Abstract
Objectives
To identify the main problem areas in the applicability of the current TNM staging system (8th ed.) for the radiological staging and reporting of rectal cancer and provide practice recommendations on how to handle them.
Methods
A global case-based online survey was conducted including 41 image-based rectal cancer cases focusing on various items included in the TNM system. Cases reaching < 80% agreement among survey respondents were identified as problem areas and discussed among an international expert panel, including 5 radiologists, 6 colorectal surgeons, 4 radiation oncologists, and 3 pathologists.
Results
Three hundred twenty-one respondents (from 32 countries) completed the survey. Sixteen problem areas were identified, related to cT staging in low-rectal cancers, definitions for cT4b and cM1a disease, definitions for mesorectal fascia (MRF) involvement, evaluation of lymph nodes versus tumor deposits, and staging of lateral lymph nodes. The expert panel recommended strategies on how to handle these, including advice on cT-stage categorization in case of involvement of different layers of the anal canal, specifications on which structures to include in the definition of cT4b disease, how to define MRF involvement by the primary tumor and other tumor-bearing structures, how to differentiate and report lymph nodes and tumor deposits on MRI, and how to anatomically localize and stage lateral lymph nodes.
Conclusions
The recommendations derived from this global survey and expert panel discussion may serve as a practice guide and support tool for radiologists (and other clinicians) involved in the staging of rectal cancer and may contribute to improved consistency in radiological staging and reporting.
Key Points
• Via a case-based online survey (incl. 321 respondents from 32 countries), we identified 16 problem areas related to the applicability of the TNM staging system for the radiological staging and reporting of rectal cancer.
• A multidisciplinary panel of experts recommended strategies on how to handle these problem areas, including advice on cT-stage categorization in case of involvement of different layers of the anal canal, specifications on which structures to include in the definition of cT4b disease, how to define mesorectal fascia involvement by the primary tumor and other tumor-bearing structures, how to differentiate and report lymph nodes and tumor deposits on MRI, and how to anatomically localize and stage lateral lymph nodes.
• These recommendations may serve as a practice guide and support tool for radiologists (and other clinicians) involved in the staging of rectal cancer and may contribute to improved consistency in radiological staging and reporting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “tumor node metastasis” (TNM) system is the most applied staging system in oncology. Although not specifically designed for radiological staging, TNM has been widely adopted by radiologists for diagnostic reporting of cancer, including rectal cancer. Still, there are several controversies in the radiological application of the TNM system for rectal cancer, leading to heterogeneity in reporting [1].
This study aims to gain further insight into these controversies and identify the main problem areas in using the current TNM (8th ed.) for the radiological reporting of rectal cancer. To this end, a global online survey with an emphasis on MRI for local staging was undertaken. This paper reports the outcome of this survey and the recommendations from a multidisciplinary expert panel on how to address the identified problem areas.
Methods
This study included five main steps (Fig. 1):
1—Online survey
An online survey (using Google forms) was constructed by two of the organizing authors (D.M.J.L., N.B.) including 41 cases/questions covering the main staging items included in TNM8 [2]. Every case included a single MRI (or CT) image and schematic representation and description of the imaging findings. Respondents were asked to answer each question based on these pre-specified imaging findings without having to interpret the images themselves. Cases were organized in 6 sections focused on clinical T staging (cT), anal canal involvement, mesorectal fascia (MRF) involvement, lymph nodes and tumor deposits, regional versus non-regional lymph nodes, and M staging. Respondents were also asked some general questions about their background and use of TNM in their clinical practice. The survey was distributed via the organizing authors’ professional networks and via member mailings of various (inter)national radiological and clinical societies. The full survey is provided in Supplement 1.
2—Analysis of survey results
Two of the organizing authors (D.M.J.L., N.B.) analyzed the survey results and calculated for each case/question the percentage agreement between respondents. Cases reaching ≥ 80% agreement were classified as “non-problem” areas; cases reaching < 80% agreement were classified as problem areas.
