Abstract
Purpose
This study was conducted in order to evaluate if iso- or hyperintensity of HCAs on HBP is systematically related to a high uptake of hepatospecific contrast agent, using a quantitative approach.
Methods
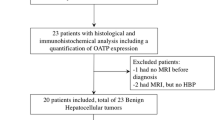
This bicentric retrospective study included all patients with histologically confirmed and subtyped HCA from 2009 to 2017 who underwent MRI with HBP after Gd-BOPTA injection and who showed iso- or hyperintensity on HBP. The signal intensity of tumors on pre- and postcontrast images and the presence of hepatic steatosis were noted. Contrast uptake on HBP was quantified using the liver-to-lesion contrast enhancement ratio (LLCER) and compared between HCA subtypes (Wilcoxon signed-rank test). Categorical variables were compared using chi-square tests.
Results
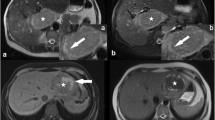
Twenty-four HCAs showed iso- or hyperintensity on HBP, specifically 17 inflammatory (IHCAs) and 7 β-catenin HCAs (BHCAs). Eighteen HCAs (75%) (17 IHCAs and 1 BHCAs) had a LLCER < 0% (median − 13.6%, group 1), of which 94% were hyperintense on precontrast T1-W images, with background hepatic steatosis. Six HCAs (25%) had LLCER ≥ 0% (median 2.9%, group 2), and all were BHCAs. A LLCER ≥ 1.6% was associated with the diagnosis of BHCA with a sensitivity of 86% and a specificity of 100%.
Conclusion
In conclusion, iso- or hyperintensity of hepatocellular adenomas on HBP does not necessarily correspond to an increased hepatospecific contrast-agent uptake. In IHCA, tumor hyperintensity on precontrast images and the underlying steatosis likely explain such iso- or hyperintensity, which do show reduced HBP contrast-agent uptake. On the other hand, marked contrast uptake can be observed, especially in BHCA.
Key Points
• Iso- or hyperintensity on HBP does not necessarily reflect a high uptake of hepatospecific contrast agent.
• Discrepancies between qualitative signal intensity and quantitative hepatospecific contrast uptake can be explained in IHCA by a combination of tumor hyperintensity on precontrast images and underlying hepatic steatosis.
• In BHCA, iso- or hyperintensity on HBP does actually correspond to a greater contrast uptake than that of the liver, demonstrated by an increased lesion-to-liver contrast enhancement ratio (LLCER).





Similar content being viewed by others
Abbreviations
- BHCA:
-
β-Catenin-mutated hepatocellular adenoma
- FNH:
-
Focal nodular hyperplasia
- HBP:
-
Hepatobiliary phase
- HCA:
-
Hepatocellular adenoma
- HHCA:
-
HNF1-α-inactivated HCA
- IHCA:
-
Inflammatory HCA
- LLCER:
-
Liver-to-lesion contrast enhancement ratio
- OATP:
-
Organic anion transporting polypeptide
- SI:
-
Signal intensity
- SIR:
-
Signal intensity ratio
References
Edmondson HA, Henderson B, Benton B (1976) Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med 294:470–472. https://doi.org/10.1056/NEJM197602262940904
Nault JC, Bioulac-Sage P, Zucman-Rossi J (2013) Hepatocellular benign tumors—from molecular classification to personalized clinical care. Gastroenterology 144:888–902. https://doi.org/10.1053/j.gastro.2013.02.032
Belghiti J, Cauchy F, Paradis V, Vilgrain V (2014) Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol 11:737–749. https://doi.org/10.1038/nrgastro.2014.151
European Association for the Study of the Liver (EASL) (2016) EASL clinical practice guidelines on the management of benign liver tumours. J Hepatol 65:386–398. https://doi.org/10.1016/j.jhep.2016.04.001
Nault JC, Paradis V, Cherqui D, Vilgrain V, Zucman-Rossi J (2017) Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol 67:1074–1083. https://doi.org/10.1016/j.jhep.2017.07.009
Dokmak S, Paradis V, Vilgrain V et al (2009) A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137:1698–1705. https://doi.org/10.1053/j.gastro.2009.07.061
Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V (2011) Changing trends in malignant transformation of hepatocellular adenoma. Gut 60:85–89. https://doi.org/10.1136/gut.2010.222109
Nault JC, Couchy G, Balabaud C et al (2017) Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology 152:880–894.e6. https://doi.org/10.1053/j.gastro.2016.11.042
Lewin M, Handra-Luca A, Arrivé L et al (2006) Liver adenomatosis: classification of MR imaging features and comparison with pathologic findings. Radiology 241:433–440. https://doi.org/10.1148/radiol.2412051243
Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H (2008) Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 48:808–818. https://doi.org/10.1002/hep.22417
Ronot M, Bahrami S, Calderaro J et al (2011) Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53:1182–1191. https://doi.org/10.1002/hep.24147
Ronot M, Vilgrain V (2014) Imaging of benign hepatocellular lesions: current concepts and recent updates. Clin Res Hepatol Gastroenterol 38:681–688. https://doi.org/10.1016/j.clinre.2014.01.014
