Abstract
Objectives
Oesophageal adenocarcinoma has a poor prognosis and relies on multi-modality assessment for accurate nodal staging. The aim of the study was to determine the prognostic significance of nodal concordance between PET/CT and EUS in oesophageal adenocarcinoma.
Methods
Consecutive patients with oesophageal adenocarcinoma staged between 2010 and 2016 were included. Groups comprising concordant node–negative (C−ve), discordant (DC), and concordant node–positive (C+ve) patients were analysed. Survival analysis using log-rank tests and Cox proportional hazards model was performed. The primary outcome was overall survival. A p value < 0.05 was considered statistically significant.
Results
In total, 310 patients (median age = 66.0; interquartile range 59.5–72.5, males = 264) were included. The median overall survival was 23.0 months (95% confidence intervals (CI) 18.73–27.29). There was a significant difference in overall survival between concordance groups (X2 = 44.91, df = 2, p < 0.001). The hazard ratios for overall survival of DC and C+ve patients compared with those of C−ve patients with cT3 tumours were 1.21 (95% CI 0.81–1.79) and 1.79 (95% CI 1.23–2.61), respectively. On multivariable analysis, nodal concordance was significantly and independently associated with overall survival (HR 1.44, 95% CI 1.12–1.83, p = 0.004) and performed better than age at diagnosis (HR 1.02, 95% CI 1.003–1.034, p = 0.016) and current cN-staging methods (HR 1.20, 95% CI 0.978–1.48, p = 0.080).
Conclusions
Patients with discordant nodal staging on PET/CT and EUS represent an intermediate-risk group for overall survival. This finding was consistent in patients with cT3 tumours. These findings will assist optimum treatment decisions based upon perceived prognosis for each patient.
Key Points
• Clinicians are commonly faced with results of discordant nodal staging in oesophageal adenocarcinoma.
• There is a significant difference in overall survival between patients with negative, discordant, and positive lymph node staging.
• Patients with discordant lymph node staging between imaging modalities represent an intermediate-risk group for overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oesophageal cancer is newly diagnosed in over 9000 people in the United Kingdom (UK) each year and predominately of adenocarcinoma cell type, with around 7 in 10 diagnosed at an advanced stage [1]. Radiological staging is central to management, planning, and prognosis, and usually involves a combination of computed tomography (CT), positron emission tomography combined with CT (PET/CT), and endoscopic ultrasound (EUS) [2].
Lymph node metastases are a major prognostic indicator in oesophageal cancer [3, 4]. Nodal assessment therefore is a key factor in radiological staging but the accuracy of these individual modalities is suboptimal [5, 6]. EUS is generally regarded as the gold standard for assessment of regional lymph nodes, but controversy regarding the role of EUS in staging exists. Studies have shown limited benefits versus risk [7] whilst others suggest EUS can impact treatment decisions in 29% of patients [8] and reduce edge of radiotherapy field relapses when EUS measurements are used to define gross tumour volume [9].
There is a lack of studies investigating the prognostic significance of nodal concordance between imaging modalities in oesophageal cancer staging. Diagnostic confidence is improved when a node has malignant characteristics on more than one imaging modality. The high specificity and positive predictive value compared with low sensitivity and negative predictive value of CT and PET/CT [10, 11] mean that a lymph node is often considered to be involved if malignant characteristics are demonstrated on multiple modalities.
However, clinicians face a difficult diagnostic conundrum in cases where there are discordant findings of malignancy in lymph nodes between different modalities. Important treatment decisions often hinge on the classification of nodal metastases on PET/CT and EUS examinations. In a previous study, Dhupar et al [12] reviewed 615 patients with oesophageal cancer from a single centre who underwent oesophagectomy for survival outcomes based on concordance of staging investigations for nodal disease. In summary, the study found that patients with discordance in nodal staging between imaging modalities had a better overall survival than patients with positively concordant nodal staging. However, more data is required to validate these findings.
Therefore, the aim of this study was to determine the prognostic significance of nodal concordance between PET/CT and EUS in oesophageal adenocarcinoma.
Materials and methods
Institutional review board approval was obtained for this study (reference 13//DMD5769). We performed a retrospective review of a prospectively collected database of patients with oesophageal adenocarcinoma in a regional upper GI cancer network comprising four health boards and eight different centres.
