Abstract
Objectives
MYC gene rearrangements in diffuse large B cell lymphomas (DLBCLs) result in high proliferation rates and are associated with a poor prognosis. Strong proliferation is associated with high metabolic demand and tumour necrosis. The aim of this study was to investigate differences in the presence of necrosis and semiquantitative 18F-FDG PET metrics between DLBCL cases with or without a MYC rearrangement. The prognostic impact of necrosis and semiquantitative 18F-FDG PET parameters was investigated in an explorative survival analysis.
Methods
Fluorescence in situ hybridisation analysis for MYC rearrangements, visual assesment, semiquantitative analysis of 18F-FDG PET scans and patient survival analysis were performed in 61 DLBCL patients, treated at a single referral hospital between 2008 and 2015.
Results
Of 61 tumours, 21 (34%) had a MYC rearrangement (MYC+). MYC status was neither associated with the presence of necrosis on 18F-FDG PET scans (necrosisPET; p = 1.0) nor associated with the investigated semiquantitative parameters maximum standard uptake value (SUVmax; p = 0.43), single highest SUVmax (p = 0.49), metabolic active tumour volume (MATV; p = 0.68) or total lesion glycolysis (TLG; p = 0.62). A multivariate patient survival analysis of the entire cohort showed necrosisPET as an independent prognostic marker for disease-specific survival (DSS) (HR = 13.9; 95% CI 3.0–65; p = 0.001).
Conclusions
MYC rearrangements in DLBCL have no influence on the visual parameter necrosisPET or the semi-quantiative parameters SUVmax, MATV and TLG. Irrespective of MYC rearrangements, necrosisPET is an independent, adverse prognostic factor for DSS.
Key Points
• Retrospective analysis indicates that MYC rearrangement is not associated with necrosis on 18 F-FDG PET (necrosis PET ) scans or semiquantitative 18 F-FDG PET parameters.
• Necrosis PET is a potential independent adverse prognostic factor for disease-specific survival in patients with DLBCL and is not influenced by the presence of MYC rearrangements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphoma (DLBCL) accounts for 35% of all B cell non-Hodgkin lymphomas (B-NHL) [1]. Approximately 10–15% of DLBCL cases harbour a MYC gene rearrangement (MYC+), as assessed by fluorescence in situ hybridisation (FISH) [2]. These lymphomas are characterised by a very high proliferation rate. Patients bearing a MYC+ lymphoma experience an aggressive clinical course and have a poor prognosis when treated with the standard regimen of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) [3]. In 2017, the World Health Organization (WHO) established a new entity for MYC rearranged DLBCL, called ‘high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements’ [1, 4].
MYC is an oncogenic transcription factor regulating a vast array of cellular processes and pathways [5, 6]. Tumour cells overexpressing MYC meet their high energy demands by increased glucose uptake, glycolysis, lactate production and amino acid consumption [7, 8]. However, unlike physiological tissues, cancer cells frequently have acquired resistance to apoptosis and cannot regulate their energy expenditure during metabolic stress, resulting in cell death via necrosis when nutrient supply is compromised [9,10,11].
In B-NHL patients, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) scans are used for staging and response assessment [12]. Tumour necrosis can be assessed by visual inspection of 18F-FDG PET scans (necrosisPET) [13]. Necrosis can be observed in 14–20% of DLBCL cases and has been associated with an adverse prognosis [14, 15]. Semiquantitative assessment of 18F-FDG PET allows for relative comparison of parameters based on the spatial distribution and degree of 18F-FDG uptake, and is currently being investigated as a tool for therapy monitoring and assessing prognosis in B-NHL [16,17,18]. Still, data on the prognostic value of the semiquantitative parameters maximum standardised uptake value (SUVmax) and metabolically active tumour volume (MATV) in DLBCL are conflicting [19,20,21].
