Abstract
Objectives
To evaluate changes in diaphragmatic function in Pompe disease using MRI over time, both during natural disease course and during treatment with enzyme replacement therapy (ERT).
Methods
In this prospective study, 30 adult Pompe patients and 10 healthy controls underwent pulmonary function tests and spirometry-controlled MRI twice, with an interval of 1 year. In the sagittal view of 3D gradient echo breath-hold acquisitions, diaphragmatic motion (cranial-caudal ratio between end-inspiration and end-expiration) and curvature (diaphragm height and area ratio) were calculated using a machine learning algorithm based on convolutional neural networks. Changes in outcomes after 1 year were compared between Pompe patients and healthy controls using the Mann-Whitney test.
Results
Pulmonary function outcomes and cranial-caudal ratio in Pompe patients did not change significantly over time compared to healthy controls. Diaphragm height ratio increased by 0.04 (−0.38 to 1.79) in Pompe patients compared to −0.02 (−0.18 to 0.25) in healthy controls (p = 0.02). An increased diaphragmatic curvature over time was observed in particular in untreated Pompe patients (p = 0.03), in those receiving ERT already for over 3 years (p = 0.03), and when severe diaphragmatic weakness was found on the initial MRI (p = 0.01); no progression was observed in Pompe patients who started ERT less than 3 years ago and in Pompe patients with mild diaphragmatic weakness on their initial MRI.
Conclusions
MRI enables to detect small changes in diaphragmatic curvature over 1-year time in Pompe patients. It also showed that once severe diaphragmatic weakness has occurred, improvement of diaphragmatic muscle function seems unlikely.
Key Points
• Changes in diaphragmatic curvature in Pompe patients over time assessed with 3D MRI may serve as an outcome measure to evaluate the effect of treatment on diaphragmatic function.
• Diaphragmatic curvature showed a significant deterioration after 1 year in Pompe patients compared to healthy controls, but the curvature seems to remain stable over this period in patients who were treated with enzyme replacement therapy for less than 3 years, possibly indicating a positive effect of ERT.
• Improvement of diaphragmatic curvature over time is rarely seen in Pompe patients once diaphragmatic motion shows severe impairment (cranial-caudal inspiratory/expiratory ratio < 1.4).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pompe disease is a genetic metabolic disorder caused by the deficiency of the lysosomal enzyme alpha-glucosidase which leads to glycogen accumulation in many tissues, particularly muscles. In adults, it usually manifests as a limb girdle myopathy with respiratory insufficiency [1]. Due to predominant diaphragmatic weakness, respiratory insufficiency is especially present in the supine position and therefore nocturnal mechanical ventilation is needed in 32–80% of adult patients [2,3,4,5,6].
Monitoring of respiratory muscle weakness is important to evaluate the need for nocturnal mechanical ventilation and to assess treatment effects. Currently, enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (alglucosidase alfa, myozyme®) is the only approved therapy for adult Pompe patients, but next-generation enzyme replacement therapies and gene therapies are being developed [7,8,9]. Surprisingly, the effect of ERT on respiratory muscles seems smaller than the effect on skeletal muscle function [10,11,12,13]. At group level, pulmonary function outcomes after 5–10 years of ERT were better than the expected natural course. Individuals however may show progressive respiratory weakness despite start of ERT or may have an initial positive effect followed by a secondary decline [5, 11, 13]. The pathophysiological background of the progressive pulmonary dysfunction in these patients is currently unknown.
Pulmonary function tests are used to evaluate vital capacity or forced vital capacity (FVC) in upright seated and supine position, and to assess respiratory muscle strength by using maximum inspiratory and expiratory mouth pressures (MIP and MEP) [10]. A drawback of these tests is that they do not provide specific insight into the function of the individual muscles involved in respiration. Using MRI, it is possible to study diaphragmatic function and thoracic movements separately. In cross-sectional MRI studies in Pompe patients, it was shown that motion of the diaphragm was decreased while motion of the thoracic wall was normal [14,15,16]. In healthy controls, the curvature of the diaphragm during inspiration was decreasing, while in Pompe patients, the curvature was increasing, indicating insufficient diaphragmatic contraction. Importantly, early changes in diaphragmatic motion could already be observed while FVC was still within the normal range [17].
