Abstract
Objectives
To investigate whether clinical condition, imaging session, and locations affect repeatability of amide proton transfer–weighted (APTw) magnetic resonance imaging (MRI) in the brain.
Materials and methods
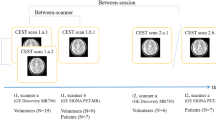
Three APTw MRI data sets were acquired, involving two intrasession scans and one intersession scan for 19 healthy, 15 glioma, and 12 acute stroke adult participants (mean age 53.8, 54.6, and 68.5, respectively) on a 3T MR scanner. The mean APTw signals from five locations in healthy brain (supratentorial and infratentorial locations) and from entire tumor and stroke lesions (supratentorial location) were calculated. The within-subject coefficient of variation (wCV) and intraclass correlation coefficient (ICC) were calculated for each clinical conditions, image sessions, and anatomic locations. Differences in APTw signals between sessions were analyzed using repeated-measures analysis of variance.
Results
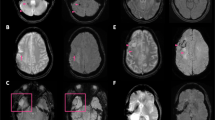
The ICC and wCV were 0.96 (95% confidence interval [CI], 0.91–0.99) and 16.1 (12.6–21.3) in glioma, 0.93 (0.82–0.98) and 15.0 (11.4–20.6) in stroke, and 0.84 (0.72–0.91) and 34.0 (28.7–41.0) in healthy brain. There were no significant differences in APTw signal between three sessions, irrespective of disease condition and location. The ICC and wCV were 0.85 (0.68–0.94) and 27.4 (21.8–35.6) in supratentorial, and 0.44 (− 0.18 to 0.76) and 32.7 (25.9 to 42.9) in infratentorial locations. There were significant differences in APTw signal between supra- (mean, 0.49%; 95% CI, 0.38–0.61) and infratentorial locations (1.09%, 0.98–1.20; p < 0.001).
Conclusion
The repeatability of APTw signal was excellent in supratentorial locations, while it was poor in infratentorial locations due to severe B0 inhomogeneity and susceptibility which affects MTR asymmetry.
Key Points
• In supratentorial locations, APTw MRI showed excellent intrasession and intersession repeatability in brains of healthy controls and patients with glioma, as well as in stroke-affected regions.
• APTw MRI showed excellent repeatability in supratentorial locations, but poor repeatability in infratentorial locations.
• Considering poor repeatability in the infratentorial locations, the use of APTw MRI in longitudinal assessment in infratentorial locations is not indicated.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- APTw:
-
Amide proton transfer–weighted
- CEST:
-
Chemical exchange saturation transfer
- CI:
-
Confidence interval
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- MTR:
-
Magnetization transfer ratio
- RF:
-
Radiofrequency
- ROI:
-
Region of interest
- TE:
-
Echo time
- TR:
-
Repetition time
- TSE:
-
Turbo spin echo
- wCV:
-
Within-subject coefficient of variation
- WHO:
-
World Health Organization
References
Ward KM, Aletras AH, Balaban RS (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143:79–87
van Zijl PC, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 65:927–948
McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC (2006) Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly‐L‐lysine and a starburst dendrimer. Magn Reson Med 55:836–847
Harston GW, Tee YK, Blockley N et al (2014) Identifying the ischaemic penumbra using pH-weighted magnetic resonance imaging. Brain 138:36–42
Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Zhou J, Lal B, Wilson DA et al (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Wu B, Warnock G, Zaiss M et al (2016) An overview of CEST MRI for non-MR physicists. An overview of CEST MRI for non-MR physicists. EJNMMI Phys 3:19
Choi Y, Ahn S, Lee SK et al (2017) Amide proton transfer imaging to discriminate between low- and high-grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volume. Eur Radiol 27:3181–3189
Zhou J, Zhu H, Lim M et al (2013) Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128
Zhou J, Tryggestad E, Wen Z et al (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130
Wang M, Hong X, Chang CF et al (2015) Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magn Reson Med 74:42–50
Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA (2016) Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 278:514–523
