Abstract
Objectives
The aim of this study was to compare the quality of images obtained using single-energy computed tomography (SECT) performed with automated tube voltage adaptation (TVA) with dual-energy CT (DECT) weighted average images.
Methods
Eighty patients were prospectively randomized to undergo either SECT with TVA (n = 40, ref. mAs 200) or radiation dose–matched DECT (n = 40, 80/Sn150 kV, ref. mAs tube A 91/tube B 61) on a dual-source CT scanner. Objective image quality was evaluated as dose-normalized contrast-to-noise ratio (CNRD) for the jugular veins relative to fatty tissue and muscle tissue and for muscle tissue relative to fatty issue. For subjective image quality, reproduction of anatomical structures, image artifacts, image noise, spatial resolution, and overall diagnostic acceptability were evaluated at sixteen anatomical substructures using Likert-type scales.
Results
Effective radiation dose (ED) was comparable between SECT and DECT study groups (2.9 ± 0.6 mSv/3.1 ± 0.7 mSv, p = 0.5). All examinations were rated as excellent or good for clinical diagnosis. Compared to the CNRD in the SECT group, the CNRD in the DECT group was significantly higher for the jugular veins relative to fatty tissue (7.51/6.08, p < 0.001) and for muscle tissue relative to fatty tissue (4.18/2.90, p < 0.001). The CNRD for the jugular veins relative to muscle tissue (3.33/3.18, p = 0.51) was comparable between groups. Image artifacts were less pronounced and overall diagnostic acceptability was higher in the DECT group (all p = 0.01).
Conclusions
DECT weighted average images deliver higher objective and subjective image quality than SECT performed with TVA in head and neck imaging.
Key Points
• Weighted average images derived from dual-energy CT deliver higher objective and subjective image quality than single-energy CT using automated tube voltage adaptation in head and neck imaging.
• If available, dual-energy CT acquisition may be preferred over automated low tube voltage adopted single-energy CT for both malignant and non-malignant conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to its wide availability, fast acquisition, and relatively low costs, computed tomography (CT) examinations are becoming more common worldwide [1, 2]. A major drawback of this technique is the use of x-rays, which are responsible for the majority of the radiation burden in diagnostic imaging and may increase individual cancer risk [3].
Different technical solutions like tube current modulation (TCM) and tube voltage adaptation (TVA) have been developed and introduced into clinical routine to minimize patient exposure while optimizing image quality [4], which is mainly defined by image noise and image contrast. With the widespread availability of powerful x-ray tubes, the combination of TCM and TVA is extensively used in clinical routine and generates images with high contrast-to-noise ratios (CNRs) [5]. Another promising technique to increase CNR is dual-energy CT (DECT). However, implementation of predicted low-kilo-electron-volt reconstructions (virtual monoenergetic images; VMI) in clinical routine is time consuming and therefore cost intensive. In contrast to VMI, weighted average image (WAI) reconstructions derived from DECT are instantly available at the scanner site. WAI are comparable to standard single-energy images acquired with 120 kV and offer a balanced trade-off between image contrast and image noise [6, 7]. In dual-source CT, the two x-ray tubes can be used either with the same tube voltage (single-energy CT; SECT) or with different tube voltages (DECT) [8]. While TCM is available in both settings, TVA is restricted to SECT. Thus, it remains unclear whether WAI reconstructions from DECT may generate equal or even superior image quality compared to single-energy images acquired with TVA on a dual-source CT scanner. This is of particular interest in head and neck imaging, which naturally suffers from low native contrast between soft tissues, which makes the administration of an iodine-based contrast agent almost indispensable [9].
The aim of this study was to evaluate the inter-individual image quality between dose-matched dual-energy-based WAI reconstructions and automatically selected low-tube-voltage single-energy CT images of the head and neck.
Materials and methods
Patient population
A total of 80 patients scheduled for a head and neck CT were included in this prospective, single-center study. The patients’ diseases covered a mixed spectrum including malignant and non-malignant conditions (Table 1); most patients with suspected malignancy underwent head and neck CT due to staging completion. Four patients had a primary malignancy in the head and neck region (Fig. 1). Patients were randomly assigned to either the DECT or the SECT study group prior to examination (40 patients per study group). Subjects with contraindications for CT imaging with intravenous administration of iodine contrast agents, such as history of allergies or intolerance to iodine contrast agents, nephropathy grade 4 or higher (estimated glomerular filtration rate < 30 mL/min/1.73 m2), hyperthyroidism, age < 18 years, and pregnancy, were excluded. All patients signed written informed consent for CT examination and study participation. The study was approved by the local Institutional Review Board and adheres to the Health Insurance Portability and Accountability Act (HIPAA) criteria and the Declaration of Helsinki.
