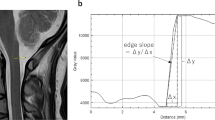
Abstract
Objectives
To investigate whether deep learning reconstruction (DLR) provides improved cervical spine MR images using a 1.5 T unit in the evaluation of degenerative changes without increasing imaging time.
Methods
This study included 21 volunteers (age 42.4 ± 11.9 years; 17 males) who underwent 1.5 T cervical spine sagittal T2-weighted MRI. From the imaging data with number of acquisitions (NAQ) of 1 or 2, images were reconstructed with DLR (NAQ1-DLR) and without DLR (NAQ1) or without DLR (NAQ2), respectively. Two readers evaluated the images for depiction of structures, artifacts, noise, overall image quality, spinal canal stenosis, and neuroforaminal stenosis. The two readers read studies blinded and randomly. Values were compared between NAQ1-DLR and NAQ1 and between NAQ1-DLR and NAQ2 using the Wilcoxon signed-rank test.
Results
The analyses showed significantly better results for NAQ1-DLR compared with NAQ1 and NAQ2 (p < 0.023), except for depiction of disc and foramina by one reader and artifacts by both readers in the comparison between NAQ1-DLR and NAQ2. Interobserver agreements (Cohen’s weighted kappa [97.5% confidence interval]) in the evaluation of spinal canal stenosis for NAQ1-DLR/NAQ1/NAQ2 were 0.874 (0.866–0.883)/0.778 (0.767–0.789)/0.818 (0.809–0.827), respectively, and those in the evaluation of neuroforaminal stenosis were 0.878 (0.872–0.883)/0.855 (0.849–0.860)/0.852 (0.845–0.860), respectively.
Conclusions
DLR improved the 1.5 T cervical spine MR images in the evaluation of degenerative spine changes.
Key Points
• Two radiologists demonstrated that deep learning reconstruction reduced the noise in cervical spine sagittal T2-weighted MR images obtained using a 1.5 T unit.
• Reduced noise in deep learning reconstruction images resulted in a clearer depiction of structures, such as the spinal cord, vertebrae, and zygapophyseal joint.
• Interobserver agreement in the evaluation of spinal canal stenosis and foraminal stenosis on cervical spine MR images was significantly improved using deep learning reconstruction (0.874 and 0.878, respectively) versus without deep learning (0.778–0.818 and 0.852–0.855, respectively).




Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- DLR:
-
Deep learning reconstruction
- NAQ:
-
Number of acquisitions
References
Brinjikji W, Luetmer PH, Comstock B et al (2015) Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 36(4):811–816
Theodore N (2020) Degenerative cervical spondylosis. N Engl J Med 383(2):159–168
Korzan JR, Gorassini M, Emery D, Taher ZA, Beaulieu C (2002) In vivo magnetic resonance imaging of the human cervical spinal cord at 3 Tesla. J Magn Reson Imaging 16(1):21–27
Galley J, Sutter R, Germann C, Wanivenhaus F, Nanz D (2021) High-resolution in vivo MR imaging of intraspinal cervical nerve rootlets at 3 and 7 Tesla. Eur Radiol 31(7):4625–4633
Takahashi M, Uematsu H, Hatabu H (2003) MR imaging at high magnetic fields. Eur J Radiol 46(1):45–52
Meacock J, Schramm M, Selvanathan S et al (2021) Systematic review of radiological cervical foraminal grading systems. Neuroradiology 63(3):305–316
Park HJ, Kim SS, Lee SY et al (2013) A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol 86(1025):20120515
Engel G, Bender YY, Adams LC et al (2019) Evaluation of osseous cervical foraminal stenosis in spinal radiculopathy using susceptibility-weighted magnetic resonance imaging. Eur Radiol 29(4):1855–1862
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O (2018) Deep learning with convolutional neural network in radiology. Jpn J Radiol 36(4):257–272
Chartrand G, Cheng PM, Vorontsov E et al (2017) Deep learning: a primer for radiologists. Radiographics 37(7):2113–2131
Joo B, Ahn SS, Yoon PH et al (2020) A deep learning algorithm may automate intracranial aneurysm detection on MR angiography with high diagnostic performance. Eur Radiol 30(11):5785–5793
Park S, Lee SM, Kim W et al (2021) Computer-aided detection of subsolid nodules at chest CT: improved performance with deep learning-based CT section thickness reduction. Radiology 299(1):211–219
Yasaka K, Akai H, Abe O, Kiryu S (2018) Deep learning with convolutional neural network for differentiation of liver masses at dynamic contrast-enhanced CT: a preliminary study. Radiology 286(3):887–896
Kiryu S, Yasaka K, Akai H et al (2019) Deep learning to differentiate parkinsonian disorders separately using single midsagittal MR imaging: a proof of concept study. Eur Radiol 29(12):6891–6899
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Liver fibrosis: deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images. Radiology 287(1):146–155
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Deep learning for staging liver fibrosis on CT: a pilot study. Eur Radiol 28(11):4578–4585
Higaki T, Nakamura Y, Tatsugami F, Nakaura T, Awai K (2019) Improvement of image quality at CT and MRI using deep learning. Jpn J Radiol 37(1):73–80
Tamada D, Kromrey ML, Ichikawa S, Onishi H, Motosugi U (2020) Motion artifact reduction using a convolutional neural network for dynamic contrast enhanced MR imaging of the liver. Magn Reson Med Sci 19(1):64–76
Kidoh M, Shinoda K, Kitajima M et al (2020) Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci 19(3):195–206
Kang Y, Lee JW, Koh YH et al (2011) New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol 197(1):W134–W140
Cohen J (1968) Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 70(4):213–220
Lee JE, Park HJ, Lee SY et al (2017) Interreader reliability and clinical validity of a magnetic resonance imaging grading system for cervical foraminal stenosis. J Comput Assist Tomogr 41(6):926–930
Funding
This study was financially supported by Canon Medical Systems Corporation. Any data and information included in this study was not controlled by Canon Medical Systems Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shigeru Kiryu.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Shigeru Kiryu got research grants from Canon Medical Systems Corporation.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Yasaka, K., Tanishima, T., Ohtake, Y. et al. Deep learning reconstruction for 1.5 T cervical spine MRI: effect on interobserver agreement in the evaluation of degenerative changes. Eur Radiol 32, 6118–6125 (2022). https://doi.org/10.1007/s00330-022-08729-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08729-z