Abstract
Objectives
In patients with rectal cancer, the size and location of lateral lymph nodes (LLNs) are correlated to increased lateral local recurrence rates. Sufficient knowledge and accuracy when measuring these features are therefore essential. The objective of this study was to evaluate the variation in measurements and anatomical classifications of LLNs before and after training.
Methods
Fifty-three Dutch radiologists examined three rectal MRI scans and completed a questionnaire. Presence, location, size, and suspiciousness of LLNs were reported. This assessment was repeated after a 2-hour online training by the same radiologists with the same three cases plus three additional cases. Three expert radiologists independently evaluated these 6 cases and served as the standard of reference.
Results
Correct identification of the anatomical location improved in case 1 (62 to 77% (p = .077)) and in case 2 (46 to 72% (p = .007)) but decreased in case 3 (92 to 74%, p = .453). Compared to the first three cases, cases 4, 5, and 6 all had a higher initial consensus of 73%, 79%, and 85%, respectively. The mean absolute deviation of the short-axis measurements in cases 1–3 were closer—though not significantly—to the expert reference value after training with reduced ranges and standard deviations. Subjective determination of malignancy had a high consensus rate between participants and experts.
Conclusion
Though finding a high consensus rate for determining malignancy of LLNs, variation in short-axis measurements and anatomical location classifications were present and improved after training. Adequate training would support the challenges involved in evaluating LLNs appropriately.
Key Points
• Variation was present in the assessment of the anatomical location and short-axis size of lateral lymph nodes.
• In certain cases, the accuracy of short-axis measurements and anatomical location, when compared to an expert reference value, improved after a training session.
• Consensus before and after training on whether an LLN was subjectively considered to be suspicious for malignancy was high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low, locally advanced rectal carcinomas (LARCs) have an increased chance of spreading towards lateral lymph nodes (LLNs), located in the lateral compartments [1]. These lateral compartments encompass the lymphatic tissue situated laterally of the mesorectal fascia and are not removed during standard total mesorectal excision (TME) surgery [2, 3]. While overall LR rates have decreased since the introduction of standardized neoadjuvant treatment and TME surgery, the proportion of lateral local recurrences (LLR) is increasing and currently accounts for approximately 50% of LRs [4]. This increase is most likely due to the still insufficient treatment of malignant LLNs.
Almost all nodal imaging studies have focused on mesorectal lymph nodes. Considering only the size of mesorectal nodes results in a low sensitivity and specificity for predicting malignancy (55–75%), but this can be improved to 85–100% when also considering morphological criteria (border contour, shape, signal heterogeneity) [5,6,7,8]. Contrastingly, recent literature regarding LLNs indicates that size and anatomical location, but not morphological criteria, are essential criteria to assess malignancy [9, 10]. Several studies have shown that the presence of enlarged LLNs on primary imaging increases the LLR rate to around 40% after 5 years [11,12,13,14,15]. A recent international, retrospective study including the re-review of MR-images for 1216 patients found significantly higher LLR rates for LLNs with a primary short-axis diameter of ≥ 7 mm; 19.5% after 5 years compared to 4.9% for patients without enlarged LLNs [10]. Furthermore, size and anatomical location were vital when also considering the restaging MRI. LLNs which remained > 4 mm and were located in the internal iliac compartment had a 5-year LLR rate of 52.3%. Obturator LLNs which remained > 6 mm had a 5-year LLR rate of 17.8% [9]. Morphological features were not found to have any significant influence on the prediction of malignancy.
There is, however, continued discussion as to the significance of LLNs, especially in Western cultures [2, 16]. Eastern clinics have long considered LLNs to be clinically significant and perform lateral lymph node dissections (LLND) for patients with LARC. This operation can lead to significant peri- and postoperative morbidity due to the possible damage of multiple blood vessels and/or nerves. While not performing an LLND in ‘high-risk’ patients may cause high LR rates, as found by Ogura et al [9, 10], an inadequate patient selection caused by, for example, over-staging LLNs on imaging, may mean that some patients unnecessarily undergo an LLND. An appropriate balance between these two, led by a suitable indication of malignancy by radiologists and active discussion by multidisciplinary teams (MDT) may help select the appropriate ‘high-risk’ patients who can benefit from an LLND, while sparing those for whom it is unnecessary. LLNs are still sparingly investigated, resulting in limited knowledge and awareness [2, 16, 17]. Accurate diagnosis of malignant LLNs remains therefore a challenging subject, even though subsequent treatment decisions for these patients are primarily based on the assessment of preoperative imaging [9, 10, 16, 18, 19].