CT technique and acquisition protocol
All examinations were performed on a third-generation dual-source CT scanner (Siemens SOMATOM Force, Siemens Healthcare GmbH) equipped with two 192-row energy integrating detectors (Siemens Stellar-Infinity, Siemens Healthcare GmbH) arranged in an orthogonal direction. The scanner provides a maximum of 240 kW generator power (2 × 120 kW).
In both study groups, CT acquisition of the neck was performed with both arms lowered and placed beside the trunk. After performing the localizer and determining the field of view, 80 mL of iodine contrast agent (Imeron 350 mg/mL, Bracco GmbH) was intravenously injected at a flow rate of 3 mL/s, followed by a saline bolus (30 mL, 3 mL/s). Both the DECT and the SECT examination protocols were performed after a fixed delay of 80 s for all study participants. A real-time automatic tube current modulation algorithm (CARE Dose4D, Siemens Healthcare GmbH) was used for all examinations in both study groups.
Single-energy CT acquisition
An automatic TVA algorithm (CARE kV, Siemens Healthcare GmbH) was used for the SECT study group. TVA is capable of tube voltage settings from 70 to 150 kV in discrete steps of 10 kV. A preselection of TVA presets for different applications is available and can be directly adjusted by the user on a 12-point slider. In this study, a mid-level TVA setting (slider position 7 of 12) was chosen to keep the CNR balanced. To avoid excess radiation dose, TVA was limited from 70 to 120 kV.
Dual-energy CT acquisition
DECT acquisition was performed with tube voltages of 80 kV (tube A) and 150 kV (tube B). Both x-ray beams were pre-filtered with an aluminum bowtie filter, with tube B equipped with an additional 0.5-mm tin prefiltration in order to refine the spectral separation. Table 2 provides a detailed overview of the acquisition and reconstruction parameters for the SECT and DECT groups.
Weighted average images
Based on DECT raw data, WAI series were reconstructed in-line on the scanner console with a slice thickness of 3 mm in axial orientation and soft (Bf40) and sharp (Br64) convolution kernels. In principle, WAIs are intended to provide image quality equivalent to that of conventional SECT reconstructions. A multiband filtered algorithm was applied to DECT raw data as recommended by the vendor (F-type reconstruction 0.7, nonlinearly merging 70% of the 80 kV and 30% of the 150 kV data spectra).
Single-energy CT images
Like the WAIs, SECT data were reconstructed with 3-mm slice thickness in axial orientation using equivalent soft (Bf40) and sharp (Br64) convolution kernels. Convolution kernels for WAI and SECT images were vendor matched to ensure comparability. Concordant to WAI, no iterative reconstruction algorithm was used in order to limit the bias between SECT and DECT images.
Objective image quality
After anonymization, evaluation of image quality was performed on a dedicated workstation with a server/client-based software (Syngo.via VB 20, Siemens Healthcare GmbH) by two board-certified radiologists with 6 and 11 years of experience in head and neck imaging. All imaging series were presented in a randomized order, and both readers were blinded to other imaging data and the patients’ medical history. Regions of interest (ROIs) were placed on axial slices in both jugular veins, in both sternocleidomastoid muscles, and in both fat-containing parapharyngeal spaces. All ROIs were manually drawn as large as possible while carefully avoiding the inclusion of neighboring structures and artifacts. Reproducibility was ensured by defining specific anatomic positions on the axial slices: ROIs in jugular vein and sternocleidomastoid muscle were drawn between the lower boundary of the mandible and the hyoid bone, ROIs in the parapharyngeal space were drawn between the angle and the head of the mandible. In both positions, the readers had to check for artifacts before ROI measurements were performed. See Fig. 2 for an illustration of ROI placement. In order to obtain objective parameters of image quality, mean attenuation (A) of every ROI was measured in Hounsfield units (HU). Fatty tissue within the parapharyngeal space was used as the reference for image noise (N) by measuring the standard deviation of the mean attenuation. Image review began with a default soft tissue (center 50 HU/width 400 HU) and bone window (450/1500 HU) that could be adjusted at the reader’s discretion. CNR was calculated using Eq. 1 and dose-normalized CNR (CNRD) was calculated using Eq. 2.