The purpose of this study was to evaluate changes in diaphragmatic function over time in patients with Pompe disease using MRI. Pompe patients and healthy controls were studied twice, with an interval of 1 year, using a standardized MRI protocol with automatic machine learning–derived imaging analysis techniques.
Materials and methods
Study design
This prospective cohort study was performed at the Center for Lysosomal and Metabolic Diseases at Erasmus University Medical Center, the reference center for Pompe disease in The Netherlands. In the initial cross-sectional study, 35 patients with Pompe disease and 18 gender- and age-matched healthy controls were included [17]. Inclusion criteria were a confirmed diagnosis of non-classic Pompe disease (based on two disease-causing variants in the acid alpha-glucosidase gene and/or decreased enzyme activity in fibroblasts) and the ability to lie in a supine position for at least 30 min without mechanical ventilation. Exclusion criteria were comorbidities or devices that did not permit MRI investigations, and claustrophobia. For the current prospective study, all Pompe patients and 10 of the healthy controls were invited to repeat the study procedures after 1 year, comprising regular pulmonary function tests and spirometry-controlled MRI scans using a 20–25-min MRI protocol [17]. Of the 35 Pompe patients that took part in the initial cross-sectional study, five patients could not be included in this follow-up study for the following reasons: living abroad (n = 1), withdrawn participation (n = 2), or participation in studies using other treatment (n = 2). All study procedures were performed between January 2016 and February 2019. The protocol was approved by the Medical Ethical Committee at our hospital (MEC-2007-103, amendment 7) and all participants provided written informed consent.
Pulmonary function tests
All study procedures were performed on 1 day in the same order, starting with pulmonary function tests. Pulmonary function tests comprised FVC in upright seated and supine position and MIP and MEP in upright seated position. All tests were performed according to American Thoracic Society/European Respiratory Society standards and results were expressed as a percentage of predicted normal values [18,19,20]. During pulmonary function tests, participants were trained to perform specific breathing maneuvers during MRI scanning. The MRI was always obtained directly after this training.
MRI analysis
All participants were examined on a 3-T Signa 750 MRI scanner (General Electric Healthcare) using the whole-body coil for radiofrequency excitation and a 32-channel torso coil for signal reception [17]. In the current study, we used three-dimensional (3D) spoiled-gradient echo (SPGR) breath-hold acquisitions (repetition time/echo time = 1.2/0.5 ms, flip angle 2°, voxel resolution 3 × 3 × 3 mm) at end-inspiration and end-expiration. Breathing maneuvers during MRI were standardized using an MR-compatible spirometer to ensure the best performance of each subject for inspiratory and expiratory scans [21]. The maximum breath-hold time was 10 s and scans were only repeated if the image quality was poor.
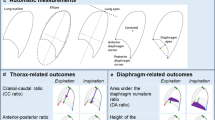
For image analysis, we developed automatic lung segmentations using a machine learning algorithm based on convolutional neural networks [22]. In these segmentations, we calculated 3D outcomes [1–2] and two-dimensional (2D) outcomes [3–8]: (1) the lung volume ratio to evaluate the relative increase in lung volume from end-expiration to end-inspiration, (2) the diaphragm volume ratio to evaluate the relative volume displaced by the diaphragm, (3) the lung area ratio to evaluate the relative increase in the lung area, (4) the cranial-caudal ratio to evaluate the motion of the diaphragm, (5) the anterior-posterior ratio to evaluate the motion of the thoracic wall, (6) the relative cranial-caudal ratio compared to the anterior-posterior ratio (CC-AP ratio) to evaluate the diaphragm motion relative to motion of the thoracic wall, and (7) the diaphragm height ratio and (8) the diaphragm area ratio to evaluate the diaphragmatic curvature (Fig. 1). Previously, we obtained 2D outcomes from 2D dynamic images [17]. In the current study, analysis of the 2D outcomes was obtained from the sagittal view at the right mid hemi-diaphragm of 3D breath-hold scans. Based on image registration of the 3D scans, the sagittal levels of the initial and follow-up MRI were automatically matched. For all MRI outcomes, we used a ratio between inspiration and expiration to adjust for possible differences in age, sex, height, and weight of the patients. All outcomes were automatically calculated using in-house developed software in Python (Python 3.6.3, https://www.python.org/, ©2001–2019; Python Software Foundation; SciPy 1.1.0, https://www.scipy.org/, ©2003–2019 SciPy developers) for the 3D segmentation and 2D outcomes, with postprocessing in MATLAB (MathWorks) for the 3D outcomes. All automatic segmentations and measurements were manually checked, and segmentations were corrected if necessary. A detailed description of the procedures is presented in the supplementary material.