3—Panel selection
An international expert panel was composed, including five radiologists (L.K.B., M.J.G., S.A.T., D.J.M.T., R.G.H.B-T.), six colorectal surgeons (J.G-A., T.K., P.J.N., R.O.P., A.W., G.L.B.), four radiation oncologists (E.F., B.G., C.A.M., V.V.), and three pathologists (I.D.N., P.S., N.P.W.), each with recognized expertise in the field.
4—Preparation for panel meeting
Two of the organizing authors (D.M.J.L., N.B.) performed a focused review of the available literature related to the identified problem areas. For each problem area, a draft recommendation (when feasible) was constructed. These were sent to all panelists to acquire their input prior to the face-to-face meeting. Panelists could indicate whether they agreed with the proposed recommendation and provide their comments and suggestions.
5—Panel meeting
The face-to-face panel meeting took place online on June 1, 2021; 15/18 panelists attended. Each problem area (+ input acquired in step 4) was discussed and voted on. This process was repeated until a single recommendation was decided on. Two non-voting observers (D.M.J.L., N.B.) documented key discussion points and outcomes of the voting rounds. The three panelists who did not attend approved the documented recommendations afterwards via email.
Results
Respondents
The survey was completed by 321 respondents (from 32 countries), including 255 radiologists and 66 other clinicians. Further details are provided in Table 1. TNM8 was routinely used by 63% of respondents; 25% used previous TNM editions and 13% did not use TNM or did not know which TNM edition was being used in their center.
Survey outcomes
Detailed survey outcomes are provided in Table 2. Respondents reached ≥ 80% agreement for 25/41 (61%) of cases. The remaining 16 (39%) were classified as problem areas, related to:
-
cT staging in anal canal involvement
-
Definitions for cT4b disease
-
cT staging in MRF vs. peritoneal involvement
-
Definitions for MRF involvement
-
Definitions for lymph nodes versus tumor deposits
-
Definitions to assess regional and non-regional lymph nodes
-
Definitions for M1a disease
Specified subgroup results (per profession and experience level) are provided in Supplement 2. In 4 out of 16 problem cases, borderline agreement (73–79%) was reached, with ≥ 80% agreement for the subgroups of MRI experts and/or abdominal radiologists.
Panel recommendations
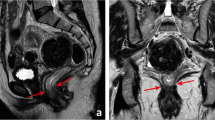
The panel recommendations for each problem area are detailed in Table 3. Figures 2 and 3 illustrate recommendations on cT staging in low-rectal cancers, and for MRF versus peritoneal involvement. Figure 4 provides an anatomical MRI map for lateral lymph node stations.
Left: survey results showing substantial variation in assessment of cT staging in cases with various degrees of anal sphincter or pelvic floor invasion. Right: panel recommendations stating not to include the internal sphincter (IS) and intersphincteric space (ISS) in cT-stage categorization, and to consider invasion of external sphincter (ES), puborectalis, and levator ani muscles (i.e., skeletal muscles) as cT4b disease
Anatomical overview of the lining of the mesorectal compartment by the MRF and peritoneum in the low, middle, and high parts of the rectum. Above the anterior peritoneal reflection, the mesorectum is lined by peritoneum anteriorly (mid) and anterolaterally (high). The remaining mesorectum is lined by the MRF. Invasion of the MRF constitutes cT3 MRF+ disease, while invasion of the peritoneum or peritoneal reflection constitutes cT4a disease. When both the peritoneum and MRF are involved, this constitutes cT4a MRF+ disease
Anatomical boundaries of lateral lymph node stations (external iliac, internal iliac, obturator) on MRI. EIA = external iliac artery, EIV = external iliac vein, IIA = internal iliac artery, IIV = internal iliac vein. The border between the internal iliac and obturator compartments is defined by the lateral border of the main trunk of the internal iliac vessels (II–IV). The posterior wall of the EIV defines the border between the external iliac and obturator plus internal iliac compartments (II–VI). *The infrapiriformis foramen represents the transit point of the internal iliac vessels from the internal iliac compartment into the pudendal canal (V). This figure is largely based on a map previously published by Ogura et al JAMA Surg 2019;254: e192172 (supplement) [26]
Discussion
Results of a global online survey with > 300 respondents on the application of TNM8 for the radiological staging of rectal cancer revealed several problem areas where TNM definitions are either ambiguous or difficult to apply to a radiological setting. Some problem areas were identified that mainly occurred for less experienced respondents, indicating a need for further education.