MYC rearrangement, tumour necrosis (necrosisPET) and parameters derived from semiquantitative analysis of 18F-FDG PET are fundamentally linked to metabolism, yet the relationship between these factors remains unknown. We hypothesise that the higher metabolic activity mediated by MYC rearrangements might result in a higher incidence of necrosisPET and increased semiquantitative parameters. The previously suggested prognostic impact of necrosisPET [15] and semiquantitative parameters [16,17,18] in DLBCL might be accredited to their potential association with MYC rearrangements.
Therefore, the aim of this study was to investigate differences in the presence of necrosisPET and semiquantitative 18F-FDG PET metrics between DLBCL cases with or without a MYC rearrangement. The prognostic impact of these factors was explored by means of survival analysis.
Materials and methods
Study design and case selection
For this retrospective single-centre study, consecutive patients with newly diagnosed, histologically confirmed DLBCL between 2008 and 2015 were identified in the electronic healthcare database of the University Medical Center Groningen (UMCG), a reference centre for aggressive B cell lymphomas. Cases of primary cutaneous DLBCL, primary central nervous system lymphoma, primary mediastinal B cell lymphoma and immunodeficiency-associated lymphomas were excluded. The selection of cases for this study is summarised in Fig. 1. Patients were stratified according to the National Comprehensive Cancer Network international prognostic index (NCCN-IPI) [22]. End of treatment response was assessed by 18F-FDG PET/CT scan. Tumour responses were classified according to Lugano criteria [12]. Follow-up was registered until early October 2017. According to Dutch regulations, no medical ethical committee approval was required for this retrospective, non-interventional study. A waiver was obtained from the medical ethics committee of the UMCG on November 13, 2018. The study utilised rest material from patients, the use of which is regulated under the code for good clinical practice in the Netherlands and does not require informed consent in accordance with Dutch regulations.
Pathology review
Pathology review was done using the 2008 WHO classification of haematopoietic and lymphoid tissues (AD) [23]. Histological scoring for necrosis (necrosisHist) was done by microscopic assessment of haematoxylin and eosin–stained slides. Only microscopic areas with definite histopathological signs of necrosis (i.e. karyolysis) were scored as positive for necrosisHist.
MYC fluorescence in situ hybridisation
For evaluation of a MYC rearrangement, formalin-fixed paraffin-embedded tissue blocks of primary tumour samples were used. Interphase fluorescence in situ hybridisation (FISH) was performed on 4-μm-thick whole tissue sections, using Vysis break apart probes (Abbot Technologies) and standard FISH protocols as previously described [24]. Researchers performing MYC FISH analyses were blinded for results from visual scoring, microscopic assessment of necrosis (necrosisHist) and clinical outcome.
18F-FDG PET imaging
All 18F-FDG PET scans were performed prior to therapy. Patients were allowed to continue all medication and fasted for at least 6 h before whole-body (from the skull vertex to mid-thigh level) three-dimensional PET images were acquired. This was done 60 min after intravenous administration of a standard dose of 3 MBq/kg (0.081 mCi/kg) bodyweight 18F-FDG on a Biograph mCT (Siemens Healthineers), according to the European Association of Nuclear Medicine (EANM) procedure guidelines for tumour imaging with FDG PET/CT (version 2.0) [25]. Acquisition was performed in seven bed positions of 2-min emission scans for patients 60–90 kg. Patients with body weight less than 60 kg and more than 90 kg body weight were scanned with 1 min and 3 min per bed position, respectively. Low-dose transmission CT was used for attenuation correction. Low-dose CT and 18F-FDG PET scans were automatically fused by the use of three-dimensional fusion software (Siemens Healthineers) with manual fine adjustments. Raw data were reconstructed through ultra-high definition (Siemens Healthineers).
Computed tomography
Diagnostic CTs were acquired via integrated 18F-FDG PET/CT scans according to the European Association of Nuclear Medicine (EANM) procedure guidelines for tumour imaging with FDG PET/CT (version 2.0) [25]. Bulky disease was defined as any nodal lymphoma lesion > 10 cm in coronal, axial or sagittal planes.
