Abstract
Objectives
To investigate whether amide proton transfer-weighted imaging (APTWI) and diffusion kurtosis imaging (DKI) can be used to evaluate endometrial carcinoma (EC) in terms of clinical type, histological grade, subtype, and Ki-67 index.
Methods
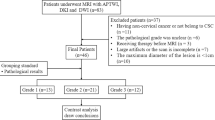
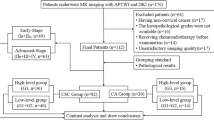
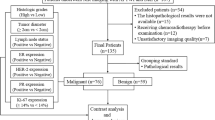
Eighty-eight patients with EC underwent pelvic DKI and APTWI. The non-Gaussian diffusion coefficient (Dapp), apparent kurtosis coefficient (Kapp), and magnetization transfer ratio asymmetry (MTRasym (3.5 ppm)) were calculated and compared based on the clinical type (type I, II), histological grade (high- and low-grade), and subtype (endometrioid adenocarcinoma (EA) and non-EA). Correlation coefficients were calculated for each parameter with histological grades and the Ki-67 index.
Results
The MTRasym (3.5 ppm) and Kapp values were higher in the type II group and high-grade group than in the type I and low-grade groups, respectively, while the Dapp values were lower in the type I and low-grade groups, respectively (all p < 0.05). The Kapp value was higher in the EA group than in the non-EA group (p = 0.022). The Kapp value was the only independent predictor for the histological grade of EA and the clinical type of EC. The AUC (DKI) was higher than the AUC (APTWI) in the identification of type I and II EC and high- and low-grade EA (Z = 2.042, 2.013, p = 0.041, 0.044), while in the identification of EA and non-EA, only the difference in Kapp was statistically significant. Moreover, the Kapp and MTRasym (3.5 ppm) values and Dapp values correlated positively and negatively, respectively, with histological grade (r = 0.759, 0.555, 0.624, and 0.462, all p < 0.05) and Ki-67 index (r = −0.704, −0.507, all p < 0.05).
Conclusion
Both DKI- and APTWI-related parameters have potential as imaging markers in estimating the histological features of EC, while DKI shows better performance than APTWI in this study.
Key Points
• DKI and APTWI can be used to preliminarily evaluate the histological characteristics of endometrial carcinoma (EC).
• The Kapp was the only independent predictor for the histological grade of EA and the clinical type of EC.
• The Kapp, MTRasym (3.5 ppm), and Dapp correlated positively and negatively, respectively, with histological grade and Ki-67 index.





Similar content being viewed by others
Abbreviations
- APTWI:
-
Amide proton transfer-weighted imaging
- D app :
-
Non-Gaussian diffusion coefficient
- DKI:
-
Diffusion-kurtosis imaging
- EA:
-
Endometrioid adenocarcinoma
- EC:
-
Endometrial carcinoma
- FIGO:
-
International Federation of Gynecology and Obstetrics
- Kapp :
-
Apparent kurtosis coefficient
- MTRasym (3.5 ppm):
-
Magnetization transfer ratio asymmetry at 3.5 ppm
- SI:
-
Signal intensity
References
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Fontham E, Wolf A, Church TR et al (2020) Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin 70:321–346.
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17
Scholten AN, Creutzberg CL, Noordijk EM, Smit VT (2002) Long-term outcome in endometrial carcinoma favors a two- instead of a three-tiered grading system. Int J Radiat Oncol Biol Phys 52:1067–1074
Kitson S, Sivalingam VN, Bolton J et al (2017) Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol 30:459–468
Werner HM, Trovik J, Marcickiewicz J et al (2013) A discordant histological risk classification in preoperative and operative biopsy in endometrial cancer is reflected in metastatic risk and prognosis. Eur J Cancer 49:625–632
Garcia TS, Appel M, Rivero R et al (2017) Agreement between preoperative endometrial sampling and surgical specimen findings in endometrial carcinoma. Int J Gynecol Cancer 27:473–478
Nougaret S, Horta M, Sala E et al (2019) Endometrial cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur Radiol 29:792–805
Ahmed M, Al-Khafaji JF, Class CA et al (2018) Can MRI help assess aggressiveness of endometrial cancer. Clin Radiol 73:833.e11–833.e18
Bhosale P, Ma J, Iyer R et al (2016) Feasibility of a reduced field-of-view diffusion-weighted (rFOV) sequence in assessment of myometrial invasion in patients with clinical FIGO stage I endometrial cancer. J Magn Reson Imaging 43:316–324
Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S (2014) MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol 24:1327–1338
Beddy P, Moyle P, Kataoka M et al (2012) Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 262:530–537
Inoue C, Fujii S, Kaneda S et al (2015) Correlation of apparent diffusion coefficient value with prognostic parameters of endometrioid carcinoma. J Magn Reson Imaging 41:213–219
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710
Qi XX, Shi DF, Ren SX et al (2018) Histogram analysis of diffusion kurtosis imaging derived maps may distinguish between low and high grade gliomas before surgery. Eur Radiol 28:1748–1755