cT staging in low-rectal cancers involving the anal canal
cT staging in tumors involving the anal canal was among the topics that reached the least agreement (45–73%) between respondents. Definitions on how to incorporate anal involvement into cT stage are either not reported or vary between different TNM editions [3, 4]. The TNM system is primarily driven by prognostic outcome stratification, and evidence on how invasion into different layers of the anal canal translates into patient outcomes is largely lacking. Although several classification systems to address low-rectal cancer have been proposed [5, 6], none have been unanimously adopted into guidelines. There is now a growing tendency among professional societies to use descriptive prose to inform clinicians about involvement of the anal canal, rather than to rely solely on cT stage. This is a strategy that was also strongly supported by our panel. The panel further agreed that cT staging should primarily be informed by the extent of tumor invasion at the level of the rectum and that involvement of the internal anal sphincter and intersphincteric space should not be taken into account in cT-stage categorization. Considering that pathologists consider skeletal muscle invasion as pT4b disease and aiming to avoid inconsistencies between radiology and pathology reports, the panel agreed that involvement extending into the external anal sphincter, puborectalis, or levator ani muscles (i.e., skeletal muscles) should be classified as cT4b. The panel also stressed the need for good-quality MRI, including a high-resolution coronal T2-weighted sequence parallel to the anal canal. Finally, the panel recommended to include a statement or suffix in the conclusion of the radiological report when there is involvement of the anal canal (e.g., “anal+”) and to provide a detailed prose description on the extent of invasion in the body of the report given the evident impact on surgical treatment [7, 8] and radiotherapy planning [9].
Definitions of cT4b disease
The survey included a case with tumor invasion beyond the MRF into the fat of the obturator space; 57% of respondents considered this as deep cT3 infiltration, while 15% classified this as cT4b disease. This discrepancy can be explained by the fact that TNM does not provide a clear definition of what is covered by the umbrella term “structures” in their classification of cT4b disease as “any tumour with invasion of another organ or structure.” The panel agreed that from a surgical point of view, cT4b disease should include any tumor with direct invasion of either another organ and/or any anatomical compartment or structure (except peritoneum alone) outside the mesorectum, as this would require adaptation of the standard surgical resection plane. This rendered the proposed definitions for cT4b disease as outlined in Table 3.
Definitions for MRF involvement
The tumor-MRF distance is sometimes referred to by radiologists as the “circumferential resection margin” (CRM), which is not accurate. Unlike MRF, which is an anatomical term, the CRM is the margin the surgeon creates when performing a resection, and what pathologists report when describing the smallest distance between the tumor and the outer plane of the resected specimen. Ideally, this plane will be along the MRF, but the CRM may be smaller when the MRF is breached during surgery or wider when the resected specimen includes additional tissue outside the MRF. In such cases, the MRF may be free of tumor but with an involved CRM, or vice versa. To avoid confusion, radiologists should therefore not use CRM but describe the tumor in relation to the MRF [10].
Respondents reached ≥ 80% agreement that macroscopic MRF invasion (i.e., a 0-mm margin) defines an involved MRF, but cases with a margin of ≤ 1 mm or 1–2 mm lacked clear consensus. In most guidelines, a cut-off of ≤ 1 mm is currently adopted as a criterion for MRF involvement [2, 11,12,13]. A pathology report from Nagtegaal et al (note: describing CRM and not MRF margins) proposed a cut-off of ≤ 2 mm as these tumors still show a significantly increased risk for local recurrence (16% versus 6% for tumors with a > 2-mm margin), although tumors with a ≤ 1-mm margin clearly constituted the highest-risk group (36% local recurrences) [14]. The consensus guidelines on rectal MRI published by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) proposed a margin of ≤ 1 mm to define an involved MRF but also mentioned a margin of 1–2 mm (or ≤ 2 mm) as a “threatened” MRF [15]. This sub-classification has not been widely adopted, and according to the survey results, these ambiguous definitions are a potential source of confusion. The panel therefore agreed to adopt the ≤ 1-mm threshold as a uniform criterion to define an involved MRF, and discard the definition of a threatened MRF.
