome Atlas
- TCIA:
-
The Cancer Imaging Archive
- WES:
-
Whole-exome sequencing
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol 22:iv1-iv96
Stupp R, Mason WP, van den Bent MJ et al (2005) European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Park AK, Kim P, Ballester LY, Esquenazi Y, Zhao Z (2019) Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro Oncol 21:59–70
Sottoriva A, Spiteri I, Piccirillo SG et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A 110:4009–4014
The Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Verhaak RG, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Mazurowski MA (2015) Radiogenomics: what it is and why it is important. J Am Coll Radiol 12:862–866
Shen D, Wu G, Suk HI (2017) Deep learning in medical image analysis. Annu Rev Biomed Eng 19:221–248
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Bismeijer T, van der Velden BH, Canisius S et al (2020) Radiogenomic analysis of breast cancer by linking MRI phenotypes with tumor gene expression. Radiology 296:277–287
Fan M, Xia P, Clarke R, Wang Y, Li L (2020) Radiogenomic signatures reveal multiscale intratumour heterogeneity associated with biological functions and survival in breast cancer. Nat Commun 11:4861
Yan J, Zhang S, Li KK et al (2020) Incremental prognostic value and underlying biological pathways of radiomics patterns in medulloblastoma. EBioMedicine 61:103093
Beig N, Bera K, Prasanna P et al (2020) Radiogenomic-based survival risk stratification of tumor habitat on Gd-T1w MRI is associated with biological processes in glioblastoma. Clin Cancer Res 26:1866–1876
Beig N, Singh S, Bera K et al (2021) Sexually dimorphic radiogenomic models identify distinct imaging and biological pathways that are prognostic of overall survival in Glioblastoma. Neuro Oncol 23:251–263
Sun Q, Chen Y, Liang C et al (2021) Biologic pathways underlying prognostic radiomics phenotypes from paired MRI and RNA sequencing in glioblastoma. Radiology 14:203281
Park JE, Kim HS, Park SY et al (2020) Prediction of core signaling pathway by using diffusion-and perfusion-based MRI radiomics and next-generation sequencing in isocitrate dehydrogenase wild-type glioblastoma. Radiology 294:388–397
Truhn D, Schrading S, Haarburger C, Schneider H, Merhof D, Kuhl C (2019) Radiomic versus convolutional neural networks analysis for classification of contrast-enhancing lesions at multiparametric breast MRI. Radiology 290:290–297
Sun Q, Lin X, Zhao Y et al (2020) Deep learning vs. radiomics for predicting axillary lymph node metastasis of breast cancer using ultrasound images: don’t forget the peritumoral region. Front Oncol 10:53
Yan J, Zhao Y, Chen Y et al (2021) Deep learning features from diffusion tensor imaging improve glioma stratification and identify risk groups with distinct molecular pathway activities. EBioMedicine 72:103583
Tomaszewski MR, Gillies RJ (2021) The biological meaning of radiomic features. Radiology 5:202553
Itakura H, Achrol AS, Mitchell LA, et al (2015) Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci Transl Med 7:303ra138
Bi WL, Hosny A, Schabath MB et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 69:127–157
Kickingereder P, Burth S, Wick A et al (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280:880–889
Kickingereder P, Neuberger U, Bonekamp D et al (2018) Radiomic subtyping improves disease stratification beyond key molecular, clinical, and standard imaging characteristics in patients with glioblastoma. Neuro Oncol 20:848–857
Bae S, Choi YS, Ahn SS, et al (2018) Radiomic MRI Phenotyping of Glioblastoma: Improving Survival Prediction. Radiology 289:797-806
Lao J, Chen Y, Li ZC et al (2017) A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep 7:10353
Han W, Qin L, Bay C et al (2020) Deep transfer learning and radiomics feature prediction of survival of patients with high-grade gliomas. AJNR Am J Neuroradiol 41:40–48
Nie D, Lu J, Zhang H et al (2019) Multi-channel 3D deep feature learning for survival time prediction of brain tumor patients using multi-modal neuroimages. Sci Rep 9:1103
Tang Z, Xu Y, Jin L et al (2020) Deep learning of imaging phenotype and genotype for predicting overall survival time of glioblastoma patients. IEEE Trans Med Imaging 39:2100–2109
Yoon HG, Cheon W, Jeong SW et al (2020) Multi-parametric deep learning model for prediction of overall survival after postoperative concurrent chemoradiotherapy in glioblastoma patients. Cancers (Basel) 12:2284
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Kotliarova S, Fine HA (2012) SnapShot: glioblastoma multiforme. Cancer Cell 21:710–710
Pearson JRD, Regad T (2017) Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther 2:17040
Bangalore Yogananda CG, Shah BR, Vejdani-Jahromi M et al (2020) A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol 22:402–411
Li ZC, Bai H, Sun Q, et al (2018) Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: a multicentre study. Eur Radiol 28:3640–3650
Hong EK, Choi SH, Shin DJ et al (2018) Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur Radiol 28:4350–4361
Chen CH, Chen PY, Lin YY et al (2019) Suppression of tumor growth via IGFBP3 depletion as a potential treatment in glioma. J Neurosurg 132:168–179
Si D, Yin F, Peng J, Zhang G (2020) High expression of CD44 predicts a poor prognosis in glioblastomas. Cancer Manag Res 12:769–775
Shin CH, Robinson JP, Sonnen JA et al (2017) HBEGF promotes gliomagenesis in the context of Ink4a/Arf and Pten loss. Oncogene 36:4610–4618
Kulkarni S, Goel-Bhattacharya S, Sengupta S, Cochran BH (2018) A large-scale RNAi screen identifies SGK1 as a key survival kinase for GBM stem cells. Mol Cancer Res 16:103–114
Chen J, Hou C, Zheng Z, Lin H, Lv G, Zhou D (2019) Identification of secreted phosphoprotein 1 (SPP1) as a prognostic factor in lower-grade gliomas. World Neurosurg 130:e775–e785
Demuth T, Reavie LB, Rennert JL et al (2007) MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther 6:1212–1222
Code availability
The Python codes for implementation of the CNN used to calculate the DLIS score, as well as the validation imaging data, were deposited into a publicly accessible repository available at https://doi.org/10.24433/CO.5979724.v1.
Funding
This study has received funding by the Key-Area Research and Development Program of Guangdong Province (2021B0101420006), the National Natural Science Foundation of China (Nos. U20A20171, 82102149, 61901458, 61571432, 81702465, 8217111948, 82173090, U1804172, U1904148), the Excellent Youth Talent Cultivation Program of Innovation in Health Science and Technology of Henan Province (No. YXKC2022061), the Key Program of Medical Science and Technique Foundation of Henan Province (No. SBGJ202002062), the Joint Construction Program of Medical Science and Technique Foundation of Henan Province (No. LHGJ20190156), the Science and Technology Program of Henan Province (No. 202102310136, 202102310138, 202102310113, 202102310083), Guangdong Basic and Applied Basic Research Foundation (2020B1515120046), and Guangdong Key Project (2018B030335001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhenyu Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Zhi-Cheng Li) has significant statistical expertise.
Informed consent
Written informed consents were obtained from patients whose fresh tumor specimens were used for RNA-seq and WES. For the rest patients, written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multi-center study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(RAR 5.60 MB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, J., Sun, Q., Tan, X. et al. Image-based deep learning identifies glioblastoma risk groups with genomic and transcriptomic heterogeneity: a multi-center study. Eur Radiol 33, 904–914 (2023). https://doi.org/10.1007/s00330-022-09066-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09066-x