18F-FDG PET analysis
All 18F-FDG PET scans were visually assessed for the presence of tumour necrosis (necrosisPET) by an experienced reader (TCK), who was blinded to clinical, laboratory, biopsy and follow-up findings, as previously described [15]. Areas within any nodal or extranodal 18F-FDG PET–avid lymphomatous lesions that showed no 18F-FDG uptake were registered as having necrosisPET (Fig. 2); no specific visual scale was used. Semiquantitative analysis was performed using an in-house tool for quantitative 18F-FDG PET/CT analysis, as previously described [26,27,28]. This programme automatically preselects lesions using a SUVmax threshold of 4 and a metabolic volume threshold of 2.5 ml. Unwanted preselected FDG-avid regions, such as the bladder and brain, are removed by user interaction. Finally, remaining FDG-avid segmentations are processed using a background-corrected 50% of SUV peak region growing method, as described by Frings et al [26], to obtain the final tumour segmentations. In case obvious lymphoma lesions were not selected (n = 3), they were manually added after automatic tumour segmentation. From the final segmentation, the metabolic active tumour volume (MATV, in ml), total lesion glycolysis (TLG = MATV × SUVmean) and SUVs are derived for each lesion independently as well as summed over all lesions. Lesion selection and semiquantitative analysis was performed by MH under direct supervision of an experienced nuclear medicine physician (WN) and a nuclear physicist (RB). SUVmax was defined as the highest SUV per voxel within one lymphomatous lesion. In this paper, SUVmax is reported as the mean of SUVmax across all lesions of an individual patient. SUVmax single highest was defined as the highest SUVmax of all lesions within an individual patient.
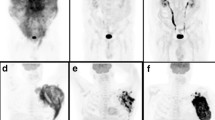
Visual assessment of necrosis and semiquantitative 18F-FDG PET review process. a A 65-year-old man with diffuse large B cell lymphoma (DLBCL) and tumour masses in the left dorsal chest wall and left pelvis, as shown on the coronal maximum intensity projection (MIP) 18F-FDG PET image (arrows). Coronal fused 18F-FDG PET/CT (b), axial CT (c) and axial fused 18F-FDG PET/CT (d) show the tumour mass with photopenic areas (arrow heads), in keeping with tumour necrosis. Coronal and sagittal MIP 18F-FDG PET images (e and f) show tumour segmentation (marked in red colour) for the calculation of metabolically active tumour volume (MATV), total lesion glycolysis (TLG), maximum standard uptake value (SUVmax), and single highest SUVmax
Statistical analysis
Comparison between continuous, non-normally distributed variables was estimated by Wilcoxon rank-sum test. Differences between two nominal variables were evaluated using Pearson’s chi-square or Fisher’s exact test (for expected groups sizes ≤ 5). For exploratory survival analysis, the primary endpoints were overall survival (OS), progression-free survival (PFS) and disease-specific survival (DSS). OS was defined as the time from diagnosis until death (from any cause). PFS was defined as the time from diagnosis until death or relapse or progression [12]. DSS was defined as the time from diagnosis until death from DLBCL. Surviving patients were censored at the last date of follow-up. Survival curves were estimated according to the Kaplan-Meier method. Cox regression was used for univariate and multivariate survival analyses and results were reported as hazard ratio (HR), 95% confidence interval (CI) and p value based on statistical Wald test. A two-tailed p value of less than 0.05 indicated statistical significance. All analyses were performed using R version 3.4.1 and R-studio version 1.0.153 software.
Results
Patient characteristics
Characteristics of the entire cohort (61 patients) are summarised in Table 1. A total of 21 patients (34%) had a DLBCL harbouring a MYC rearrangement. MYC rearrangement was observed in 11 patients (21.6%) primarily seen in the UMCG (n = 51) and 10 patients (100%) referred from affiliated hospitals (n = 10). MYC groups did not differ with regard to baseline characteristics (Table 1) except for serum LDH levels, which were higher in the MYC-positive group (p = 0.036) than in cases without MYC rearrangement.
MYC status, necrosis and semiquantitative 18F-FDG PET parameters
necrosisPET was observed in 15 patients (25%). The relationships between MYC status and necrosisPET, necrosisHist and semiquantitative 18F-FDG PET parameters are summarised in Table 2. MYC+ cases did not differ from cases without MYC rearrangement with regard to necrosisPET (p = 1.0) or necrosisHist (p = 0.52).