What the size and anatomical location is of an LLN and whether it is considered malignant are vital pieces of information that should be accurately investigated, mentioned in radiology reports and discussed during MDT meetings [20, 21]. This study investigated if inter-physician variation was present for the measurement of LLN short-axis size, anatomical location, and for the judgement of whether an LLN is suspicious. This study also considered whether dedicated training resulted in significant improvements in these aspects.
Methods
Participants in this study included radiologists who were either collaborators in the national ‘Snapshot rectal cancer 2016 study’ or interested colleagues. The Snapshot study is a national cross-sectional retrospective study investigating all patients who underwent an operation for rectal cancer in 2016 and includes re-assessment of all MR-imaging after a dedicated online training. The methodology of a Snapshot study is described in detail elsewhere [22].
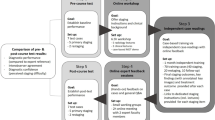
A total of 90 Dutch abdominal radiologists from 62 hospitals participated in the online training session; 69/90 radiologists from 51/62 hospitals completed the necessary assessments before the training and 53/69 radiologists from 51/62 hospitals also completed the repeat assessments after the training session (Fig. 1). This latter group fulfilled the inclusion criteria for participation in the current study.
Pre-training assessment
Participants were asked to complete a short questionnaire (Appendix 1) and then examine the primary MR-images of three cases with visible LLNs. All series of MRIs were provided. Cases 1 and 2 had one significantly enlarged LLN (internal iliac right and obturator left respectively), while case 3 had one LLN located in the external iliac compartment. Radiologists were asked to indicate the presence and number of LLNs, to measure the short-axis diameter of each LLN in mm, and to classify the anatomical compartment (Fig. 2a–f). Whether they deemed this LLN to be malignant was also reported.
A–F Six cases with enlarged lateral lymph nodes used for the training. A. Right internal iliac compartment, expert reference measurement: 12.1 mm (11.6–12.5, SD 0.5 mm). B. Left obturator compartment, expert reference measurement: 12.1 mm (11.3–12.5, SD 0.6 mm). C. Left external iliac compartment, expert reference measurement: 7.2 mm (6.9–7.4, SD 0.2 mm). D. Left obturator compartment, expert reference measurement: 5.2 mm (4.8–5.9, SD 0.6 mm). E. Right internal iliac compartment, expert reference measurement: 9.1 mm (8.5–9.9, SD 0.7 mm). F. Right obturator compartment, expert reference measurement: 14.8 mm (14.3–15.4, SD 0.6 mm)
Training
Participants then partook in a 2-hour, interactive training led by two expert radiologists (K.H., R.B.T.) with 17 and 24 years’ experience, respectively. During the training, relevant background literature concerning LLNs was presented and 10 example MRI scans were shown, with particular emphasis on explaining how to discern between the different lateral compartments. Definitions of the anatomical compartments were described in accordance with previous literature [9, 18, 23]. An example is shown in Fig. 3.
Axial view of T2-MRI depicting the lateral compartments. Red—external iliac compartment, green - obturator compartment, blue—internal iliac compartment. Brown spot: primary trunk of the internal iliac artery. The internal iliac compartment is defined as medial to the lateral border of the primary trunk of the internal iliac artery. The obturator compartment is the tissue located laterally of the primary trunk of the internal iliac artery and all tissue still present after the internal iliac artery exits the pelvis
Post-training assessment
After the training, 53 participants scored the first three cases again, plus an additional three cases. Case 4 had multiple small LLNs (obturator left), while cases 5 (internal iliac right) and 6 (obturator right) had one significantly enlarged LLN (Fig. 2a–f). These three additional cases were included to evaluate the assessment in ‘new’ cases and also because one of the three primary cases (case 2) was shown and discussed during the training session.