A second identified problem was that there are no validated definitions on how to classify MRF involvement by tumor-bearing structures other than the primary tumor. As outlined in a review by Gollub et al [1], pathologic lymph nodes causing positive margins at histopathology do not confer an added risk for local recurrence compared to control cases with non-involved margins [14]. Moreover, it is uncommon that mesorectal lymph nodes are the only factor responsible for margin involvement on histopathological examination [16]. Conclusive data on the prognostic importance of margin involvement by tumor deposits or extramural vascular invasion (EMVI) are currently not available, although a study by Birbeck et al suggested that margin involvement caused by EMVI or tumor deposits adds a 20% and 31% risk for local recurrence, respectively, versus a 42% added risk for direct tumor invasion [17].
Current guidelines do not include any specific recommendations on whether to stratify patients for neoadjuvant treatment based on MRF involvement by the primary tumor or by nodes, deposits, or EMVI, recognizing that further studies are strongly needed. The panel agreed that for now the MRF should be considered as involved in case of a margin of ≤ 1 mm from either the primary tumor; any irregularly enlarged lymph nodes or tumor deposits; or EMVI. The panel also recommended that radiologists should no longer consider the MRF as involved when potentially malignant smooth enlarged lymph nodes (i.e., with an apparently intact capsule) are contacting the MRF. The panel considered the prognostic implications of these nodes as low and recognized the risk of overstaging and potential overtreatment in such cases, considering the limited accuracy of MRI for nodal staging [18, 19]. Finally, the panel agreed that MRF involvement should be included in the conclusion of the radiology report indicated as a suffix (or description) which specifies whether invasion is caused by the primary tumor or other structures, e.g., “MRF+ (primary)” or “MRF+ (non-primary)”.
MRF involvement and cT staging
There was insufficient agreement (73–79%) among survey respondents for cT staging in cases with MRF versus peritoneal invasion. As outlined in Fig. 3, the mesorectum is fully covered by the MRF below the anterior peritoneal reflection. The MRF is a separate anatomical structure and not a synonym for peritoneum. MRF involvement should thus be classified as cT3 MRF+ and not cT4a disease (as erroneously done by 22% of respondents). At and above the level of the peritoneal reflection, the mesorectum is partly covered by peritoneum (anteriorly). When there is anterior invasion at these levels, this constitutes cT4a disease and the MRF should not be classified as involved (as erroneously done by 41% of respondents), except when there is simultaneous invasion of the MRF dorsally (i.e., cT4a MRF+). The suboptimal agreement in the survey results indicates a knowledge gap requiring further teaching, supported by the fact that the most experienced respondents did reach consensus in these cases.
Lymph nodes and tumor deposits
Definitions of what constitutes a node or a deposit vary between different TNM editions [2, 20, 21], and the appropriateness of these definitions has been argued extensively. A meta-analysis of histopathology data demonstrated that, though tumor deposits correlated with the presence of lymph nodes and EMVI, they have distinctly different prognostic implications [22]. In a recent Delphi-consensus study, an international panel of pathologists agreed that tumor deposits are prognostically worse than lymph node metastases and that the N1c staging position as outlined in TNM8 is suboptimal as it does not properly reflect this risk status in the staging hierarchy [23].