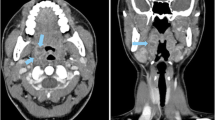
Image illustrating the regions of interest drawn in predefined anatomic structures. Image from a 68-year-old female patient who underwent single-energy CT. The image on the left side (a) shows the level chosen for measurement in the parapharyngeal space (marked as white circles), and the image on the right side (b) depicts the level chosen for measurement in the internal jugular veins (marked as black circles) and the adjacent sternocleidomastoid muscles (marked as white circles). Regions of interest were carefully drawn to avoid artifacts from dental hardware
Subjective image quality
Sixteen anatomical substructures were evaluated using a default soft tissue window setting (center 50 HU/width 400 HU) according to the European Guidelines on Quality Criteria for CT [10]. The substructures analyzed were as follows: pharynx (1: wall of the pharynx, 2: mucosal margins, 3: parapharyngeal fat spaces, 4: parapharyngeal muscles), larynx (5: wall of the larynx, 6: mucosal folds, 7: perimucosal fat spaces, 8: intrinsic laryngeal muscles, 9: paralaryngeal muscles), salivary glands (10: glandular tissue, 11: margins of normal glands, 12: paraglandular fat spaces, 13: mandible and associated muscles), and lymphatic tissue (14: regional lymph node areas of the pharynx, 15: regional lymph node areas of the larynx, 16: regional lymph node areas of the salivary glands). Overall image noise, spatial resolution, and diagnostic acceptability were also evaluated.
In compliance with the guidelines mentioned above, each reader assessed the visually sharp reproduction of anatomical substructures on a two-point Likert-type scale (1: no, 2: yes), the image noise and spatial resolution on a three-point Likert-type scale (1: too little, 2: optimum, 3: too much), and the overall diagnostic acceptability on a four-point Likert-type scale (1: fully confident for diagnostic interpretation, 2: probably confident for interpretation, 3: confident only under limited conditions for visualization of abnormalities, 4: unacceptable). Image artifacts were also graded on a four-point Likert-type scale (1: no artifacts, 2: minor artifacts not affecting the visualization of any structure, 3: major artifacts affecting the visualization of normal structures, 4: artifacts affecting diagnostic information). Beam hardening and photon starvation artifacts caused by metal dental hardware were not evaluated. Inter-rater agreement was calculated based on the results obtained for each category and each acquisition technique.
Radiation exposure
The radiation exposure was quantified as pitch-corrected computed tomography dose index (CTDIvol) and dose length product (DLP) as provided by the scanner. The effective radiation dose (ED) associated with the CT examination was calculated using the specific conversion factor for neck examinations in adults (0.0051 mSv × mGy−1 × cm−1) [11]. The estimated radiation exposure in the SECT group was matched to that of the DECT group in an ex ante trial using a dedicated 16-cm acrylic CTDI phantom by adjusting the reference tube current time product stepwise. Single-energy examinations were referenced to a 32-cm phantom; thus, values had to be converted to match the 16-cm phantom [11]. According to the vendor (Siemens Healthcare GmbH), the respective conversion factor for the CT system used in this study is 2.0 for 120 kV, but it must be adapted according to the chosen kilovolt value by TVA to a maximum of 2.4 at 70 kV.
Statistical analysis
Interval-level data were evaluated for normal distribution using the Shapiro–Wilk test. If the data were assumed to be normally distributed, values are given as mean ± standard deviation; otherwise, and in cases of ordinal-level data, values are given as median and interquartile range. For comparison of objective image quality data and radiation exposure between the SECT and DECT groups, a non-parametric Mann–Whitney U test was performed, as normal distribution could not be assumed according to the Shapiro–Wilk test. Comparison of subjective image quality ratings was performed using a Wilcoxon rank sum test. Inter-rater agreement was evaluated by calculating Cohen’s kappa value (κ); κ was interpreted according to Landis and Koch [12]. Results were accepted as statistically significant for p values < 0.05. The Statistical Package for the Social Sciences (SPSS) was used for randomization process and data analysis (IBM SPSS for Windows, Version 24.0 released 2016, IBM Corp.).