When the semiquantitative parameters SUVmax, SUVmax single highest, MATV and TLG were studied, no difference between MYC groups was observed. There was no relation between the presence of necrosisPET and necrosisHist (p = 0.1; Supplementary Figure 1).
NecrosisPET and tumour volume
In 14 of 15 necrosisPET cases, necrosis was observed in the largest lesion. In comparison, the largest individual lesion of cases without necrosisPET had a significantly lower MATV (p = 0.0006) and SUVmax (p = 0.02), irrespective of MYC status (Supplementary Figure 2). Bulky disease was observed in 24 patients (39%). Bulky disease was significantly correlated with necrosisPET (p = 0.005), but not with MYC status (p = 0.9) or necrosisHist (p = 0.8). Extranodal growth of lesions was not significantly correlated with the presence of necrosisPET (p = 0.26).
Survival analysis
The median follow-up was 34 months. At 5 years, OS was 67% (95% CI 54–83%), PFS was 65% (95% CI 53–81%) and DSS was 81% (95% CI 70–93%) for the entire cohort. Of the seven deaths unrelated to lymphoma, two were caused by metastatic adenocarcinoma, two were due to cardiac failure, one was due to acute on chronic renal failure and there were two cases of sudden deaths in patients in complete remission of DLBCL.
Results of the univariate Cox regression analysis (HR, 95% CI and p value) are shown in Table 3. The univariate analysis for OS identified MYC, NCCN-IPI and SUVmax single highest as associated factors. In univariate analysis for PFS, only NCCN-IPI was associated with outcome. In the univariate analysis for DSS MYC, NCCN-IPI, SUVmax single highest and necrosisPET were associated. Both SUVmax and SUVmax single highest showed negative beta-coefficients throughout the univariate survival analysis.
For multivariate analysis, the parameters MYC, NCCN-IPI, necrosisPET and SUVmax single highest were used due to their prognostic impact on lymphoma-related deaths in univariate analysis (Table 4). NecrosisPET did not contribute to the prognostic model for OS and PFS. However, for DSS, necrosisPET had a large adverse prognostic impact and proved to be independent (HR = 13.9; 95% CI 3.0–65; p = 0.001). The Kaplan-Meier analysis for DSS showed no events during the 5-year follow-up period for patients who neither had MYC rearrangements nor had necrosisPET (n = 30) (Fig. 3).
Discussion
Based on the current investigation, there is no association of MYC rearrangements with the presence of tumour necrosis assessed by 18F-FDG PET or the semiquantitative 18F-FDG PET parameters SUVmax, SUVmax single highest, MATV and TLG. We therefore rejected the hypothesis that metabolic changes induced by MYC rearrangements might increase the incidence of necrosisPET or alter the profile of semiquantitative parameters in DLBCL. NecrosisPET was significantly associated with the MATV of the single largest tumour lesion. The SUVmax of the single largest necrosisPET lesion was significantly higher compared with the lesions without necrosisPET. Both of these observations support the notion of larger, more metabolically active tumours being more susceptible to necrosis, irrespective of MYC status.
Our analyses demonstrate that necrosisPET had a significant impact on DSS, thereby substantiating previous findings about the prognostic value of this visual marker [15]. The presented data show that the presence of MYC rearrangement, in itself a powerful predictive factor, is not related to necrosisPET. This allows for integration of MYC status and necrosisPET into a prognostic model for DLBCL. When combined with MYC, NCCN-IPI and SUVmax single highest in the multivariate analysis, necrosisPET had the highest significance in predicting death due to lymphoma and a higher prognostic impact than NCCN-IPI, the currently most accurate prognostic index for DLBCL [22]. Thus, our results support the potential additive value of necrosisPET as an important biomarker for risk stratification in the clinical setting [14, 15].