Outcome measures. Examples of 3D outcomes and 2D outcomes in the sagittal view at the right mid hemi-diaphragm of 3D breath-hold scans. 2D outcomes were obtained from previously conducted dynamic 2D imaging studies [17]. For all outcomes, we calculated a ratio between end-expiration and end-inspiration outcomes to adjust for differences in sex, age, and size of participants
Statistical analysis
Differences in demographics (sex, age, height, weight, BMI), pulmonary function test outcomes, and MRI outcomes between Pompe patients and healthy controls were analyzed using the chi-square test for sex and the Mann-Whitney test for the other variables. To calculate changes over time in pulmonary function test outcomes and MRI outcomes, we calculated the differences between follow-up and initial results.
To investigate the possible effect of ERT on changes over time in MRI outcomes, we analyzed subgroups of (1) patients without ERT, (2) patients with ≤ 3 years ERT at the initial MRI, and (3) patients with > 3 years ERT at the initial MRI, based on the peak clinical effect of ERT that is usually reached in the first 3 years of treatment [11, 13]. Moreover, we compared subgroups of patients with no or minor diaphragmatic weakness at the initial MRI (defined as a cranial-caudal ratio ≥ 1.4) and patients with moderate or severe diaphragmatic weakness (cranial-caudal ratio < 1.4). This cut-off of 1.4 was chosen on the basis of the lowest cranial-caudal ratio recorded in healthy controls [17]. Differences between each subgroup and healthy controls were tested using the Mann-Whitney test. Because subgroups were small and these analyses are indicative only, we did not correct for multiple testing. Statistical analysis was performed with SPSS for Windows (version 24, SPSS Inc). The significance level was set at p ≤ 0.05.
Results
Participant characteristics
We included 30 Pompe patients (14 men, median age 37 years (range 18–70), 16 women, median age 44 years (range 17–70)) and 10 healthy controls (5 men, median age 32 years (range 26–61), 5 women, median age 42 years (range 25–62)) (Table 1). There were no significant differences in sex, age, height, and weight between Pompe patients and healthy controls. One Pompe patient used nocturnal mechanical ventilation and none of the patients used a wheelchair. Median time between initial and follow-up MRI was longer in healthy controls than in Pompe patients (1.13 vs 1.02 years, p = 0.018). Individual outcomes in each subject are presented in the supplementary material.
Pulmonary function test outcomes
Pompe patients had lower values of pulmonary function test results than healthy controls at the initial measurement (Table 2). The changes in FVC upright and supine, MIP, and MEP after 1 year were not significantly different between Pompe patients and healthy controls (Table 2).
MRI outcomes
Lung volume ratio, diaphragm volume ratio, lung area ratio, cranial-caudal ratio, and CC-AP ratio at the initial MRI were lower in Pompe patients than in healthy controls (p < 0.01 for all outcomes) (Table 3, Fig. 2). Changes in lung volume ratio, diaphragm volume ratio, lung area ratio, cranial-caudal ratio, anterior-posterior ratio, and CC-AP ratio after 1-year follow-up were not significantly different between Pompe patients and healthy controls, indicating no change in overall lung volume or diaphragmatic motion over 1-year time.
Example of 3D breath-hold scans at end-expiration and end-inspiration in a Pompe patient and in a healthy control, including automatic segmentations of the left lung (green) and the right lung (red). This Pompe patient has a decreased motion of the diaphragm, decreased volume displaced by the diaphragm, and an increased curvature of the diaphragm during inspiration
Diaphragm height ratio and diaphragm area ratio at the initial MRI were not significantly different between Pompe patients and healthy controls (Table 3). Interestingly, the median change in diaphragm height ratio after 1 year was 0.04 (range −0.38 to 1.79) in Pompe patients versus −0.02 (range −0.18 to 0.25) in healthy controls (p = 0.019) and the median change in diaphragm area ratio was 0.04 (range −0.28 to 4.40) in Pompe patients versus −0.07 (range −0.27 to 0.39) in healthy controls (p = 0.034). If we assume that the change in diaphragm height and area ratio after 1 year in all healthy controls was 0, the difference between Pompe patients and healthy controls in the change in diaphragm height ratio remained significant (p = 0.012), but the change in diaphragm area ratio was not significant (p = 0.208). The increasing ratios in Pompe patients indicate an increased diaphragmatic curvature after 1 year. This corresponds to a more insufficient diaphragmatic contraction and may indicate progressive diaphragmatic dysfunction.