Standard of reference: expert evaluation
Three expert radiologists (R.B.T., D.L., K.H.) in the field of rectal cancer imaging independently evaluated the same six cases. The average of their measurements was used as the ‘expert reference value’ (ERV).
Statistics
Data was analyzed in SPSS Statistics (version 26.0). Categorical data are described in n and percentages, continuous data as means and standard deviation (SD) or medians and inter-quartile range (IQR). The primary outcomes were the agreement with the expert reference regarding the short-axis diameter (in mm), location of LLNs, and suspiciousness. This was examined with either the McNemar change test or a single/paired t-test, depending on the type of data. Paired t-tests considered the mean absolute deviation (MAD) of the measurements from cases 1, 2, and 3, and these were compared to the ERV. A p value of < 0.05 was considered statistically significant.
Results
Baseline characteristics of the 53 abdominal radiologists who participated in the study can be found in Table 1. Participants had a mean experience of 9.6 years (SD 5.4).
Questionnaire
Participants first completed a questionnaire regarding LLNs. Participants estimated that LLNs were present in 16% (range 5–70%, SD 9) of patients with low, cT3/4 rectal cancer and various definitions were used to describe suspicious LLNs. A total of 30% used ≥ 7 mm as proposed by the Lateral Node Consortium Study [14, 15], 43% used the same criteria as for mesorectal lymph nodes and 27% gave different definitions such as 8mm, 10mm, or only based solely on the anatomical compartments. Only 22% of radiologists always mentioned LLNs in their reports. Finally, when asked if suspicious LLNs were a sign of metastatic disease, 54% replied no, 25% yes, and 21% remained neutral. Further results from the questionnaire can be found in Table 1.
Short-axis measurements
Short-axis measurements of LLNs by participants before and after training compared to the expert reference value (ERV) are provided in Table 2. For cases 1, 2, and 3, no significant differences were observed in short-axis diameters or mean absolute deviation (MAD) by the participants compared to the expert reference before and after the training (p = 0.134–0.925). Reductions in MAD from the ERV, ranges, and SD were seen for all cases after training compared to before training (Fig. 4). Cases 4, 5, and 6 were only measured after the training (Table 2). Single t-tests compared to the ERV revealed significant differences for case 4 (ERV 5.2 (p = .003)) and case 6 (ERV 14.8 (p < .001)) but not for case 5 (ERV 9.1 (p = .079)).
Variations and standard deviations in measurements between participants decreased after training, and smaller ranges and standard deviations were seen between the three expert radiologists compared to the participants (Appendix 1).
Location of LLNs according to anatomical compartments
The three experts reached full agreement on the anatomical locations of the LLNs in all six cases (Table 2 and Appendix 1). Consensus regarding the location of the largest LLN in case 1 was initially 62% (right internal iliac) and increased to 78% after training (p = .077). Consensus for the location of the largest LLN in case 2 was initially 46% (left obturator) and increased to 72% after training (p = .007). The external iliac LLN in case 3 had an initial consensus of 92%, but this decreased to 74% after training (p = .453). Consensus for the anatomical locations of cases 4, 5, and 6 was 74, 79%, and 85%, respectively (similar to the post-training results of cases 1–3) and all were in line with the expert reference.
Malignant LLNs
There was a high consensus between the participating radiologists and experts regarding whether an LLN was considered suspicious. The three experts stated independently from each other that cases 1, 2, and 6 were suspicious on both the primary and restaging images. Case 5 was suspicious primarily, but responded well to neoadjuvant treatment, while cases 3 and 4 were not deemed as suspicious for malignancy. For cases 1, 2, and 6, the consensus between participants was 98.1–100% that the LLNs here were suspicious, while 15–23.7% also considered LLNs in cases 3 and 4 to be suspicious. All participants regarded the LLN in case 5 as initially suspicious (100%), and 23.1% also stated its suspiciousness when considering restaging images (Table 2).