The lack of a relationship between MYC rearrangements and semiquantitative 18F-FDG PET metrics might have several causes. First, proliferation in DLBCL could be independent of MYC rearrangement. This would only partially explain the lack of relationship, since the median proliferation index (Ki-67 staining) of MYC+ DLBCL is universally high (> 90%) in contrast to the much broader range observed in MYC− DLBCL [29]. Second, overexpression of MYC via other mechanisms such as epigenetic pathways might explain increased glucose uptake in MYC FISH–negative DLBCL. This is supported by studies showing high MYC protein expression in 19–40% of DLBCL cases [30,31,32]. Cottereau et al previously reported a lack of relation between MYC protein expression and 18F-FDG PET parameters in DLBCL [19]. However, FISH analysis, which is considered the gold standard examination for MYC rearrangements [33,34,35], was not performed. Third, high metabolic activity might be induced by alternative changes in metabolic drivers, such as mutations in PTEN (observed in approximately 15% of DLBCL) that lead to activation of the P13K/AKT/mTOR pathway [29, 36,37,38].
Intriguingly, the univariate survival analysis indicated a protective effect for cases with SUVmax and SUVmax single highest measurements above the median. Studies on the prognostic impact of these variables are conflicting [20, 39,40,41]. Gallicchio et al published results similar to ours, alluding to lymphomas with high metabolic activity being more responsive to chemotherapy [20]. In light of conflicting data on the prognostic value of semiquantitative 18F-FDG PET parameters [19,20,21, 42, 43], our results underline the need for larger, prospective studies with external validation cohorts [42].
This study has several limitations. First there is a referral bias with a high incidence of MYC+ cases (34%) in our dataset. The enrichment in our study can largely be explained by the fact that, as a reference centre, aggressive and MYC+ DLBCL cases (including suspected cases of Burkitt lymphoma which subsequently prove to be MYC+ DLBCL) are referred to our site. Second, the total number of cases with necrosisPET is small, which increases the risk of a sampling error. Nevertheless, the incidence of necrosisPET in our study is in line with previous studies [13,14,15]. Furthermore, patients were included irrespective of their comorbidities. Factors like differences in treatment regimen and non-cancer-related deaths might thus have a large impact on the statistical analysis. This is supported by the difference between DSS and OS. Despite its limitations, the prognostic potential of MYC status and NCCN-IPI was reproduced in this dataset, making it a representative set of DLBCL cases. Larger prospective studies are warranted to validate the prognostic value of necrosisPET.
Conclusion
In this comprehensive analysis of MYC rearranged DLBCL, we showed that a fundamental pathological change such as MYC rearrangement, which by itself has a significant impact on prognosis, has no influence on the presence of necrosisPET or semiquantitative 18F-FDG PET metrics. An explorative survival analysis suggests that the presence of necrosis determined by visual assessment of 18F-FDG PET scans is an independent predictor of disease-specific survival in patients with DLBCL, regardless of MYC status.
Abbreviations
- 18F-FDG:
-
18F-fluorodeoxyglucose
- B-NHL:
-
B cell non-Hodgkin lymphoma
- CT:
-
Computed tomography
- DLBCL:
-
Diffuse large B cell lymphoma
- DSS:
-
Disease-specific survival
- FISH:
-
Fluorescence in situ hybridisation
- LDH:
-
Lactate dehydrogenase
- MATV:
-
Metabolically active tumour volume (sum of all lesions within an individual patient)
- NCCN-IPI:
-
National Comprehensive Cancer Network international prognostic index
- necrosisHist :
-
Necrosis as assessed by histological scoring
- necrosisPET :
-
Necrosis as assessed by 18F-FDG PET
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- PFS:
-
Progression-free survival
- SUV:
-
Standard uptake value
- SUVmax :
-
Highest SUV per voxel within 1 lymphoma lesion (reported here as the mean of SUVmax of all lesions within an individual patient)
- SUVmax single highest:
-
Highest SUVmax of all lesions within an individual patient
- TLG:
-
Total lesion glycolysis (sum of all lesions within an individual patient)
- WHO:
-
World Health Organization.