In one Pompe patient (treated > 3 years with ERT), the increase in diaphragm height and area ratio (1.79 and 4.40) was outside the range of the results of other patients. FVC upright decreased 4% and FVC supine remained stable. The MRI results were manually checked, and not caused by an error in automatic measurements; therefore, this patient was not excluded from analysis. However, if we would have excluded this particular patient, the median change in diaphragm height ratio (0.03 (range −0.38 to 0.42), p = 0.024) and diaphragm area ratio (0.03 (range −0.28 to 0.49), p = 0.043) in Pompe patients remained significant compared to healthy controls.
Subgroup analysis
Diaphragm curvature significantly increased in non-treated Pompe patients (median change in diaphragm height ratio 0.04 (range −0.01 to 0.23), p = 0.025), and also in Pompe patients treated > 3 years with ERT (median change in diaphragm height ratio 0.07 (range −0.38 to 1.79), p = 0.031). The curvature remained stable in healthy controls and in Pompe patients treated ≤ 3 years with ERT (median change in diaphragm height ratio 0.02 (range −0.28 to 0.07), p > 0.05). Results of the change in diaphragm area ratio were similar to the change in diaphragm height ratio (Figs. 3 and 4).
Changes in diaphragm curvature after 1-year follow-up compared to the initial MRI in subgroups of Pompe patients related to ERT duration and compared to healthy controls. An increasing diaphragm height and area ratio after 1-year follow-up (values > 0) correspond to an increasing diaphragm curvature ratio indicating a deterioration of diaphragm function over time; decreasing ratios indicate improvement. ERT, enzyme replacement therapy; ns, not significant
The diaphragm height is shown in white arrows. The diaphragm area is shown in blue (expiration) or yellow (inspiration). Ratios are outcomes at inspiration divided by outcomes at expiration. ERT, enzyme replacement therapy. Patient A is a female Pompe patient of 17 years old, not treated with ERT because she is asymptomatic and stable over time. At the initial MRI, both diaphragm height ratio and diaphragm ratio are increasing during inspiration (ratio > 1), indicating an insufficient diaphragmatic contraction. After 1-year follow-up, the ratios are increasing, corresponding to a deterioration of diaphragmatic curvature. At the initial measurement, forced vital capacity (FVC) upright was 93% predicted and FVC supine 95%, at follow-up FVC upright was 94% predicted and FVC supine 89%. Patient B is a female Pompe patient of 25 years old and 2.5 years treated with ERT at the initial MRI. FVC upright was 119% predicted and FVC supine 115%. The diaphragmatic curvature is increasing during inspiration (diaphragm height ratio and diaphragm ratio > 1), indicating an insufficient diaphragmatic contraction. After 1-year follow-up, FVC upright was 115% predicted and FVC supine 113%, but the diaphragmatic height and area ratios are decreased, corresponding to an improved diaphragmatic curvature
We investigated whether the changes in diaphragmatic curvature are related to diaphragmatic weakness at the initial MRI. We found an increased diaphragmatic curvature after 1-year follow-up in Pompe patients with moderate or severe diaphragmatic weakness (cranial-caudal ratio < 1.4 at the initial MRI, n = 14), as the diaphragm height ratio increased with 0.03 (range −0.38 to 0.29) and the diaphragm area ratio with 0.04 (range −0.25 to 0.29) compared to healthy controls (p = 0.014 and p = 0.04). However, in Pompe patients with no or only minor diaphragmatic weakness (a cranial-caudal ratio ≥ 1.4 at the initial MRI, n = 16), the diaphragmatic curvature did not change significantly over time compared to healthy controls: median change in diaphragm height ratio 0.05 (range −0.28 to 1.79) and diaphragm area ratio 0.06 (range −0.28 to 4.40), both p = 0.082.