Discussion
This study, including 53 Dutch abdominal radiologists from 51 hospitals, demonstrates that inter-physician variation is present for the short-axis measurements of LLNs and for classifying the appropriate lateral compartment. This variation decreased when repeating the assessment after completing a 2-hour training provided by expert radiologists. Contrastingly, the overall consensus between participants and compared to the experts was very high for the subjective determination of whether an LLN was suspicious of malignancy.
Participants indicated prior to the training that they used different definitions to diagnose suspicious LLNs in their clinical practice (Table 1) and there was a wide range in whether the participants routinely reported the presence or absence of LLNs in their reports; 3% said never and 22% always. It is possible that radiologists only mention their presence if deemed suspicious, even though heterogeneity existed within the group regarding their definitions for ‘suspiciousness’. This reflects variation between participating hospitals and exposes a lack of knowledge and awareness for LLNs by a proportion of participants. Another difference was the interpretation of whether LLNs are indicative of metastatic or locoregional disease; 23% believed them to represent metastatic disease, 56% locoregional and 21% indicated that they did not know how to answer this question. Recent literature has suggested that LLNs represent locoregional disease, considering that distant metastasis rates appear not to be influenced by the presence of enlarged LLNs [2, 9, 10].
Since research has found that size, and not morphological features, is primarily relevant to LLNs [9, 10]; it is vital that size measurements are reported accurately. Based on the most recent retrospective research, 7 mm (short-axis) has been suggested as the threshold for malignancy [9, 10]. One issue with selecting a specific size threshold is the inter-physician variation in measurements found in this study. While a small range of variation is to be expected when comparing measurements of multiple specialists, the ranges found here were larger than expected. For example, in case 2, while the mean measurement was 11.4 mm, and thus close to the ERV of 12.1 mm, measured values between participants ranged from 5 to 14 mm (SD 1.5). This range significantly decreased after the training to 9–14 mm (SD 1.0). This broad variation may be explained by a significant outer ‘shell’ illustrated in Fig. 5. In fact, for all cases, the ranges and standard deviations in measurements decreased after the training. This is most likely a direct result of the training, during which dedicated time was spent explaining short-axis measurements with MRI examples.
An important consideration should be the variation in measurements for the LLNs which straddle the 7 mm size. For these LLNs, the chances of discrepant measurements and opinions on suspiciousness increased, compared to larger LLNs for which consensus in this study was almost 100% that it was suspicious. This variation was also visible in the range of measurements between the three experts for LLNs around 7 mm in size (Appendix 1). For both the participants and experts, it is possible that the measurements from one individual vary just below or just above the 7 mm threshold compared to a colleague; insinuating that such a strict guideline may be subject to inter-physician variation. In such cases, peer review by a colleague could be desirable and included in protocols [24, 25].
This study also found that initial consensus regarding the anatomical location of LLNs in the lateral compartments was low; suggesting that various borders are currently used to define the compartments. During the training session, the anatomical definitions were described according to Ogura et al [9] and Gollub et al [23] and were explained with the help of multiple visual examples. For the cases completed after the training, a noticeable improvement was seen in the consensus regarding anatomical location. This implies that these borders are understandable and translatable into routine daily practice. However, correct identification of the compartments was still not consistently > 80% after the training, with the highest consensus of 86% for case 6. Considering the oncological implications for LLNs in different locations [18, 26], it is important that LLNs are classified correctly. Possibilities could include color atlases with detailed explanations combined with interactive training sessions, as this does appear to be advantageous. On the other hand, while cases 1 and 2 improved after training, case 3 consensus worsened. This is due to the fact that during the training session, experts explained that elongated LLNs located close to the external iliac artery were rarely found to be malignant. Consequently, less radiologists mentioned the presence of the node in case 3 because they did not consider it to be a lateral lymph node anymore.