Consecutive patients (n = 420) were considered for this study. Inclusion criteria were patients with biopsy-confirmed adenocarcinoma of the oesophagus or gastro-oesophageal junction (GOJ) who were staged with contrast-enhanced CT, PET/CT, and EUS between 2010 and 2016. Exclusion criteria were a histological cell type other than adenocarcinoma, those who did not have PET/CT (n = 2) or EUS (n = 89; n = 71 because of M1 disease on PET/CT and n = 18 due to non-traversable stenotic tumour), patients with missing TNM staging data (n = 17), and patients with missing survival data (n = 2). Following exclusion criteria application, 310 patients were included. During the study period, patients were staged according to TNM 7th edition [13]. Use of the TNM 8th edition [14] would not have altered stage groupings.
Staging pathway
Patients are usually diagnosed with oesophageal cancer following upper GI endoscopy and biopsy. Patients then undergo a contrast-enhanced CT of the thorax, abdomen, and pelvis to assess for distant metastases. If the patient was considered to have potentially curable disease after staging CT (i.e. absence of M1 disease), then more detailed staging with PET/CT and EUS was performed. EUS was not completed in patients with a non-traversable stenotic tumour and not performed in patients with confirmed M1 disease on PET/CT. The PET/CT and EUS protocols are available in the supplementary material.
Clinical T-stage
Following multi-disciplinary team (MDT) review, clinical T-stage (cT-stage) was assigned to each patient following consideration of the contrast-enhanced CT and EUS findings, with the latter being considered the most accurate modality for cT-stage [15].
Definition of positive lymph node metastasis
On PET/CT, nodes were classed as involved if identified on the CT component and showed FDG uptake appreciably higher than background values. No specific standardised uptake value was used to diagnose regional nodes because this decision is subjective and multi-factorial. Lymph nodes considered physiological or related to an alternative aetiology were excluded from the N-stage. On EUS, the criteria for malignant lymphadenopathy specified a hypoechoic pattern, spherical contour, distinct border, and a short-axis diameter of 6 mm or more. PET/CT and EUS cN-staging were collected from the clinical radiology reports which were used to decide the subsequent treatment. Overall clinical N-stage (cN-stage) was assigned following MDT review after consideration of the combined CT, PET/CT, and EUS findings.
Definition of concordance
Three classifications were defined for this study. Nodal concordance was defined as either negative (C−ve) or positive (C+ve). C−ve patients had cN0 disease on both PET/CT and EUS and C+ve patients had cN+ disease on both modalities. Discordant patients (DC) had cN0 disease on one modality but not the other (Fig. 1). Further subgroups were constructed based on modality findings; PET/CT−ve:EUS+ve and EUS−ve:PET/CT+ve.
Clinical example of discordant radiological lymph node staging. A patient with a primary distal oesophageal adenocarcinoma had a 9-mm lymph node (white arrows) on (a) a contrast-enhanced CT and (b) a PET/CT with minimally increased SUV (2.6) compared with background. An (c) EUS was performed which shows a malignant appearance (8 mm, round and hypoechoic), and a fine needle aspiration (FNA) was performed. The (d) FNA with papanicolaou stain at × 40 magnification showed adenocarcinoma cells from the lymph node (large pleomorphic cells with prominent nucleoli in a cohesive group)
Survival data
The primary outcome of the study was overall survival, defined as the length of survival following diagnosis until death or date of last follow-up. Survival data was obtained from the Cancer Network Information System database. Patients are followed up at regular intervals following radical treatment, every 3 months for the first year and then 6-monthly thereafter for the next 4 years.
Statistical analysis
Descriptive statistics were expressed as frequency (percentage) for categorical variables and median (interquartile range (IQR)) for continuous variables. Differences between categorical variables and continuous variables were assessed using chi-square tests and Mann-Whitney U tests, respectively. Median overall survival was estimated using the Kaplan-Meier life-table method [16]. Mean overall survival was calculated when median overall survival was not reached. Cumulative survival curves were generated and differences between groups evaluated with the log-rank test. The prognostic significance of cT-stage and cN-stage were evaluated using this method. Hazard ratios were calculated with a Cox proportional hazards model and compared with the baseline group. A subgroup analysis for curative versus palliative treatment groups was pre-specified and performed separately. Multivariable analysis evaluated whether age at diagnosis, nodal concordance, or current cN-staging methods were the better predictor of overall survival. Statistical analysis was performed using SPSS v23.0 (IBM). A p value < 0.05 was considered statistically significant.