We investigated a possible effect of ERT on the change in diaphragmatic curvature in relation to the level of diaphragmatic weakness at the initial MRI (Fig. 5). Noteworthy, we found that two out of eight Pompe patients with a normal cranial-caudal ratio (≥ 1.4) at the initial MRI (suggesting no or minor weakness of the diaphragm), who were treated ≤ 3 years with ERT, showed a clear decrease of diaphragmatic curvature, indicating improvement of diaphragmatic function. This is in contrast to the absence of improvement of diaphragmatic curvature over time, observed in all seven Pompe patients who did not receive ERT, six of whom also had a cranial-caudal ratio ≥ 1.4 at the initial MRI. Of all Pompe patients with a cranial-caudal ratio < 1.4 at the initial MRI, indicating moderate to severe weakness of the diaphragm, only one patient showed a clear improvement of the diaphragmatic curvature over time. This particular female patient aged 68 years, was yet treated over 10 years with ERT, had mild limb girdle weakness and a fairly stable FVC upright (86%) and FVC supine (41%) over the last years.
A positive change in diaphragmatic height ratio (increased diaphragmatic curvature over time) is classified as deterioration. A negative change in diaphragmatic height ratio (decreased diaphragmatic curvature over time) is classified as improvement. A cranial-caudal ratio < 1.4 is classified as moderate to severe diaphragmatic weakness at the initial MRI, and a cranial-caudal ratio ≥ 1.4 is classified as mild to no diaphragmatic weakness at the initial MRI. One Pompe patient (cranial-caudal ratio 1.48, change in diaphragmatic height ratio 1.79, > 3 years ERT) is not included in this figure because these results were outside the range of the results of other patients. ERT, enzyme replacement therapy
Discussion
Pompe patients may have progressive pulmonary dysfunction despite treatment with ERT. Changes in diaphragmatic function are difficult to detect with standard pulmonary function tests. This is the first study using MRI to evaluate diaphragmatic function in Pompe patients over time. We showed that the curvature of the diaphragm during inspiration may increase in Pompe patients already after 1-year follow-up, while standard pulmonary function tests did not change. Interestingly, the diaphragmatic curvature seems to remain stable in Pompe patients who were treated with ERT for less than 3 years, suggesting a positive effect on diaphragmatic function in the first years after starting ERT. Additionally, we found that Pompe patients with a severely decreased motion of the diaphragm, despite ERT, rarely show improvement of the diaphragmatic curvature over time.
Our current MRI protocol enabled simultaneous evaluation of both the entire diaphragm and the thoracic wall during dynamic inspiration with a relatively large field of view. For this study, we developed an advanced automatic segmentation technique and all outcome measures were calculated automatically and thus are operator independent. By using 3D MRI sequences, we were able to compare MRI outcomes on a matching sagittal level, leading to a more robust analysis.
The curvature of the diaphragm during inspiration, evaluated using diaphragm height and area ratio, increased in Pompe patients over a 1-year period. Changes in diaphragmatic curvature after 1 year are small, which could be expected because Pompe disease is a slowly progressive disorder. Because the changes in Pompe patients were significantly larger than in healthy controls, we concluded that these minor changes after 1 year are possibly an early sign of progressive diaphragmatic weakness. In contrast, the change in other MRI outcomes and pulmonary function test outcomes were not significantly different between Pompe patients and healthy controls. Therefore, we assume that the curvature of the diaphragm may increase before the obvious changes in the cranial-caudal ratio and pulmonary function tests can be observed. The increased curvature during inspiration in Pompe patients is likely caused by weakness of the diaphragm, due to insufficient muscle contraction. Because Pompe patients also have abdominal muscle weakness, abdominal pressure will insufficiently increase during inspiration and diaphragmatic weakness is not causing a paradoxical motion but only a paradoxical increased curvature [17, 23].
At the initial MRI, diaphragm height and area ratio were not significantly different between Pompe patients and healthy controls. This might be explained by a large range of disease severity between Pompe patients we evaluated, including six patients (20%) without an indication for ERT because these patients had no or only very mild symptoms of muscle weakness. However, in Pompe patients with impaired pulmonary function, the diaphragm height and area ratio were decreased compared to healthy controls [17].