Interestingly, high consensus was seen between participants when asked whether an LLN was considered malignant, demonstrating a good ability to judge this phenomenon. Mentioning this in reports is also essential so to influence potential treatment decisions by radiation oncologists and surgeons. Results from the questionnaire found that only 22% always reported LLNs, a rate that must be improved. Methods to facilitate this could be structured reporting, focused training, or computer-aided detection to ensure that suspicious LLNs are properly identified as signaled to the multidisciplinary team [27]. A clear international guideline and/or universal definition for when an LLN is considered malignant is currently missing. Considering the multidisciplinary treatment implications of LLNs discussed here, concise guidelines should be created and implemented.
This study has a number of limitations. Firstly, not all participants who completed the pre-training assessment also participated in the post-training assessment, reflecting a selection bias for the 53 participants, possibly representing the more enthusiastic participants. The fact that the total case number before training was only three is a limiting factor, restricting our ability to properly evaluate their ability and knowledge. Additionally, while the addition of three new cases afterwards was carefully considered to allow an analysis of the ‘new’ consensus after training, this method creates heterogeneity within the small sample of cases. Furthermore, the ERV is based on the measurements of three experts for which variation is also possible. Lastly, many of the hospitals had one representative radiologist, while intra-institution variation would also be very interesting to investigate.
Conclusion
This study found that while radiologists have a high consensus rate for the subjective determination of malignancy of lateral lymph nodes (LLNs), variation was present in the classification of LLNs into lateral compartments and their short-axis measurements. These aspects improved after participating in an online training session led by expert radiologists. Considering the oncological implications that LLNs hold; especially with regard to the differences found between lateral compartments and for varying short-axis sizes, care should be taken to ensure a minimal amount of variation between specialists. Increased knowledge and awareness through training, instruction documents, and visual guides may help improve this further.
Abbreviations
- ERV:
-
Expert reference value
- LLND:
-
Lateral lymph node dissection
- LLNs:
-
Lateral lymph nodes
- LLR:
-
Lateral local recurrence
- LR:
-
Local recurrence
- MAD:
-
Mean absolute deviation
- MRI:
-
Magnetic resonance imaging
- TME:
-
Total mesorectal excision
References
Steup WH, Moriya Y, van de Velde CJ (2002) Patterns of lymphatic spread in rectal cancer. A topographical analysis on lymph node metastases. Eur J Cancer 38(7):911–918
Christou N, Meyer J, Toso C, Ris F, Buchs NC (2019) Lateral lymph node dissection for low rectal cancer: Is it necessary? World J Gastroenterol 25(31):4294–4299
Sammour T, Chang GJ (2018) Lateral pelvic lymph node dissection and radiation treatment for rectal cancer: Mutually exclusive or mutually beneficial? Ann Gastroenterol Surg 2(5):348–350
Iversen H, Martling A, Johansson H, Nilsson PJ, Holm T (2018) Pelvic local recurrence from colorectal cancer: surgical challenge with changing preconditions. Color Dis 20(5):399–406
Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG (2004) High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 52(1):78–83
Lahaye MJ, Engelen SM, Nelemans PJ et al (2005) Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26(4):259–268
Brown G, Richards CJ, Bourne MW et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227(2):371–377
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 232(3):773–783
Ogura A, Konishi T, Beets GL et al (2019) Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg 154(9):e192172
Ogura A, Konishi T, Cunningham C et al (2019) Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol 37(1):33–43
Ueno H, Mochizuki H, Hashiguchi Y, Hase K (2001) Prognostic determinants of patients with lateral nodal involvement by rectal cancer. Ann Surg 234(2):190–197
Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T (2005) Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg 92(6):756–763
