Abstract
Objective
To assess whether the main genetic differences observed in high-grade gliomas (HGG) will present different MR imaging and MR spectroscopy correlates that could be used to better characterize lesions in the clinical setting.
Methods
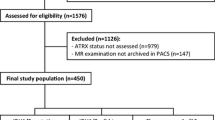
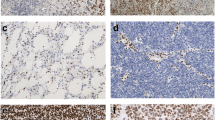
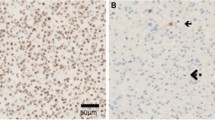
Seventy-nine patients with histologically confirmed HGG were recruited. Immunohistochemistry analyses for isocitrate dehydrogenase gene 1 (IDH1), alpha thalassemia mental retardation X-linked gene (ATRX), Ki-67, and p53 protein expression were performed. Tumour radiological features were examined on MR images. Metabolic profile and infiltrative pattern were assessed with MR spectroscopy. MR features were analysed to identify imaging-molecular associations. The Kaplan-Meier method and the Cox regression model were used to identify survival prognostic factors.
Results
In total, 17.7% of the lesions were IDH1-mutated, 8.9% presented ATRX-mutated, 70.9% presented p53 unexpressed, and 22.8% had Ki-67 > 5%. IDH1 wild-type tumours had higher levels of mobile lipids (p = 0.001). The tumour-infiltrative pattern was higher in HGG with unexpressed p53 (p = 0.009). Mutated ATRX tumours presented higher levels of glutamate and glutamine (Glx) (p = 0.001). An association was observed between Glx tumour levels (p = 0.038) and Ki-67 expression (p = 0.008) with the infiltrative pattern. Survival analyses identified IDH1 status, age, and tumour choline levels as independent predictors of prognostic significance.
Conclusions
Our results suggest that IDH1-wt tumours are more necrotic than IDH1-mut. And that the presence of an infiltrative pattern in HGG is associated with loss of p53 expression, Ki-67 index, and Glx levels. Finally, tumour choline levels could be used as a predictive factor in survival in addition to the IDH1 status to provide a more accurate prediction of survival in HGG patients.
Key Points
• IDH1-wt tumours present higher levels of mobile lipids than IDH1-mut.
• Mutated ATRX tumours exhibit higher levels of glutamate and glutamine.
• Loss of p53 expression, Ki-67 expression, and glutamate and glutamine levels may contribute to the presence of an infiltrative pattern in HGG.





Similar content being viewed by others
Abbreviations
- 2-D-HG:
-
(D)-2-Hydroxyglutarate
- ATRX:
-
Alpha thalassemia mental retardation X-linked gene
- Cho:
-
Choline
- Cr:
-
Creatine
- GBM:
-
Glioblastoma
- Glx:
-
Glutamate and glutamine
- HGG:
-
High-grade gliomas
- IDH1:
-
Isocitrate dehydrogenase gene 1
- IRR:
-
Inter-rater reliability analysis
- Lac:
-
Lactate
- Lip:
-
Mobile lipids
- mIno:
-
Myo-inositol
- MRSI:
-
3D chemical shift imaging sequence
- mut:
-
Mutated
- NAA:
-
N-Acetyl aspartate
- NAWM:
-
Normal-appearing white matter
- wt:
-
Wild-type
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Ostrom QT, Gittleman H, Fulop J et al (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772. https://doi.org/10.1158/1078-0432.CCR-12-3002
Homma T, Fukushima T, Vaccarella S et al (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65:846–854. https://doi.org/10.1097/01.jnen.0000235118.75182.94
Gusyatiner O, Hegi ME (2018) Glioma epigenetics: from subclassification to novel treatment options. Semin Cancer Biol 51:50–58. https://doi.org/10.1016/j.semcancer.2017.11.010
Antonelli M, Buttarelli FR, Arcella A et al (2010) Prognostic significance of histological grading, p53 status, YKL-40 expression, and IDH1 mutations in pediatric high-grade gliomas. J Neurooncol 99:209–215. https://doi.org/10.1007/s11060-010-0129-5
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Belden CJ, Valdes PA, Ran C et al (2011) Genetics of glioblastoma: a window into its imaging and histopathologic variability. Radiographics 31:1717–1740. https://doi.org/10.1148/rg.316115512
Sanson M, Marie Y, Paris S et al (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154. https://doi.org/10.1200/JCO.2009.21.9832
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. https://doi.org/10.1126/science.1164382
Millward CP, Brodbelt AR, Haylock B et al (2016) The impact of MGMT methylation and IDH-1 mutation on long-term outcome for glioblastoma treated with chemoradiotherapy. Acta Neurochir (Wien) 158:1943–1953. https://doi.org/10.1007/s00701-016-2928-8
Juratli TA, Kirsch M, Geiger K et al (2012) The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol 110:325–333. https://doi.org/10.1007/s11060-012-0977-2