Clear guidelines on how the presence of tumor deposits versus or in addition to nodal metastases should impact treatment stratification are also lacking, although in general both are considered adverse prognostic features that frequently imply a necessity for some form of (neo)adjuvant treatment. In line with the inconsistency in TNM definitions, validated definitions on what defines a lymph node or tumor deposit on imaging are lacking. The UK group of Brown et al have proposed a definition where tumor deposits are classified as “discontinuous EMVI” and characterized as nodules arising within/along venous channels, in continuity with major venous branches within the mesorectum and discontinuous from the main tumor, while nodes are characterized by the familiar shape and capsule typical of lymph nodes. The COMET trial is currently investigating the reproducibility of these definitions and the concordance between MRI and histopathology, along with the prognostic implications [24]. The panel agreed that we need to await the results of this trial to discover if the proposed criteria are reproducible and prognostically significant enough to warrant adoption into routine radiological reporting. Meanwhile, the panel proposes that any nodules discontinuous from the tumor (regardless of whether considered as nodes or deposits) are included in the cN-stage category and a prose description of the size and morphology of these lesions should be included in the report.
Lateral lymph nodes
According to TNM definitions, any nodes within the mesorectum and in the distal sigmoid mesocolon, as well as nodes in the obturator space and alongside the internal iliac vessels, are considered regional lymph nodes. Although these nodes are all embedded in the N stage, the panel unanimously agreed that further specification of which regional lymph node stations are involved is important to inform surgical and radiotherapy planning. Specifically, the presence of “high” lymph nodes along the superior rectal blood vessels impacts the upper borders of the radiotherapy volume [9] while N+ nodes in the “lateral” (obturator, internal iliac) compartments are associated with a higher risk for local recurrence, which can be improved by lateral lymph node dissection and/or targeted (chemo)radiotherapy [25]. As such, these nodes should be mentioned explicitly. Lymph nodes along the external iliac vessels are also considered part of the lateral nodes, but like lymph nodes along the common iliac vessels, lymph node involvement is much less common in these regions and would constitute non-regional (M1-stage) nodal disease. Elongated (oval) nodes in the posterior external iliac compartment, i.e., directly dorsal to the external iliac vein, are commonly encountered on MRI and have been demonstrated to be of little or no clinical significance [1]. Inguinal lymph nodes are typically also considered non-regional nodes. As an exception, the AJCC version of the TNM specifies that for distal tumors extending below the dentate line, inguinal nodes should be considered regional nodes similar to anal cancer staging.
Despite these relatively straightforward definitions, differentiation of regional versus non-regional lymph nodes was identified as an area of much disagreement in the survey results, probably reflecting a knowledge gap. A contributing factor may be the limited availability of radiological guidelines to define the various anatomical compartments for nodal staging on MRI. In an online supplement to a publication in JAMA surgery, Ogura et al published a color map defining the lateral lymph node compartments on MRI [26]. In Fig. 4, the panel proposed a slightly adapted version of this map with specified oblique-axial views (as typically encountered during radiological staging), also considering previously published definitions from the radiation oncologists society [9]. The panel believes that such maps can offer useful support to radiologists and can contribute to improved consistency in reporting of lateral lymph nodes.
Evidence on which criteria to use for evaluation of lateral lymph nodes is very limited. In the most recent consensus publication from ESGAR, the panel proposed specific criteria based on a combination of size and morphology features for mesorectal nodes, but acknowledged that for lateral lymph nodes, no specific criteria could be derived from literature at that time [15]. Subsequently, the Lateral Node Study Consortium published a pooled retrospective multicenter analysis of 741 patients, proposing a cut-off of ≥ 7 mm for obturator and internal iliac nodes at primary staging to define cN+ nodes, combined with a cut-off of > 4 mm (internal iliac) and > 6 mm (obturator) after chemoradiotherapy as criteria associated with a higher-than-acceptable risk for lateral lymph node recurrence [26]. The same group also showed that in contrast to mesorectal nodes, morphologic features are not of added benefit for lateral nodal staging [27]. Considering the current level of evidence, the panel agreed that for primary staging, the ≥ 7-mm threshold may for now be adopted, although further validation is obviously needed. The panel did not support the proposed size thresholds after chemoradiotherapy as the evidence provided was considered too preliminary. Reasons for concern included under-investigation of confounding effects (e.g., varying intervals between neoadjuvant treatment and radiological re-assessment/surgery, varying radiation volumes/doses). Nevertheless, the panel acknowledged that at the moment no alternative criteria are available.