Yin J, Sun H, Wang Z, Ni H, Shen W, Sun PZ (2018) Diffusion kurtosis imaging of acute infarction: comparison with routine diffusion and follow-up MR imaging. Radiology 287:651–657
Sun K, Chen X, Chai W et al (2015) Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277:46–55
Meng N, Wang X, Sun J et al (2020) Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer. Eur Radiol 30:5758–5767
Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Ohno Y, Yui M, Koyama H et al (2016) Chemical exchange saturation transfer MR imaging: preliminary results for differentiation of malignant and benign thoracic lesions. Radiology 279:578–589
Krikken E, Khlebnikov V, Zaiss M et al (2018) Amide chemical exchange saturation transfer at 7 T: a possible biomarker for detecting early response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res 20:51
Takayama Y, Nishie A, Togao O et al (2018) Amide proton transfer MR imaging of endometrioid endometrial adenocarcinoma: association with histologic grade. Radiology 286:909–917
Yamada I, Sakamoto J, Kobayashi D et al (2019) Diffusion kurtosis imaging of endometrial carcinoma: correlation with histopathological findings. Magn Reson Imaging 57:337–346
Jia G, Abaza R, Williams JD et al (2011) Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging 33:647–654
Mitra AP, Birkhahn M, Cote RJ (2009) Re: Joseph Chin. In Search of the Perfect Crystal Ball for Ta Urothelial Cancer. Eur Urol. 10.1016/j.eururo.2009.09.014. Eur Urol 57:23–24
Voss MA, Ganesan R, Ludeman L et al (2012) Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol 124:15–20
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol 16:441–448
Zheng S, van der Bom IM, Zu Z, Lin G, Zhao Y, Gounis MJ (2014) Chemical exchange saturation transfer effect in blood. Magn Reson Med 71:1082–1092
Zaino RJ, Kurman RJ, Diana KL, Morrow CP (1995) The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 75:81–86
Dobrzycka B, Mackowiak-Matejczyk B, Kinalski M, Terlikowski SJ (2013) Pretreatment serum levels of bFGF and VEGF and its clinical significance in endometrial carcinoma. Gynecol Oncol 128:454–460
Fukunaga T, Fujii S, Inoue C et al (2015) Accuracy of semiquantitative dynamic contrast-enhanced MRI for differentiating type II from type I endometrial carcinoma. J Magn Reson Imaging 41:1662–1668
Li B, Sun H, Zhang S, Wang X, Guo Q (2019) The utility of APT and IVIM in the diagnosis and differentiation of squamous cell carcinoma of the cervix: a pilot study. Magn Reson Imaging 63:105–113
Zhao X, Wen Z, Zhang G et al (2013) Three-dimensional turbo-spin-echo amide proton transfer MR imaging at 3-Tesla and its application to high-grade human brain tumors. Mol Imaging Biol 15:114–122
Heo HY, Zhang Y, Lee DH et al (2017) Accelerating chemical exchange saturation transfer (CEST) MRI by combining compressed sensing and sensitivity encoding techniques. Magn Reson Med 77:779–786
Rutgers JK (2015) Update on pathology, staging and molecular pathology of endometrial (uterine corpus) adenocarcinoma. Future Oncol 11:3207–3218
Xiao Z, Zhong Y, Tang Z et al (2018) Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 28:2923–2933
Khlebnikov V, Polders D, Hendrikse J et al (2017) Amide proton transfer (APT) imaging of brain tumors at 7 T: The role of tissue water T1 -Relaxation properties. Magn Reson Med 77:1525–1532
Zhang S, Keupp J, Wang X et al (2018) Z-spectrum appearance and interpretation in the presence of fat: Influence of acquisition parameters. Magn Reson Med 79:2731–2737
Liu R, Zhang H, Niu W et al (2020) Erratum to: improved chemical exchange saturation transfer imaging with real-time frequency drift correction (Magn Reson Med. 2019; 81: 2915-2923). Magn Reson Med 83:1884
Acknowledgements
We acknowledge the support received from the National Natural Science Foundation of China and Henan Medical Science and Technology Research Program. In addition, Nan Meng wants to say to Jing Sun: It is graceful grief and sweet sadness to think of you, but in my heart, there is a kind of soft warmth that can’t be expressed with any choice of words.
Funding
This study has received funding by the National Key R&D Program of China (2017YFE0103600), the Henan Medical Science and Technology Research Program (2018020357 and 2018020367), the National Natural Science Foundation of China (81720108021 and 31470047), the Zhongyuan Thousand Talents Plan Project-Basic Research Leader Talent (ZYQR201810117), and the Zhengzhou Collaborative Innovation Major Project (20XTZX05015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Meiyun Wang.
Conflict of interest
One of the authors of this manuscript (Kaiyu Wang) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in radiology.
Methodology
• prospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, N., Wang, X., Sun, J. et al. Evaluation of amide proton transfer-weighted imaging for endometrial carcinoma histological features: a comparative study with diffusion kurtosis imaging. Eur Radiol 31, 8388–8398 (2021). https://doi.org/10.1007/s00330-021-07966-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07966-y