Abstract
Objectives
To identify useful features to predict hidden pancreatic malignancies in patients with main pancreatic duct (MPD) abrupt cutoff and dilatation, but without visible focal pancreatic lesions on CT.
Methods
This retrospective study included 92 patients (mean age, 63.4 ± 10.6 years, 63 men and 29 women) with MPD abrupt cutoff and dilatation, but without visible focal pancreatic lesion on contrast-enhanced CT between 2009 and 2021. Two radiologists independently evaluated the CT imaging features. Multivariable logistic regression analysis was performed to identify clinical and CT imaging features for hidden pancreatic malignancies. A nomogram was developed based on these results and assessed its performance.
Results
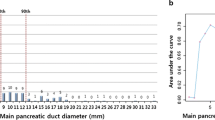
Thirty-eight (41.3%) and 54 (58.7%) were classified into the malignant and benign groups, respectively. In the multivariable analysis, CA19-9 elevation (odds ratio [OR] 7.5, p = 0.003), duct cutoff site at the head/neck (OR 7.6, p = 0.006), parenchymal contour abnormality at the duct cutoff site (OR 13.7, p < 0.001), and presence of acute pancreatitis (OR 11.5, p = 0.005) were independent predictors of pancreatic malignancy. A combination of any two significant features showed an accuracy of 77.2%, and a combination of any three features exhibited a specificity of 100%. The CT-based nomogram showed an area under the curve (AUC) of 0.84 (95% confidence interval, 0.77–0.90).
Conclusions
The three CT imaging features and CA19-9 elevation translated into a nomogram permit a reliable estimation of hidden pancreatic malignancies in patients with MPD abrupt cutoff without visible focal pancreatic lesion. It may facilitate determining whether to proceed to further diagnostic tests.
Key Points
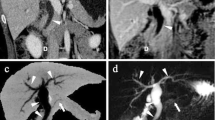
• Isoattenuating pancreatic ductal adenocarcinoma can manifest only as an isolated main pancreatic duct (MPD) dilatation with abrupt cutoff, making it difficult to distinguish from benign strictures.
• Along with the serum CA 19-9 elevation, MPD cutoff site at the pancreas head or neck, parenchymal contour abnormality at the duct cutoff site, and associated acute pancreatitis indicated a higher probability of the malignant MPD strictures.
• The CT-based nomogram provided excellent diagnostic performance (AUC of 0.84) for hidden pancreatic malignancies in patients with MPD abrupt cutoff and dilatation.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the ROC curve
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- EUS:
-
Endoscopic ultrasonography
- FNA:
-
Fine needle aspiration
- ICC:
-
Intraclass correlation coefficients
- IPMN:
-
Intraductal papillary mucinous neoplasm
- LR (+):
-
Positive likelihood ratio
- LR (−):
-
Negative likelihood ratio
- MPD:
-
Main pancreatic duct
- OR:
-
Odds ratio
- PDAC:
-
Pancreatic ductal adenocarcinoma
- ROC curve:
-
Receiver operating characteristic curve
- CA 19-9:
-
Carbohydrate antigen 19-9
References
Takeshita K, Kutomi K, Haruyama T et al (2010) Imaging of early pancreatic cancer on multidetector row helical computed tomography. Br J Radiol 83:823–830
Kim JH, Park SH, Yu ES et al (2010) Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 257:87–96
Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB Jr (2002) Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 224:764–768
Yoon SH, Lee JM, Cho JY et al (2011) Small (</= 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 259:442–452
Edge MD, Hoteit M, Patel AP, Wang X, Baumgarten DA, Cai Q (2007) Clinical significance of main pancreatic duct dilation on computed tomography: single and double duct dilation. World J Gastroenterol 13:1701–1705
Ahn SS, Kim MJ, Choi JY, Hong HS, Chung YE, Lim JS (2009) Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol 19:2448–2455
Tanaka S, Nakao M, Ioka T et al (2010) Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: a prospective study. Radiology 254:965–972
Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL (2003) Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 138:427–423 discussion 433-424
Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y (1995) Analysis of small cystic lesions of the pancreas. Int J Pancreatol 18:197–206
Kim SW, Kim SH, Lee DH et al (2017) Isolated main pancreatic duct dilatation: CT differentiation between benign and malignant causes. AJR Am J Roentgenol 209:1046–1055
Tanaka S, Nakaizumi A, Ioka T et al (2002) Main pancreatic duct dilatation: a sign of high risk for pancreatic cancer. Jpn J Clin Oncol 32:407–411
Johnston A, Serhal A, Lopes Vendrami C et al (2020) The abrupt pancreatic duct cutoff sign on MDCT and MRI. Abdom Radiol (NY) 45:2476–2484
Nakahodo J, Kikuyama M, Nojiri S et al (2020) Focal parenchymal atrophy of pancreas: an important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 20:1689–1697
Yamao K, Takenaka M, Ishikawa R et al (2020) Partial pancreatic parenchymal atrophy is a new specific finding to diagnose small pancreatic cancer (≤10 mm) including carcinoma in situ: comparison with localized benign main pancreatic duct stenosis patients. Diagnostics 10:445
Turkvatan A, Erden A, Turkoglu MA, Secil M, Yener O (2015) Imaging of acute pancreatitis and its complications. Part 1: acute pancreatitis. Diagn Interv Imaging 96:151–160
Modolell I, Guarner L, Malagelada JR (1999) Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol 10(Suppl 4):82–84
Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371:1039–1049
Shimizu Y, Yasui K, Matsueda K, Yanagisawa A, Yamao K (2005) Small carcinoma of the pancreas is curable: new computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol 20:1591–1594
Bracci PM, Wang F, Hassan MM, Gupta S, Li D, Holly EA (2009) Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control 20:1723–1731
Kang JD, Clarke SE, Costa AF (2021) Factors associated with missed and misinterpreted cases of pancreatic ductal adenocarcinoma. Eur Radiol 31:2422–2432
Poruk KE, Gay DZ, Brown K et al (2013) The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med 13:340–351
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL (2006) Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 24:2897–2902
Yuan C, Babic A, Khalaf N et al (2020) Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol 6:e202948
Hayano K, Miura F, Wada K et al (2016) Diffusion-weighted MR imaging of pancreatic cancer and inflammation: prognostic significance of pancreatic inflammation in pancreatic cancer patients. Pancreatology 16:121–126
Srisajjakul S, Prapaisilp P, Bangchokdee S (2020) CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol Med 125:356–364
Prokesch RW, Schima W, Chow LC, Jeffrey RB (2003) Multidetector CT of pancreatic adenocarcinoma: diagnostic advances and therapeutic relevance. Eur Radiol 13:2147–2154
Macari M, Spieler B, Kim D et al (2010) Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol 194:W27–W32
Coursey CA, Nelson RC, Boll DT et al (2010) Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 30:1037–1055
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the South Korean government (MSIT) (No. 2020R1F1A1071531).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ji Hye Min in Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
A statistician (Ji Eun Moon) of medical statistics (Department of Biostatistics, Clinical Trial Center, Soonchunhyang University Bucheon Hospital, Bucheon, Korea) kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained (IRB FILE No: 2021-10-076).
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 965 kb)
Rights and permissions
About this article
Cite this article
Lim, C.Y., Min, J.H., Hwang, J.A. et al. Assessment of main pancreatic duct cutoff with dilatation, but without visible pancreatic focal lesion on MDCT: a novel diagnostic approach for malignant stricture using a CT-based nomogram. Eur Radiol 32, 8285–8295 (2022). https://doi.org/10.1007/s00330-022-08928-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08928-8