Other (non-TNM) staging controversies
The authors acknowledge that there are several other potential controversies in the radiological staging of rectal cancer that are not (or less directly) related to the TNM-staging system and were therefore outside the scope of the current paper. These include the radiological classification of mucinous tumors, MRI protocols and patient preparation, criteria for restaging after neoadjuvant treatment, and the anatomical localization of tumors (including the differentiation between distal-, mid-, and high-rectal cancer, and the classification of tumors near the rectosigmoid junction as either rectal or sigmoid). With respect to the latter, the authors would like to refer to recent publications describing the “sigmoid take-off” as a useful radiological landmark (recently agreed upon by expert consensus) to discriminate rectal from sigmoid cancer [28, 29]. Regarding the differentiation between distal-, mid-, and high-rectal cancer, it is mainly the management of high-rectal cancers that in some countries (like the USA) is different and usually does not involve neoadjuvant treatment. Though there are no unanimously agreed upon definitions, the anterior peritoneal reflection is a commonly used anatomical landmark that can also easily be recognized on MRI [30].
In conclusion, this paper provides recommendations derived from the outcome of a global online survey and discussed among a panel of recognized multidisciplinary experts in the field on how to handle current controversies in TNM-based staging of rectal cancer on MRI related to cT staging in low-rectal cancers, definitions for cT4b disease and MRF invasion, evaluation of tumor deposits versus nodes, and the staging of lateral lymph nodes. These recommendations may serve as a practice guide and support tool for radiologists (and other clinicians) involved in the staging of rectal cancer, help guide multidisciplinary team discussions, and will hopefully contribute to improved consistency in radiological reporting.
Abbreviations
- cN stage:
-
Clinical nodal stage
- CRM:
-
Circumferential resection margin
- CT:
-
Computed tomography
- cT stage:
-
Clinical tumor stage
- EMVI:
-
Extramural vascular invasion
- ESGAR:
-
European Society of Gastrointestinal and Abdominal Radiology
- MRF:
-
Mesorectal fascia
- MRI:
-
Magnetic resonance imaging
- TNM:
-
Tumor node metastases
References
Gollub MJ, Lall C, Lalwani N, Rosenthal MH (2019) Current controversy, confusion, and imprecision in the use and interpretation of rectal MRI. Abdom Radiol (NY) 44(11):3549–3558
Jessup MJ, Goldberg RM, Asare EA, et al (2017) Colon and rectum. In: Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al Eds. AJCC cancer staging manual (8th edition). Springer: 251–273
Wittekind C, Greene FL, Henson DE, et al (2003) Explanatory notes specific anatomical sites. In: Wittekind Ch, Greene F.L, Henson D.E et al eds. TNM supplement: a commentary on uniform use 3rd edition. New York, NY. Wiley-Liss: 40–86
Wittekind C, Brierly JD, Lee AWM, et al (2019) Explanatory notes specific anatomical sites. In: Wittekind C, Brierly J.D, Lee A.W.M, et al eds. TNM supplement: a commentary on uniform use. 5th edition. New York, NY. Wiley-Liss: 54–85
Shihab OC, How P, West N et al (2011) Can a novel MRI staging system for low rectal cancer aid surgical planning? Dis Colon Rectum 54(10):1260–1264
Bamba Y, Itabashi M, Kameoka S (2012) Preoperative evaluation of the depth of anal canal invasion in very low rectal cancer by magnetic resonance imaging and surgical indications for intersphincteric resection. Surg Today 42(4):328–333
You YN, Hardiman KM, Bafford A et al (2020) The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum 63(9):1191–1222
Bleday R, Melnitchouk N (2014) Surgical management of rectal cancer. In: Beck, D.E., Nasseri, Y., Hull, et al eds. The ASCRS manual of colon and rectal surgery (2nd ed.). Springer: 811–831
Valentini V, Gambacorta MA, Barbaro B et al (2016) International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol 120(2):195–201