Results
The baseline characteristics of included patients are detailed in Table 1. The median age of the cohort was 66.0 years (IQR 59.5–72.5). The median overall survival was 23.0 months (95% confidence intervals (CI) 18.73–27.29). One-, 2-, and 5-year overall survival rates were 73.5% (n = 228/310; 95% CI 73.45–73.55), 48.4% (n = 150/310; 95% CI 48.35–48.45), and 13.5% (n = 42/310; 95% CI 13.44–13.55), respectively. The median follow-up was 63.0 months (95% CI 57.97–68.04).
A large number of patients (n = 89) were initially excluded because of missing EUS data; therefore, comparison of baseline characteristics was performed in these patients. Age (t = 1.518, mean difference 1.738 (95% CI − 0.513–3.988), p = 0.130), gender (X2 0.003, df 1, p = 0.957), and tumour location (X2 0.239, df 1, p = 0.625) were not significantly different between patient groups with and without EUS staging.
Prognostic significance of nodal concordance between PET/CT and EUS
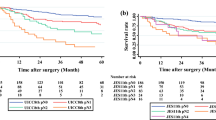
The median overall survival for C−ve, DC, and C+ve patients were 43.0 months (95% CI 33.22–52.78), 21.0 months (95% CI 9.36–32.64), and 13.0 months (95% CI 11.25–14.76), respectively. There was a significant difference in overall survival between groups (X2 44.91, df 2, p < 0.001) (Fig. 2). Furthermore, there were significant differences between C−ve and DC groups (X2 5.18, df 1, p = 0.023) and DC and C+ve groups (X2 11.11, df 1, p = 0.001). The hazard ratios for overall survival of DC and C+ve patients compared with those of C−ve patients overall were 1.46 (95% CI 1.04–2.06) and 2.66 (95% CI 1.97–3.60), respectively. This suggests that patients with discordant nodal staging between PET/CT and EUS represent an intermediate-risk group for overall survival.
Prognostic significance of cT-stage and cN-stage
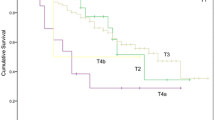
In this cohort, cT-stage (X2 54.12, df 4, p < 0.001) and cN-stage (X2 48.85, df 3, p < 0.001) were significantly associated with overall survival. The hazard ratios for overall survival of N1, N2, and N3 stages compared with those of N0 were 1.57 (95% CI 1.14–2.15), 3.13 (2.19–4.49), and 2.60 (1.65–4.09), respectively. Survival statistics are presented for each cT-stage in Table 2.
There was a significant difference between C−ve, DC, and C+ve patients staged with cT3 tumours (X2 10.19, df 2, p = 0.006) (Fig. 3). cT3 was significantly associated with overall survival but other cT-stages were not, likely due to the small numbers included. The hazard ratios for overall survival of DC and C+ve patients compared with those of C−ve patients with cT3 tumours were 1.21 (95% CI 0.81–1.79) and 1.79 (95% CI 1.23–2.61), respectively. No significant difference in overall survival was found between C−ve and DC (X2 0.94, df 1, p = 0.33), groups but there was a significant difference between DC and C+ve (X2 3.19, df 1, p = 0.048) groups, suggesting that DC patients with cT3 tumours have similar survival to C−ve patients.
Differences between PET/CT and EUS nodal staging
The relationship between PET/CT and EUS N-staging was examined by creating two groups: (1) PET/CT−ve:EUS+ve and (2) EUS-ve:PET/CT+ve. In total, 16/310 patients (5.2%) had PET/CT cN+ and EUS cN0 disease. Conversely, 57/310 patients (18.4%) had EUS cN+ and PET/CT cN0 disease. There was a significant difference in frequency between these two groups (X2 97.29, df 1, p < 0.001). In addition, there were significant differences between C−ve, PET/CT−ve:EUS+ve, EUS-ve:PET/CT+ve, and C+ve groups (X2 50.99, df 3, p < 0.001) (Fig. 4). The median overall survival for the EUS-ve:PET/CT+ve group was 14.0 months (95% CI 12.70–15.30) and 34.0 months (95% CI 13.92–54.08) for the PET/CT−ve:EUS+ve group. There was a significant difference between PET/CT−ve:EUS+ve and EUS-ve:PET/CT+ve groups (X2 6.50, df 1, p = 0.011) suggesting patients with PET/CT−ve:EUS+ve nodes had a better outcome, although the numbers in the PET/CT+ve:EUS-ve group were relatively low.