Weller M, Weber RG, Willscher E et al (2015) Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129:679–693. https://doi.org/10.1007/s00401-015-1409-0
Nam JY, de Groot JF (2017) Treatment of glioblastoma. J Oncol Pract 13:629–638. https://doi.org/10.1200/JOP.2017.025536
Biasoli D, Sobrinho MF, da Fonseca ACC et al (2014) Glioblastoma cells inhibit astrocytic p53-expression favoring cancer malignancy. Oncogenesis 3:e123. https://doi.org/10.1038/oncsis.2014.36
Jiao Y, Killela PJ, Reitman ZJ et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722. https://doi.org/10.18632/oncotarget.588
Nandakumar P, Mansouri A, Das S (2017) The role of ATRX in glioma biology. Front Oncol 7:1–8. https://doi.org/10.3389/fonc.2017.00236
Carrillo JA, Lai A, Nghiemphu PL et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol 33:1349–1355. https://doi.org/10.3174/ajnr.A2950
Usinskiene J, Ulyte A, Bjørnerud A et al (2016) Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 58:339–350. https://doi.org/10.1007/s00234-016-1642-9
Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31:269–286
Cano M, Martínez-Zalacaín I, Bernabéu-Sanz Á et al (2017) Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl Psychiatry 7:e1023. https://doi.org/10.1038/tp.2016.267
Bernabeu A, Alfaro A, García M, Fernández E (2009) Proton magnetic resonance spectroscopy (1H-MRS) reveals the presence of elevated myo-inositol in the occipital cortex of blind subjects. Neuroimage 47:1172–1176. https://doi.org/10.1016/j.neuroimage.2009.04.080
Morales S, Bernabeu-Sanz A, López-Mir F, González P, Luna L, Naranjo V (2017) BRAIM: a computer-aided diagnosis system for neurodegenerative diseases and brain lesion monitoring from volumetric analyses. Comput Methods Programs Biomed 145:167–179. https://doi.org/10.1016/j.cmpb.2017.04.006
Hallgren Kevin A (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8:23–34. https://doi.org/10.1080/11035896009449194
Aggarwa R, Ranganathan P (2018) Conducting real-world evidence studies in India. Study designs: part 1 – an overview and classification. Perspect Clin Res 10:51–56. https://doi.org/10.4103/picr.PICR
Van Den Bent MJ, Dubbink HJ, Marie Y et al (2010) IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res 16:1597–1604. https://doi.org/10.1158/1078-0432.CCR-09-2902
Denicolaï E, Tabouret E, Colin C et al (2016) Molecular heterogeneity of glioblastomas: does location matter? Oncotarget 7:902–913. https://doi.org/10.18632/oncotarget.6433
Qi S, Yu L, Li H et al (2014) Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett 7:1895–1902. https://doi.org/10.3892/ol.2014.2013
Paldor I, Drummond KJ, Kaye AH (2016) IDH1 mutation may not be prognostically favorable in glioblastoma when controlled for tumor location: a case-control study. J Clin Neurosci 34:117–120. https://doi.org/10.1016/j.jocn.2016.05.016
Ranjan DB, Rajiv T, Firoz A, Roy Arnab PK (2013) Molecular investigation of Isocitrate dehydrogenase gene (IDH) mutations in gliomas: first report of IDH2 mutations in Indian patients. Asian Pac J Cancer Prev J Cancer Prev 14:7261–7264. https://doi.org/10.7314/APJCP.2013.14.12.7261
Fack F, Tardito S, Hochart G et al (2017) Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways. EMBO Mol Med 9:1681–1695. https://doi.org/10.15252/emmm.201707729
Esmaeili M, Hamans BC, Navis AC et al (2014) Tumor and stem cell biology IDH1 R132H mutation generates a distinct phospholipid metabolite profile in glioma. Cancer Res 74:4898–4907. https://doi.org/10.1158/0008-5472.CAN-14-0008
Ozturk-Isik E, Cengiz S, Ozcan A et al (2019) Identification of IDH and TERTp mutation status using 1H-MRS in 112 hemispheric diffuse gliomas. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26964
Wenger KJ, Hattingen E, Franz K, Steinbach J, Bähr O, Pilatus U (2019) In vivo metabolic profiles as determined by 31 P and short TE 1 H MR-spectroscopy: no difference between patients with IDH wildtype and IDH mutant gliomas. Clin Neuroradiol 29:27–36. https://doi.org/10.1007/s00062-017-0630-8
Durmo F, Lätt J, Rydelius A et al (2018) Brain tumor characterization using multibiometric evaluation of MRI. Tomography 4:14–25. https://doi.org/10.18383/j.tom.2017.00020.Brain
Haase S, Garcia-Fabiani MB, Carney S et al (2018) Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets 0:14728222.2018.1487953. https://doi.org/10.1080/14728222.2018.1487953