Results
In the SECT group, the study population consisted of 16 female and 24 male patients with a mean age of 62 ± 12 years, while the DECT group consisted of 14 female and 26 male patients with a mean age of 66 ± 12 years.
Objective image quality
In SECT and DECT, ROIs for attenuation analysis could be drawn in all study participants (Fig. 2). Mean ROI size was 0.47 ± 0.18 cm2 for vessel attenuation, 0.60 ± 0.13 cm2 for muscle attenuation, and 0.33 ± 0.15 cm2 for parapharyngeal fatty tissue. For DECT, a significantly increased CNR and CNRD was found for jugular veins relative to fatty tissue and for muscle tissue relative to fatty tissue compared to SECT (all p < 0.001). No significant difference was found for vessel attenuation relative to muscle tissue (CNR p = 0.36; CNRD p = 0.35). Table 3 summarizes all results from the objective image quality assessment.
Subjective image quality
All anatomical substructures evaluated were visually sharp and definable in all SECT and DECT examinations.
Image noise and spatial resolution were comparable between DECT and SECT for both readers. In contrast, overall diagnostic acceptability and image artifacts differed significantly between the DECT and SECT groups in favor of DECT for both readers. See Table 4 for a detailed overview and specific p values. Figure 3 provides a sample comparison of artifacts at the height of the shoulders between SECT and DECT.
Streak artifacts at the cervicothoracic transition zone in single-energy CT (SECT). Streak artifact (white arrows) found at the level of the shoulders with the SECT technique. Images are from a 63-year-old male patient with a cT2N0 carcinoma of the oropharynx who underwent SECT at 70 kV (a) and a 52-year-old male patient who underwent dual-energy CT (DECT) for staging completion (b). All images are shown in the same window setting (center = 50 HU, width = 500 HU). No streak artifacts were found with DECT at the height of the shoulders
Inter-rater agreement for image noise evaluation, overall diagnostic acceptability, and image artifacts was substantial for SECT and DECT (κ > 0.7). A moderate inter-rater agreement was found for spatial resolution (SECT and DECT κ = 0.6).
Radiation exposure
In the SECT group, TVA automatically selected 70 kV in 3 of the 40 patients (7.5%), 80 kV in 10 patients (25%), 90 kV in 19 patients (47.5%), and 100 kV in 8 patients (20%). Neither 110 nor 120 kV was selected. CTDIvol, DLP, and ED were comparable between the study groups. Table 5 provides a detailed overview.
Discussion
In our study, WAI delivered superior CNR and CNRD values for the sternocleidomastoid muscle relative to fat and for the jugular vein relative to fat in head and neck imaging compared to dose-matched standard SECT images with automated TVA. Image noise and spatial resolution were comparable between the study groups, but WAIs had fewer image artifacts and better overall diagnostic acceptability than SECT images.
Head and neck imaging is especially challenging due to the relevant anatomic structures being in close proximity and low intrinsic contrast. For lesion detection and exact anatomic localization, image contrast must be maximized while image noise is minimized. To achieve a homogeneous distribution of noise over the scan range while complying with the “as low as reasonably achievable” (ALARA) principle in radiation protection, TCM is commonly implemented in all modern CT scanners. The tube current is automatically decreased in projections of low attenuation and increased in projections of high attenuation (like the cervicothoracic transition).
DECT acquires one data set, but allows for various post-processing options to simulate different energy levels and thereby generate different CNRs. Many studies have investigated the effect of using low-kilo-electron-volt VMIs like 40 keV to increase CNR, but direct comparison between low-kilovolt SECT and DECT is rare in head and neck imaging [7, 13, 14]. In a recently published study, SECT images with a fixed 70-kV setting were comparable to 40-keV VMI reconstructions for tumor delineation; however, the study focused on a limited scan region, excluding critical regions like the cervicothoracic transition [15]. Thus, in SECT, low-kilovolt scanning like 70 kV is suitable for soft tissue evaluation covering only parts of the neck, but its use is limited for challenging regions like the cervicothoracic region or the oral cavity, which may contain artifacts due to dental implants [16].
This is also reflected in the automatically selected tube voltages in our study. Only three patients were scanned with 70 kV; higher kilovolt values were used in over 90% of the patients. These settings, based on the patient anatomy, seem to be the best compromise between higher iodine attenuation and higher image noise in SECT. DECT delivered superior soft tissue contrast, as reflected by the CNR and CNRD values for sternocleidomastoid muscle relative to fat and for the jugular vein relative to fat. This is due to WAIs representing a blend of high- and low-energy information and thereby combining higher image contrast from low-kilovolt levels with lower image noise levels from higher-kilovolt levels (in our study, nonlinearly merging 70% of the 80-kV and 30% of the 150-kV data spectra). DECT not only improved objective parameters like CNRD, but also exhibited higher subjective image quality compared to SECT.