Subgroup analysis
With different treatment regimens included in this study, a subgroup analysis compared the prognostic significance of nodal concordance between patients treated with radical versus palliative treatments. There was a significant difference in overall survival between C−ve, DC, and C+ve nodal concordance when adjusting for treatment intent (X2 13.88, df 1, p = 0.001), demonstrating that the discordant group remained at intermediate risk in both radical and palliative settings. The median overall survival for patients treated with radical versus palliative intent was 37.0 months (95% CI 28.77–45.23) and 12.0 months (95% CI 10.04–13.97), respectively. Furthermore, there were no significant differences in overall survival (X2 6.977, df 4, p = 0.137) between radical treatment groups (neoadjuvant chemotherapy (NACT), definitive chemoradiotherapy (dCRT), surgery alone, neoadjuvant chemoradiotherapy (NACRT), and endoscopic mucosal resection (EMR)).
The hazard ratios for overall survival of DC and C+ve patients compared with those of C−ve in patients treated with radical intent were 1.24 (95% CI 0.84–1.84) and 1.75 (95% CI 1.16–2.64), respectively. In palliative patients, the hazard ratios for overall survival of DC and C+ve patients compared with those of C−ve patients treated palliatively were 3.13 (95% CI 1.46–6.67) and 2.38 (95% CI 1.28–4.42), respectively. These results show that the overall survival of DC patients more closely matched C−ve patients when treated with radical intent, but the opposite effect was true when treated palliatively.
Multivariable analysis
On multivariable analysis, age at diagnosis, nodal concordance, and cN-stage were entered into a multi-variable model. Nodal concordance was significantly and independently associated with overall survival (HR 1.44, 95% CI 1.12–1.83, p = 0.004) and performed better than age at diagnosis (HR 1.02, 95% CI 1.003–1.034, p = 0.016) and current cN-staging methods (HR 1.20, 95% CI 0.978–1.48, p = 0.080).
Discussion
This study has shown that patients with discordant nodal staging on PET/CT and EUS represent an intermediate-risk group for overall survival. This finding is important when deciding upon the optimum treatment decision based upon the perceived risk stratification for each patient [17].
Clinicians are commonly faced with results of discordant lymph node staging between imaging modalities. Accurate detection of lymph node metastases is important for staging and prognosis, and their detection changes treatment decisions. In this cohort, we have demonstrated the prognostic significance of cT- and cN-stage. However, clinical ambiguity is introduced into decision pathways if two modalities (PET/CT and EUS) are discordant. This ambiguity can create diagnostic and management uncertainty for clinicians who must judge the most appropriate treatment considering the best interests of each patient. In such cases, a discordant lymph node is often considered likely to be metastatic given the incidence of metastases with advanced cT-stage [3] and the higher specificity and positive predictive value of CT and PET [11].
These results are consistent with data from a cohort of oesophageal cancer patients who underwent oesophagectomy [12]. This study also described an intermediate-risk group comprising patients with discordant staging investigations, in which DC patients had better over survival than C+ve patients. Despite the findings of our current study, the literature surrounding the prognostic significance of discordant lymph node staging is sparse.
Importantly, this study has further shown the significant overall survival differences between C−ve, DC, and C+ve groups in patients with cT3 tumours. These data could be used to support clinical decisions in patients with cT3 tumours, which is the most common stage of oesophageal tumours at presentation [18]. Overall survival for patients with cT4 tumours was not found to be statistically significant between cT-stage groups, but this is likely to be an effect of small patient numbers in this subgroup. Patients with nodal discordance had an overall survival probability more closely aligned to patients with C−ve nodes, suggesting that in these cases, clinicians should place the findings in a wider clinical context and perhaps consider more radical treatment.
The subgroup analysis found that discordant nodal staging had a better prognosis in patients treated with radical intent but a worse prognosis in patients treated palliatively. These findings are likely to be biased by the retrospective nature of this study and influenced by the effects of treatment, which are broadly based on a number of clinical, physiological, radiological, and pathological factors. Endoscopic mucosal resection (EMR) is available for patients with limited early-stage disease. No survival benefit from EMR was demonstrated in this study, but only four EMR patients were included in this cohort. Fit, young patients may have been treated more aggressively than those with a significant number of comorbidities. In the latter cases, clinicians may have decided that the discordance added further uncertainty that the effects of radical treatment would be beneficial. This confounding bias cannot be adjusted for in retrospective studies, and these findings will need to be validated in prospective studies.