References
International Agency for Research on Cancer (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition 2017. WHO, Lyon
Aukema SM, Siebert R, Schuuring E et al (2011) Double-hit B-cell lymphomas. Blood 117:2319–2331. https://doi.org/10.1182/blood
Barrans S, Crouch S, Smith A et al (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28:3360–3365. https://doi.org/10.1200/JCO.2009.26.3947
Jiang M, Bennani NN, Feldman AL (2017) Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev Hematol 10:405–415. https://doi.org/10.1080/17474086.2017.1318053
Zeller KI, Jegga AG, Aronow BJ et al (2003) An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol 4:R69. https://doi.org/10.1186/gb-2003-4-10-r69
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7:11–20. https://doi.org/10.1016/j.cmet.2007.10.002
Miller DM, Thomas SD, Islam A et al (2012) c-Myc and cancer metabolism. Clin Cancer Res 18:5546–5553. https://doi.org/10.1158/1078-0432.CCR-12-0977
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483. https://doi.org/10.1158/1078-0432.CCR-09-0889
Jin S, DiPaola RS, Mathew R, White E (2007) Metabolic catastrophe as a means to cancer cell death. J Cell Sci 120:379–383. https://doi.org/10.1242/jcs.03349
Jin S, White E (2007) Role of autophagy in cancer: management of metabolic stress. Autophagy 3:28–31
Proskuryakov SY, Gabai VL (2010) Mechanism of tumor cell necrosis. Curr Pharm Des 16:56–68
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068. https://doi.org/10.1200/JCO.2013.54.8800
Song MK, Chung JS, Shin DY et al (2017) Tumor necrosis could reflect advanced disease status in patients with diffuse large B cell lymphoma treated with R-CHOP therapy. Ann Hematol 96:17–23. https://doi.org/10.1007/s00277-016-2822-8
Adams HJA, de Klerk JMH, Fijnheer R et al (2015) Prognostic value of tumor necrosis at CT in diffuse large B-cell lymphoma. Eur J Radiol 84:372–377. https://doi.org/10.1016/j.ejrad.2014.12.009
Adams HJA, de Klerk JMH, Fijnheer R et al (2016) Tumor necrosis at FDG-PET is an independent predictor of outcome in diffuse large B-cell lymphoma. Eur J Radiol 85:304–309. https://doi.org/10.1016/j.ejrad.2015.09.016
Barrington SF, Kluge R (2017) FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging 44:97–110. https://doi.org/10.1007/s00259-017-3690-8
Xie M, Wu K, Liu Y et al (2015) Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol 32:446. https://doi.org/10.1007/s12032-014-0446-1
Dührsen U, Müller S, Hertenstein B et al (2018) Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol 36:2024–2034. https://doi.org/10.1200/JCO
Cottereau A-S, Lanic H, Mareschal S et al (2016) Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res 22:3801–3809. https://doi.org/10.1158/1078-0432.CCR-15-2825
Gallicchio R, Mansueto G, Simeon V et al (2014) F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol 92:382–389. https://doi.org/10.1111/ejh.12268
Adams HJA, de Klerk JMH, Fijnheer R et al (2015) Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol 94:532–539. https://doi.org/10.1111/ejh.12467
Zhou Z, Sehn LH, Rademaker AW et al (2014) An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123:837–842. https://doi.org/10.1182/blood
International Agency for Research on Cancer (2008) WHO classification of tumours of haematopoeitic and lymphoid tissues. 4th edition 2008 WHO, Lyon
van der Wekken AJ, Pelgrim R, ’t Hart N et al (2017) Dichotomous ALK-IHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer. Clin Cancer Res 23:4251–4258. https://doi.org/10.1158/1078-0432.CCR-16-1631
Boellaard R, Delgado-Bolton R, Oyen WJG et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354. https://doi.org/10.1007/s00259-014-2961-x
Frings V, van Velden FHP, Velasquez LM et al (2014) Repeatability of metabolically active tumor volume measurements with FDG PET/CT in advanced gastrointestinal malignancies: a multicenter study. Radiology 273:539–548. https://doi.org/10.1148/radiol.14132807
Cheebsumon P, van Velden FH, Yaqub M et al (2011) Measurement of metabolic tumor volume: static versus dynamic FDG scans. EJNMMI Res 1:35. https://doi.org/10.1186/2191-219X-1-35