An important question is whether an early start of ERT can prevent irreversible damage to the diaphragm in Pompe patients. We showed that the diaphragmatic curvature in Pompe patients with no or limited impairment of the diaphragm seems to remain stable or even improved after 1 year in patients treated with ERT for a period of less than 3 years. No improvement of the diaphragmatic curvature was observed when patients already have severe dysfunction of the diaphragm. Despite subgroups were small and these findings are indicative only, they parallel previous studies on the effects of ERT on FVC that showed that the main effects of ERT could be achieved during the first years after start of treatment [5, 11, 13]. It would also be interesting to study diaphragmatic involvement and response to ERT in subgroups of children with classic and non-classic Pompe disease.
Our study has some limitations. First, the number of participants was relatively small and patients had a large range of disease and treatment duration. In addition, Pompe patients with severe pulmonary dysfunction are not included because they are not able to lay flat without ventilation. Second, we were only able to compare one initial measurement with one follow-up measurement in each single patient. However, breathing maneuvers were standardized using an MR-compatible spirometer to improve the reliability of our measurements. As the mean follow-up time in healthy controls was longer, this may have even resulted in an underestimation of the differences between Pompe patients and healthy controls. Third, we used breath-hold scans, which may be different from the clinical situation during normal breathing. The use of dynamic 3D acquisitions potentially would be preferable; however, analysis of these images appeared to be more difficult due to a lower spatial resolution. Finally, automatic algorithms are not yet widely available, currently hampering its application in daily practice.
In conclusion, using 3D MRI, small changes in diaphragmatic curvature over time could be detected in patients with Pompe disease. Once severe diaphragmatic weakness has occurred, it is unlikely that this can improve. MRI potentially can be used to identify Pompe patients with still normal standard pulmonary function tests and only minor diaphragmatic abnormalities who might benefit from an early start of ERT. Our MRI outcome measures need further validation in larger cohort studies with a longer follow-up interval before these can be introduced as new MRI markers in clinical trials to evaluate changes in diaphragmatic function over time.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- CC-AP ratio:
-
Cranial-caudal ratio compared to the anterior-posterior ratio
- ERT:
-
Enzyme replacement therapy
- FVC:
-
Forced vital capacity
- MEP:
-
Maximum expiratory pressure
- MIP:
-
Maximum inspiratory pressure
- SPGR:
-
Spoiled-gradient echo
References
Reuser AJJ, Hirschhorn R, Kroos MA (2019) Pompe disease: glycogen storage disease type II, acid α-glucosidase (acid maltase) deficiency. Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (Eds.). The online metabolic and molecular bases of inherited disease. McGraw Hill. https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225890450
Prigent H, Orlikowski D, Laforet P et al (2012) Supine volume drop and diaphragmatic function in adults with Pompe disease. Eur Respir J. 39(6):1545–1546
van der Beek NA, van Capelle CI, van der Velden-van Etten KI et al (2011) Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol Genet Metab. 104(1-2):129–136
Hagemans ML, Winkel LP, Hop WC, Reuser AJ, Van Doorn PA, Van der Ploeg AT (2005) Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 64(12):2139–2141
Harlaar L, Hogrel JY, Perniconi B et al (2019) Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 93(19):e1756–e1e67
van der Meijden JC, Kruijshaar ME, Rizopoulos D, van Doorn PA, van der Beek N, van der Ploeg AT (2018) Enzyme replacement therapy reduces the risk for wheelchair dependency in adult Pompe patients. Orphanet J Rare Dis. 13(1):82
Xu S, Lun Y, Frascella M et al (2019) Improved efficacy of a next-generation ERT in murine Pompe disease. JCI Insight 4(5)
Pena LDM, Barohn RJ, Byrne BJ et al (2019) Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalglucosidase alfa (neoGAA) in treatment-naive and alglucosidase alfa-treated patients with late-onset Pompe disease: a phase 1, open-label, multicenter, multinational, ascending dose study. Neuromuscul Disord. 29(3):167–186
Colella P, Mingozzi F (2019) Gene therapy for Pompe disease: the time is now. Hum Gene Ther.
van der Ploeg AT, Clemens PR, Corzo D et al (2010) A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 362(15):1396–1406
Stepien KM, Hendriksz CJ, Roberts M, Sharma R (2016) Observational clinical study of 22 adult-onset Pompe disease patients undergoing enzyme replacement therapy over 5years. Mol Genet Metab. 117(4):413–418
Angelini C, Semplicini C, Ravaglia S et al (2012) Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol. 259(5):952–958
Kuperus E, Kruijshaar ME, SCA W et al (2017) Long-term benefit of enzyme replacement therapy in Pompe disease: a 5-year prospective study. Neurology.