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Zhu H, Wang H, Huang Q et al (2018) Transcriptional repression of p53 by PAX3 contributes to gliomagenesis and differentiation of glioma stem cells. Front Mol Neurosci 11:187. https://doi.org/10.3389/fnmol.2018.00187
Sun J, Li D-M, Huang J et al (2017) The correlation between the expression of ADAM17, EGFR and Ki-67 in malignant gliomas. Eur Rev Med Pharmacol Sci 21:4595–4599
Oizel K, Chauvin C, Oliver L et al (2017) Efficient mitochondrial glutamine targeting prevails over glioblastoma metabolic plasticity. Clin Cancer Res 23:6292–6304. https://doi.org/10.1158/1078-0432.CCR-16-3102
Neal A, Moffat BA, Stein JM et al (2019) Glutamate weighted imaging contrast in gliomas with 7 Tesla magnetic resonance imaging. Neuroimage Clin 22:101694. https://doi.org/10.1016/j.nicl.2019.101694
Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J (2000) Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol 47:11–22. https://doi.org/10.1023/A:1006426917654
Pollack IF, Finkelstein SD, Woods J et al (2002) Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med 346:420–427. https://doi.org/10.1056/NEJMoa012224
Monticelli M, Zeppa P, Zenga F et al (2018) The post-surgical era of GBM: how molecular biology has impacted on our clinical management. A review. Clin Neurol Neurosurg 170:120–126. https://doi.org/10.1016/j.clineuro.2018.05.015
Debus C, Waltenberger M, Floca R et al (2018) Impact of 18F-FET PET on target volume definition and tumor progression of recurrent high grade glioma treated with carbon-ion radiotherapy. Sci Rep 8:7201. https://doi.org/10.1038/s41598-018-25350-7
Shaw EG, Wisoff JH (2003) Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol 5:153–160. https://doi.org/10.1215/S1152851702000601
Claus EB, Horlacher A, Hsu L et al (2005) Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 103:1227–1233. https://doi.org/10.1002/cncr.20867
Hsieh JC, Lesniak MS (2005) Surgical management of high-grade gliomas. Expert Rev Neurother 5:33–39. https://doi.org/10.1586/14737175.5.6.S33
Shimizu H, Kumabe T, Shirane R, Yoshimoto T (2000) Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am J Neuroradiol 21:659–665
Horská A, Barker PB (2010) Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin N Am 20:293–310. https://doi.org/10.1016/j.nic.2010.04.003
Yan H, Parsons DW, Jin G et al (2009) Mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Chiang IC, Kuo Y-T, Lu C-Y et al (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46:619–627. https://doi.org/10.1007/s00234-004-1246-7
Ricci R, Bacci A, Tugnoli V et al (2007) Metabolic findings on 3T 1 H-MR spectroscopy in peritumoral brain edema. AJNR Am J Neuroradiol 28:1287–1291. https://doi.org/10.3174/ajnr.A0564
Raschke F, Barrick TR, Jones TL, Yang G, Ye X, Howe FA (2019) Tissue-type mapping of gliomas. Neuroimage Clin 21:101648. https://doi.org/10.1016/j.nicl.2018.101648
Server A, Josefsen R, Kulle B et al (2010) Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol 51:316–325. https://doi.org/10.3109/02841850903482901
Wright AJ, Fellows G, Byrnes TJ et al (2009) Pattern recognition of MRSI data shows regions of glioma growth that agree with DTI markers of brain tumor infiltration. Magn Reson Med 62:1646–1651. https://doi.org/10.1002/mrm.22163
Acknowledgments
We would like to thank Mr. David González García, Ms. Evelyn Teruel Sánchez, Mr. Jonathan Monge Ivars, Ms. Sonia Álvarez Bernabéu, and Mr. Enrique García Rodriguez for their outstanding work during the acquisition of the studies; Estefania Rojas Calvente for her support in the histopathology analyses; Mr. Jorge Esteban Jarabo for his invaluable help and advice on the process of writing and improving the manuscript readability; and Mr. Javier Sánchez González (Philips, Spain) for his advice and technical support during the study. We would also like to show our gratitude to the anonymous reviewers who provided insight and expertise that greatly improved the manuscript.
Funding
This work was supported in part by the Centro para el Desarrollo Tecnológico Industrial (CDTI) under the project BRAIM (IDI- 20130020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Ángela Bernabéu-Sanz.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Bernabéu-Sanz, Á., Fuentes-Baile, M. & Alenda, C. Main genetic differences in high-grade gliomas may present different MR imaging and MR spectroscopy correlates. Eur Radiol 31, 749–763 (2021). https://doi.org/10.1007/s00330-020-07138-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07138-4