Future developments, such as instantly available low-kilo-electron-volt levels with no additional post-processing, will further establish the use of DECT, especially in anatomical regions like the head and neck that suffer from low intrinsic contrast.
Limitations
Some limitations must be considered when interpreting the results of our study:
-
First, due to ethical reasons, we could not perform an intra-individual comparison between SECT and DECT.
-
Second, only one vendor’s dual- and single-energy options were evaluated, and no direct comparison to other CT scanners is possible.
-
Third, dedicated follow-up trials must be performed to investigate whether the higher image quality of WAIs compared to SECT images also translates into increased diagnostic accuracy of lesion detection.
-
Fourth, no iterative reconstruction algorithms were used and evaluated in this study. Further studies may show additional benefits of iterative reconstruction algorithms.
Conclusion
DECT with instantly availably WAI delivers superior image quality compared to SECT with tube voltage adaptation and is beneficial for head and neck imaging, which suffers from low intrinsic contrast.
Abbreviations
- CNR:
-
Contrast-to-noise ratio
- CNRD:
-
Dose-normalized contrast-to-noise ratio
- CT:
-
Computed tomography
- HU:
-
Hounsfield units
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- STD:
-
Standard deviation
- TCM:
-
Tube current modulation
- TVA:
-
Tube voltage adaptation
References
Mettler FA Jr (2019) Medical radiation exposure in the United States: 2006-2016 Trends. Health Phys 116:126–128
Mettler FA Jr, Bhargavan M, Faulkner K et al (2009) Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology 253:520–531
Berrington de González A, Mahesh M, Kim KP et al (2009) Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169:2071–2077
Kalender WA, Buchenau S, Deak P et al (2008) Technical approaches to the optimisation of CT. Phys Med 24:71–79
May MS, Kramer MR, Eller A et al (2014) Automated tube voltage adaptation in head and neck computed tomography between 120 and 100 kV: effects on image quality and radiation dose. Neuroradiology 56:797–803
Tawfik AM, Kerl JM, Razek AA et al (2011) Image quality and radiation dose of dual-energy CT of the head and neck compared with a standard 120-kVp acquisition. AJNR Am J Neuroradiol 32:1994–1999
May MS, Wiesmueller M, Heiss R et al (2019) Comparison of dual- and single-source dual-energy CT in head and neck imaging. Eur Radiol 29:4207–4214
Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG (2008) Technical principles of dual source CT. Eur J Radiol 68:362–368
Forghani R, Mukherji SK (2018) Advanced dual-energy CT applications for the evaluation of the soft tissues of the neck. Clin Radiol 73:70–80
Panzer W, Shrimpton P, Jessen K (2000) European guidelines on quality criteria for computed tomography. In: Commission E (ed) Publications Office of the EU, pp 114
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Wichmann JL, Nöske EM, Kraft J et al (2014) Virtual monoenergetic dual-energy computed tomography: optimization of kiloelectron volt settings in head and neck cancer. Invest Radiol 49:735–741
Albrecht MH, Vogl TJ, Martin SS et al (2019) Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology 293:260–271
May MS, Bruegel J, Brand M et al (2017) Computed tomography of the head and neck region for tumor staging-comparison of dual-source, dual-energy and low-kilovolt, single-energy acquisitions. Invest Radiol 52:522–528
Gnannt R, Winklehner A, Goetti R, Schmidt B, Kollias S, Alkadhi H (2012) Low kilovoltage CT of the neck with 70 kVp: comparison with a standard protocol. AJNR Am J Neuroradiol 33:1014–1019
Acknowledgements
The authors of this manuscript declare no conflicts of interest with any company whose products or services may be related to the subject matter of this article.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Marco Wiesmueller.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med. dent.” at the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bedernik, A., Wuest, W., May, M.S. et al. Image quality comparison of single-energy and dual-energy computed tomography for head and neck patients: a prospective randomized study. Eur Radiol 32, 7700–7709 (2022). https://doi.org/10.1007/s00330-022-08689-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08689-4