Park JE, Lee JY, Kim HS et al (2018) Amide proton transfer imaging seems to provide higher diagnostic performance in post-treatment high-grade gliomas than methionine positron emission tomography. Eur Radiol 28:3285–3295
Togao O, Hiwatashi A, Keupp J et al (2015) Scan–rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J Magn Reson Imaging 42:1346–1353
Sun PZ, Benner T, Kumar A, Sorensen AG (2008) Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med 60:834–841
Li S, Dardzinski BJ, Collins CM, Yang QX , Smith MB (1996) Three-dimensional mapping of the static magnetic field inside the human head. Magn Reson Med 36:705–714
Sullivan DC, Obuchowski NA, Kessler LG et al (2015) Metrology standards for quantitative imaging biomarkers. Radiology 277:813–825
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Togao O, Yoshiura T, Keupp J et al (2012) Effect of saturation pulse length on parallel transmission based amide proton transfer (APT) imaging of different brain tumor types. Proc Intl Soc Mag Reson Med 20:744
Keupp J, Baltes C, Harvey PR, van den Brink J (2011) Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain at 3T. Proc Int Soc Magn Reson Med 19:710
Eggers H, Brendel B, Duijndam A, Herigault G (2011) Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med 65:96–107
Xiang QS (2006) Two-point water-fat imaging with partially-opposed-phase (POP) acquisition: an asymmetric Dixon method. Magn Reson Med 56:572–584
Edwards S, Gong Q, Liu C et al (1999) Infratentorial atrophy on magnetic resonance imaging and disability in multiple sclerosis. Brain 122:291–301
Guivel-Scharen V, Sinnwell T, Wolff S, Balaban RS (1998) Detection of proton chemical exchange between metabolites and water in biological tissues. J Magn Reson 133:36–45
Park JE, Han K, Sung YS et al (2017) Selection and reporting of statistical methods to assess reliability of a diagnostic test: conformity to recommended methods in a peer-reviewed journal. Korean J Radiol 18:888–897
Barnhart HX, Barboriak DP (2009) Applications of the repeatability of quantitative imaging biomarkers: a review of statistical analysis of repeat data sets. Transl Oncol 2:231–235
Sappakitkamjorn J, Niwitpong S-A (2013) Confidence intervals for the coefficients of variation with bounded parameters. Int J Math Comput Sci Eng 7:198–203
Hallgren KA (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8:23
Jiang S, Zou T, Eberhart CG et al (2017) Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn Reson Med 78:1100–1109
Yu H, Lou H, Zou T et al (2017) Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol 27:4516–4524
Zhao X, Wen Z, Huang F, et al (2011) Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3 T. Magn Reson Med 66(4):1033–1041
Park J, Kim H, Jung S et al (2017) Depiction of acute stroke using 3-tesla clinical amide proton transfer imaging: saturation time optimization using an in vivo rat stroke model, and a preliminary study in human. Investig Magn Reson Imaging 21:65–70
Zhou J, Blakeley JO, Hua J et al (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60:842–849
Jacobs MA, Horská A, Van Zijl PC, Barker PB (2001) Quantitative proton MR spectroscopic imaging of normal human cerebellum and brain stem. Magn Reson Med 46:699–705
Schmidt H, Schwenzer NF, Gatidis S et al (2016) Systematic evaluation of amide proton chemical exchange saturation transfer at 3 T: effects of protein concentration, pH, and acquisition parameters. Invest Radiol 51:635–646
Acknowledgments
The authors sincerely thank all patients with glioma and stroke, as well as the healthy volunteers, who kindly agreed to participate in the present study. We also thank Seonok Kim in the Department of Clinical Epidemiology and Biostatistics at Asan Medical Center, Seoul, South Korea, for her contributions regarding interpretation of the results.
Funding
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI12C1847).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ho Sung Kim.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
Seonok Kim kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 588 kb)
Rights and permissions
About this article
Cite this article
Lee, J.B., Park, J.E., Jung, S.C. et al. Repeatability of amide proton transfer–weighted signals in the brain according to clinical condition and anatomical location. Eur Radiol 30, 346–356 (2020). https://doi.org/10.1007/s00330-019-06285-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06285-7