Glimelius B, Beets-Tan R, Blomqvist L et al (2011) Mesorectal fascia instead of circumferential resection margin in preoperative staging of rectal cancer. J Clin Oncol 29(16):2142–2143
Glynne-Jones R, Wyrwicz L, Tiret E, et al (2017) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv22–iv40
National Comprehensive Cancer Network. Rectal cancer (version 1.2021) [www.nccn.org] Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed June 21, 2021
Federatie Medische specialisten. Richtlijn colorectaal carcinoom (2019 update). Available at: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_-_crc.html. Accessed December 2, 2020
Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, Pathology Review Committee; Cooperative Clinical Investigators (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26(3):350–357
Beets-Tan RGH, Lambregts DMJ, Maas M et al (2019) Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 28(4):1465–1475
Shihab OC, Quirke P, Heald RJ, Moran BJ, Brown G (2010) Magnetic resonance imaging-detected lymph nodes close to the mesorectal fascia are rarely a cause of margin involvement after total mesorectal excision. Br J Surg 97(9):1431–1436
Birbeck KF, Macklin CP, Tiffin NJ et al (2002) Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 235(4):449–457
Bipat S, Glas AS, Slors FJ et al (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology 232(3):773–783
Lahaye MJ, Engelen SM, Nelemans PJ et al (2005) Imaging for -predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26(4):259–268
Fleming ID, Cooper JS, Henson DE, et al (1997) Eds. General information on cancer staging and end-results reporting. In: AJCC Cancer Staging Manual (5th edition). Lippincott-Raven: 3–11
Greene FL, Page DL, Fleming ID, et al (2002). Eds. AJCC cancer staging manual (6th edition). Springer
Nagtegaal ID, Knijn N, Hugen N et al (2017) Tumour deposits in colorectal cancer: improving the value of modern staging-a systematic review and meta-analysis. J Clin Oncol 35(10):1119–1127
Lord A, Brown G, Abulafi M et al (2021) Histopathological diagnosis of tumour deposits in colorectal cancer: a Delphi consensus study. Histopathology 79(2):168–175
Lord AC, Moran B, Abulafi M et al (2020) Can extranodal tumour deposits be diagnosed on MRI? Protocol for a multicentre clinical trial (the COMET trial). BMJ Open 10(10):e033395. https://doi.org/10.1136/bmjopen-2019-033395
Schaap DP, Boogerd LSF, Konishi T et al (2021) Lateral node study consortium. Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg 108(2):205–213
Ogura A, Konishi T, Cunningham C et al (2019) Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg 154(9):e192172. https://doi.org/10.1001/jamasurg.2019.2172
Ogura A, Konishi T, Cunningham C, et al Lateral Node Study Consortium (2019) Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol 37(1):33–43
D’Souza N, de Neree Tot Babberich MPM, d’Hoore A et al (2019) Definition of the rectum: an international, expert-based delphi consensus. Ann Surg 270(6):955–959
Bogveradze N, Lambregts DMJ, El Khababi N et al (2021) The sigmoid take-off as a landmark to distinguish rectal from sigmoid tumours on MRI: reproducibility, pitfalls and potential impact on treatment stratification. Eur J Surg Oncol 20:S0748-7983(21)00735–6. https://doi.org/10.1016/j.ejso.2021.09.009
Gollub MJ, Maas M, Weiser M et al (2013) Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol 200(1):97–101
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Doenja M.J. Lambregts.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
No complex statistical methods were necessary for this paper.
Informed Consent
Not applicable
Ethical Approval
Not applicable
Methodology
• prospective
• Survey and consensus study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.95 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lambregts, D.M.J., Bogveradze, N., Blomqvist, L.K. et al. Current controversies in TNM for the radiological staging of rectal cancer and how to deal with them: results of a global online survey and multidisciplinary expert consensus. Eur Radiol 32, 4991–5003 (2022). https://doi.org/10.1007/s00330-022-08591-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08591-z