Wens SC, Ciet P, Perez-Rovira A et al (2015) Lung MRI and impairment of diaphragmatic function in Pompe disease. BMC Pulm Med. 15:54
Gaeta M, Musumeci O, Mondello S et al (2015) Clinical and pathophysiological clues of respiratory dysfunction in late-onset Pompe disease: new insights from a comparative study by MRI and respiratory function assessment. Neuromuscular Disord. 25(11):852–858
Mogalle K, Perez-Rovira A, Ciet P et al (2016) Quantification of diaphragm mechanics in Pompe disease using dynamic 3D MRI. PLoS One. 11(7):e0158912
Harlaar L, Ciet P, van Tulder G et al (2021) Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet J Rare Dis. 16(1):21
American Thoracic Society/European Respiratory Society (2002) ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 166(4):518–624
Quanjer PH, Stanojevic S, Cole TJ et al (2012) Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 40(6):1324–1343
Wilson SH, Cooke NT, Edwards RH, Spiro SG (1984) Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax. 39(7):535–538
Salamon E, Lever S, Kuo W, Ciet P, Tiddens HA (2017) Spirometer guided chest imaging in children: it is worth the effort! Pediatr Pulmonol. 52(1):48–56
Ronneberger O, Fischer P, Brox T (2015) U-net: convolutional networks for biomedical image segmentation. In: International Conference on Medical image computing and computer-assisted intervention. Springer, pp 234–241
McCool FD, Tzelepis GE (2012) Dysfunction of the diaphragm. N Engl J Med. 366(10):932–942
Acknowledgements
We thank prof. Dimitris Rizopoulos (Erasmus MC, University Medical Center Rotterdam, Department of Biostatistics) for his help with the statistical analyses of the results.
Funding
This study has received funding by the Prinses Beatrix Spierfonds (project no.W.OR15-10) and by ZonMw (Veni grant to NAME vd Beek; No. 09150161910230).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Pieter A. van Doorn, MD PhD.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
• PC received writing grants outside the submitted work from Editamed Srl.
• NAMEB received funding for research and as advisor or speaker from Sanofi-Genzyme, under agreements with Erasmus MC University Medical Center.
• HAWMT received unconditional research grants outside the submitted work from Roche, Novartis, CFF, Vertex, Chiesi, Vectura, and Gilead and participated in the last 5 years in expert panels for Vertex and Gilead. All financial aspects for the grants are handled by Sophia Research BV. HAWMT is director of the Erasmus MC Lung Analysis laboratory and acts as consultant for Thirona.
• ATP received funding for research and/or clinical trials and as advisor or speaker from Amicus, Sanofi-Genzyme, Ultragenix, Spark Therapeutics, Audentes, Biomarin, Shire, Ciesi, and Sarepta under agreements with Erasmus MC University Medical Center and the relevant industry.
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Prof. Dimitris Rizopoulos (Erasmus MC, University Medical Center Rotterdam, Department of Biostatistics) kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
In the current prospective study, 30 Pompe patients and 10 healthy controls were included. All these participants initially participated in a cross-sectional study, in which we reported a decreased motion and an abnormal curvature of the diaphragm during inspiration in Pompe patients (Harlaar L, Ciet P, van Tulder G, et al Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet Journal of Rare Diseases. 2021;16(1):21).
In this follow-up study, we repeated the MRI study after 1 year, to evaluate possible changes in the motion and curvature of the diaphragm over time and during treatment with enzyme replacement therapy. We used 3D SPGR breath-hold acquisitions at end-expiration and end-inspiration, instead of dynamic sagittal 2D images that were used in the cross-sectional study. Therefore, we were able to compare MRI outcomes on a matching sagittal level at the initial MRI and the follow-up MRI. In addition, we developed automatic lung segmentations, while in the cross-sectional study we used manual lung segmentations.
Methodology
• prospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1501 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harlaar, L., Ciet, P., van Tulder, G. et al. MRI changes in diaphragmatic motion and curvature in Pompe disease over time. Eur Radiol 32, 8681–8691 (2022). https://doi.org/10.1007/s00330-022-08940-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08940-y