The number of patients classified with positive lymph nodes (cN+) is often higher on EUS than on PET/CT [15], and this trend was shown again here. PET imaging is limited in its ability to detect small and peri-tumoural lymph node metastases because of the relatively large spatial resolution and reliance on co-registration for anatomical definition [19]. In addition, the positive correlation between primary tumour and lymph node uptake means that metastases are less likely to be detected if the primary tumour demonstrates low FDG uptake. Overall, recent data has shown that the sensitivity of CT, PET/CT, and EUS is poor (39.7%, 35.3%, and 42.6%, respectively) [5], which is consistent with data from other specialist oesophageal cancer centres [6] and different thoracic tumour sites such as non–small cell lung cancer [20].
Similarly, there was a significant difference in overall survival between PET/CT−ve:EUS+ve and EUS-ve:PET/CT+ve groups, suggesting that patients with PET/CT−ve:EUS+ve discordant nodes had a better outcome, although the numbers in the PET/CT−ve:EUS+ve were low. Overall, these results show that clinicians should refrain from instinctively concluding that a discordant lymph node is metastatic. False-positive rates of CT, PET/CT, and EUS range between 15 and 30% for cN-staging [10].
New methods to improve lymph node metastasis staging accuracy are required because radiological techniques alone are unlikely to improve sufficiently in the near future. A high proportion of micro-metastases (82%) have been detected in normal-sized lymph nodes of patients with oesophageal cancer [5], and it is believed that micro-metastases are associated with a poorer clinical outcome [21], although the evidence is conflicting. At present, micro-metastases cannot be visualised on current imaging modalities.
The false-positive rates of each modality and high proportion of metastases highlight that lymph node staging should be considered within a multi-disciplinary context, with factors such as lymph node location and number, grade of primary tumour differentiation, and underlying tumour biology known to have prognostic significance [22].
Strengths
This study includes a large consecutive cohort of fully staged patients from a large regional upper GI cancer network serving a population of approximately 1.5 million. The overall survival data are robust and no patient was lost to follow-up. A comparison of cT-stage and radical versus palliative care treatment subgroups demonstrated that the prognostic significance of discordant lymph nodes was consistent.
Limitations
As discussed above, retrospective studies are likely to be hindered by confounding biases. Individual lymph nodes were not examined; rather, the cN-stage between modalities was compared on a per-patient basis. Histopathological correlation was not possible in the majority of the cohort because relatively few patients are suitable for surgical resection. Contrast-enhanced CT data were not analysed in this study because it is known that the accuracy of CT N-staging is poor and the PET/CT has a CT component which was used at the time of reporting to evaluate individual lymph nodes. Lastly, squamous cell carcinoma was not included because this study focussed on adenocarcinoma, the most prevalent histological cell type in European and North American countries [23].
In conclusion, this study has shown that patients with discordant nodal staging on PET/CT and EUS represent an intermediate-risk group for overall survival and have better survival rates than those with positively concordant lymph nodes. This finding was consistent in patients with cT3 tumours and is important when deciding upon the optimum treatment based upon the perceived prognosis for each patient. These results should be validated in prospective studies.
Abbreviations
- C−ve:
-
Concordant negative nodal status
- C+ve:
-
Concordant positive nodal status
- CI:
-
Confidence intervals
- CT:
-
Computed tomography
- cM-stage:
-
Clinical M-stage
- cN-stage:
-
Clinical N-stage
- cT-stage:
-
Clinical T-stage
- DC:
-
Discordant nodal status
- dCRT:
-
Definitive chemoradiotherapy
- EMR:
-
Endoscopic mucosal resection
- EUS:
-
Endoscopic ultrasound
- GOJ:
-
Gastro-oesophageal junction
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- MDT:
-
Multi-disciplinary team
- NACRT:
-
Neoadjuvant chemoradiotherapy
- NACT:
-
Neoadjuvant chemotherapy
- PET:
-
Positron emission tomography
- UK:
-
United Kingdom
References
Cancer Research UK (2019) Oesophageal cancer statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-bycancer-type/oesophageal-cancer.