Cheebsumon P, Boellaard R, de Ruysscher D et al (2012) Assessment of tumour size in PET/CT lung cancer studies: PET- and CT-based methods compared to pathology. EJNMMI Res 2:56. https://doi.org/10.1186/2191-219X-2-56
Agarwal R, Lade S, Liew D et al (2016) Role of immunohistochemistry in the era of genetic testing in MYC-positive aggressive B-cell lymphomas: a study of 209 cases. J Clin Pathol 69:266–270. https://doi.org/10.1136/jclinpath-2015-203002
Johnson NA, Slack GW, Savage KJ et al (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3452–3459. https://doi.org/10.1200/JCO.2011.41.0985
Horn H, Ziepert M, Becher C et al (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121:2253–2263. https://doi.org/10.1182/blood-2012-06
Valera A, López-Guillermo A, Cardesa-Salzmann T et al (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 98:1554–1562. https://doi.org/10.3324/haematol.2013.086173
Tilly H, Gomes Da Silva M, Vitolo U et al (2015) Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl. 5):116–125. https://doi.org/10.1093/annonc/mdv304
Nguyen L, Papenhausen P, Shao H (2017) The role of c-MYC in B-cell lymphomas: diagnostic and molecular aspects. Genes (Basel) 8:E116. https://doi.org/10.3390/genes8040116
Sesques P, Johnson NA (2017) Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood 129:280–288. https://doi.org/10.1182/blood-2016-02
Tsukamoto N, Kojima M, Hasegawa M et al (2007) The usefulness of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) and a comparison of 18F-FDG-PET with 67gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer 110:652–659. https://doi.org/10.1002/cncr.22807
Frick M, Dörken B, Lenz G (2011) The molecular biology of diffuse large B-cell lymphoma. Ther Adv Hematol 2:369–379. https://doi.org/10.1177/2040620711419001
Barrington SF, Mikhaeel NG, Kostakoglu L et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–3058. https://doi.org/10.1200/JCO.2013.53.5229
Chihara D, Oki Y, Onoda H et al (2011) High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol 93:502–508. https://doi.org/10.1007/s12185-011-0822-y
Park S, Moon SH, Park LC et al (2012) The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol 87:937–940. https://doi.org/10.1002/ajh.23267
Miyazaki Y, Nawa Y, Miyagawa M et al (2013) Maximum standard uptake value of 18F-fluorodeoxyglucose positron emission tomography is a prognostic factor for progression-free survival of newly diagnosed patients with diffuse large B cell lymphoma. Ann Hematol 92:239–244. https://doi.org/10.1007/s00277-012-1602-3
Schröder H, Moskowitz C (2016) Metabolic tumor volume in lymphoma: hype or hope? J Clin Oncol 34:3591–3594
Schöder H, Zelenetz AD, Hamlin P et al (2016) Prospective study of 3’-deoxy-3’-18F-fluorothymidine PET for early interim response assessment in advanced-stage B-cell lymphoma. J Nucl Med 57:728–734. https://doi.org/10.2967/jnumed.115.166769
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is M. Nijland.
Conflict of interest
The authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study. This study utilised rest material from patients, the use of which is regulated under the code for good clinical practice in the Netherlands and does not require informed consent in accordance with Dutch regulations.
Ethical approval
According to Dutch regulations, no medical ethical committee approval was required for this retrospective, observational study. A waiver was obtained from the medical ethics committee of the UMCG on November 13, 2018.
Methodology
This is a retrospective observational study performed at one institution.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 69 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kahle, X.U., Hovingh, M., Noordzij, W. et al. Tumour necrosis as assessed with 18F-FDG PET is a potential prognostic marker in diffuse large B cell lymphoma independent of MYC rearrangements. Eur Radiol 29, 6018–6028 (2019). https://doi.org/10.1007/s00330-019-06178-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06178-9