Watanabe T, Muro K, Ajioka Y et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23(1):1–34
Kim MJ, Hur BY, Lee ES et al (2018) Prediction of lateral pelvic lymph node metastasis in patients with locally advanced rectal cancer with preoperative chemoradiotherapy: focus on MR imaging findings. PLoS One 13(4):e0195815
Kim MJ, Kim TH, Kim DY et al (2015) Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol 111(4):459–464
Williamson JS, Quyn AJ, Sagar PM (2020) Rectal cancer lateral pelvic sidewall lymph nodes: a review of controversies and management. Br J Surg 107(12):1562–1569
Kim MJ, Oh JH (2018) Lateral lymph node dissection with the focus on indications, functional outcomes, and minimally invasive surgery. Ann Coloproctol 34(5):229–233
Schaap DP, Boogerd LSF, Konishi T et al (2021) Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg 108(2):205–213
Schaap DP, Ogura A, Nederend J et al (2018) Prognostic implications of MRI-detected lateral nodal disease and extramural vascular invasion in rectal cancer. Br J Surg 105(13):1844–1852
Hazen SJA, Sluckin TC, Konishi T, Kusters M (2021) Lateral lymph node dissection in rectal cancer: State of the art review. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2021.11.003
Sluckin TC, Hazen SJA, Kusters M (2021) From “East vs West” towards international multidisciplinary collaboration: an appraisal of lateral lymph nodes in rectal cancer. Ann Gastroenterol Surg 5(6):731–737
Dutch Snapshot Research Group (2017) Benchmarking recent national practice in rectal cancer treatment with landmark randomized controlled trials. Color Dis 19(6):O219–Oo31
Gollub MJ, Lall C, Lalwani N, Rosenthal MH (2019) Current controversy, confusion, and imprecision in the use and interpretation of rectal MRI. Abdom Radiol (NY) 44(11):3549–3558
Chaudhry H, Del Gaizo AJ, Frigini LA et al (2020) Forty-one million RADPEER reviews later: what we have learned and are still learning. J Am Coll Radiol 17(6):779–785
Chetlen AL, Petscavage-Thomas J, Cherian RA et al (2020) Collaborative learning in radiology: from peer review to peer learning and peer coaching. Acad Radiol 27(9):1261–1267
Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K (2012) Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 255(6):1129–1134
Bruno MA, Walker EA, Abujudeh HH (2015) Understanding and Confronting Our Mistakes: The Epidemiology of Error in Radiology and Strategies for Error Reduction. Radiographics 35(6):1668–1676
Acknowledgements
Collaborators from the Dutch Lateral Node Imaging group:
Henk Avenarius, Fleur Bangert, Heleen M. D. Beekhuis, Shira H. de Bie, Jaap Borstlap, Sicco J. Braak, Manon N. G. J. A. Braat, Jennifer Bradshaw, Ilse M. van Dop, Kitty E. Droogh-de Greve, Hanneke Duijsens, Bram Eijlers, Saskia van Elderen, Marc Engelbrecht, Shirin S. Feshtali, Anne van Geel, Alette W. Daniëls-Gooszen, Robbert J. de Haas, Rolf van den Hoed, Erik J. R. J. van der Hoeven, Sebastiaan Jensch, Roy de Jong, Stephanie E. M. Kolderman, Fleur I. de Korte, Jasenko Krdzalic, Eveline Krul, Derk Lahuis, Manou de Lijster, Joost Nederend, Pieter Ott, Jan Peringa, Jip F. Prince, Lodewijk Roosen, Ellen A. Roskott-ten Brinke, Dennis B. Rouw, Merel M. Scheurkogel, Petra C. G. Simons, Marjan J. Slob, G. Y. Mireille The, Jeroen A. W. Tielbeek, Aukje A. J. M. van Tilborg, Fiek van Tilborg, Charlotte Tutein Nolthenius, Cornelis J. Veeken, Roy F. A. Vliegen, Marianne de Vries, Frank J. Wessels, Paul P. van Westerveld, Bart W. K. de Wit, Floor S. W. van der Wolf, Sander van der Wolk, Johannes Wybenga, Herman J. A. Zandvoort, and Tjeerd Zwaga.
Funding
This study has received funding from the Dutch Cancer fund (KWF): reference no. 12768.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Miranda Kusters.
Conflict of interest
Doenja M. J. Lambregts and Regina G. H. Beets-Tan are members of the European Radiology Scientific Editorial Board. They have not taken part in the review or selection process of this article.
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• observational
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 19 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sluckin, T.C., Hazen, SM.J.A., Horsthuis, K. et al. Significant improvement after training in the assessment of lateral compartments and short-axis measurements of lateral lymph nodes in rectal cancer. Eur Radiol 33, 483–492 (2023). https://doi.org/10.1007/s00330-022-08968-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08968-0