National Institute for Health and Clinical Excellence (NICE) (2018) NICE guideline [NG83]: Oesophago-gastric cancer: assessment and management in adults. https://www.nice.org.uk/guidance/ng83
Kayani B, Zacharakis E, Ahmed K, Hanna GB (2011) Lymph node metastases and prognosis in oesophageal carcinoma-a systematic review. Eur J Surg Oncol 37:747–753
Davies AR, Gossage JA, Zylstra J et al (2014) Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 32:2983–2990
Foley KG, Christian A, Fielding P, Lewis WG, Roberts SA (2017) Accuracy of contemporary oesophageal cancer lymph node staging with radiological-pathological correlation. Clin Radiol 72:e691–e697
Bunting D, Bracey T, Fox B, Berrisford R, Wheatley T, Sanders G (2017) Loco-regional staging accuracy in oesophageal cancer-how good are we in the modern era? Eur J Radiol 97:71–75
Findlay JM, Bradley KM, Maile EJ et al (2015) Pragmatic staging of oesophageal cancer using decision theory involving selective endoscopic ultrasonography, PET and laparoscopy. Br J Surg 102:1488–1499
Hulshoff JB, Mul VEM, de Boer HEM et al (2017) Impact of endoscopic ultrasonography on 18F-FDG-PET/CT upfront towards patient specific esophageal cancer treatment. Ann Surg Oncol 24:1828–1834
Button MR, Morgan CA, Croydon ES et al (2009) Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 73:818–823
van Vliet EP, Heijenbrok-Kal MH, Hunink MG et al (2008) Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98:547–557
Shi W, Wang W, Wang J et al (2013) Meta-analysis of 18FDG PETCT for nodal staging in patients with esophageal cancer. Surg Oncol 22:112–116
Dhupar R, Correa AM, Ajani J et al (2014) Concordance of studies for nodal staging is prognostic for worse survival in esophageal cancer. Dis Esophagus 27:770–776
Sobin LH, Gospodarowicz MK, Wittekind CH (2009) TNM classification of malignant tumours, 7th edn. Wiley, New York
Sobin LH, Gospodarowicz MK, Wittekind CH (2017) TNM classification of malignant tumours, 8th edn. Wiley, New York
Puli SR, Reddy JB, Bechtold ML et al (2008) Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 14:1479–1490
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Allum WH, Blazeby JM, Griffin SM et al (2011) Guidelines for the management of oesophageal and gastric cancer. Gut 60:1449–1472
Royal College of Surgeons of England (2013) National Oesophago-Gastric Cancer Audit (NOGCA). http://www.hscic.gov.uk/catalogue/PUB11093/clin-audi-supp-prog-oesogast-2013-rep.pdf
Kinahan PE, Fletcher JW (2010) Positron emission tomography computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CTMR 31:496–505
Frechet B, Kazakov J, Thiffault V et al (2018) Diagnostic accuracy of mediastinal lymph node staging techniques in the preoperative assessment of nonsmall cell lung cancer patients. J Bronchol Interv Pulmonol 25:17–24
Izbicki JR, Hosch SB, Pichlmeier U et al (1997) Prognostic value of immuno histochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl JMed 337:1188–1194
Frankell AM, Jammula S, Li X et al (2019) The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat Genet 51:506–516
Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64:381–387
Acknowledgements
The authors would like to thank Dr. Adam Christian (Department of Histopathology, University Hospital of Wales, Cardiff) for contributing the EUS-FNA image used in this article.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Kieran Foley.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Dr. Kieran Foley) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained (reference number 13//DMD5769).
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Foley KG, Hills RK, Berthon B, et al (2018) Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol 28:428–436. https://doi.org/10.1007/s00330-017-4973-y
This article investigated the prognostic significance of PET texture analysis. A similar cohort was used (patients with oesophageal cancer staged between 2010 and 2016, but nodal concordance between PET/CT and EUS was not included in this study and is a brand new research hypothesis).
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multi-centre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carder, C., Fielding, P., Roberts, A. et al. Discordant nodal staging identifies intermediate-risk group for overall survival in patients with cT3 oesophageal adenocarcinoma. Eur Radiol 30, 3429–3437 (2020). https://doi.org/10.1007/s00330-019-06642-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06642-6