Kreft BP, Baba Y, Tanimoto A, Finn JP, Stark DD (1993) Orally administered manganese chloride: enhanced detection of hepatic tumors in rats. Radiology 186:543–548. https://doi.org/10.1148/radiology.186.2.8421762
Ni Y, Marchal G, Yu J, Mühler A, Lukito G, Baert AL (1994) Prolonged positive contrast enhancement with Gd-EOB-DTPA in experimental liver tumors: potential value in tissue characterization. J Magn Reson Imaging 4:355–363
Ni Y, Marchal G (1998) Enhanced magnetic resonance imaging for tissue characterization of liver abnormalities with hepatobiliary contrast agents: an overview of preclinical animal experiments. Top Magn Reson Imaging 9:183–195
Grazioli L, Morana G, Kirchin MA, Schneider G (2005) Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology 236:166–177. https://doi.org/10.1148/radiol.2361040338
Grazioli L, Bondioni MP, Haradome H et al (2012) Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 262:520–529. https://doi.org/10.1148/radiol.11101742
Bieze M, van den Esschert JW, Nio CY et al (2012) Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol 199:26–34. https://doi.org/10.2214/AJR.11.7750
Suh CH, Kim KW, Kim GY, Shin YM, Kim PN, Park SH (2015) The diagnostic value of Gd-EOB-DTPA-MRI for the diagnosis of focal nodular hyperplasia: a systematic review and meta-analysis. Eur Radiol 25:950–960. https://doi.org/10.1007/s00330-014-3499-9
Neri E, Bali MA, Ba-Ssalamah A et al (2016) ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol 26:921–931. https://doi.org/10.1007/s00330-015-3900-3
Merkle EM, Zech CJ, Bartolozzi C et al (2016) Consensus report from the 7th international forum for liver magnetic resonance imaging. Eur Radiol 26:674–682. https://doi.org/10.1007/s00330-015-3873-2
Ba-Ssalamah A, Antunes C, Feier D et al (2015) Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology 277:104–113. https://doi.org/10.1148/radiol.2015142366
Agarwal S, Fuentes-Orrego JM, Arnason T et al (2014) Inflammatory hepatocellular adenomas can mimic focal nodular hyperplasia on gadoxetic acid-enhanced MRI. AJR Am J Roentgenol 203:W408–W414. https://doi.org/10.2214/AJR.13.12251
Thomeer MG, Willemssen FE, Biermann KK et al (2014) MRI features of inflammatory hepatocellular adenomas on hepatocyte phase imaging with liver-specific contrast agents. J Magn Reson Imaging 39:1259–1264. https://doi.org/10.1002/jmri.24281
Tse JR, Naini BV, Lu DSK, Raman SS (2016) Qualitative and quantitative gadoxetic acid-enhanced MR imaging helps subtype hepatocellular adenomas. Radiology 279:118–127. https://doi.org/10.1148/radiol.2015142449
Glockner JF, Lee CU, Mounajjed T (2017) Inflammatory hepatic adenomas: characterization with hepatobiliary MRI contrast agents. Magn Reson Imaging 47:103–110. https://doi.org/10.1016/j.mri.2017.12.006
Yoneda N, Matsui O, Kitao A et al (2016) Benign hepatocellular nodules: hepatobiliary phase of gadoxetic acid-enhanced MR imaging based on molecular background. Radiographics 36:2010–2027. https://doi.org/10.1148/rg.2016160037
Reizine E, Amaddeo G, Pigneur F et al (2018) Quantitative correlation between uptake of Gd-BOPTA on hepatobiliary phase and tumor molecular features in patients with benign hepatocellular lesions. Eur Radiol. https://doi.org/10.1007/s00330-018-5438-7
Yoneda N, Matsui O, Kitao A et al (2012) Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol 30:777–782. https://doi.org/10.1007/s11604-012-0115-2
Roux M, Pigneur F, Calderaro J et al (2015) Differentiation of focal nodular hyperplasia from hepatocellular adenoma: role of the quantitative analysis of gadobenate dimeglumine-enhanced hepatobiliary phase MRI. J Magn Reson Imaging 42:1249–1258. https://doi.org/10.1002/jmri.24897
Fléjou JF (2011) WHO classification of digestive tumors: the fourth edition. Ann Pathol 31:S27–S31. https://doi.org/10.1016/j.annpat.2011.08.001
Zucman-Rossi J, Jeannot E, Nhieu JT et al (2006) Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 43:515–524. https://doi.org/10.1002/hep.21068
Bioulac-Sage P, Laumonier H, Couchy G et al (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50:481–489. https://doi.org/10.1002/hep.22995
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474. https://doi.org/10.1111/j.1572-0241.1999.01377.x
Cassidy FH, Yokoo T, Aganovic L et al (2009) Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 29:231–260. https://doi.org/10.1148/rg.291075123
van Aalten SM, Thomeer MGJ, Terkivatan T et al (2011) Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology 261:172–181. https://doi.org/10.1148/radiol.11110023
Ueno A, Masugi Y, Yamazaki K et al (2014) OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol 61:1080–1087. https://doi.org/10.1016/j.jhep.2014.06.008
Thomeer MG, E Bröker ME, de Lussanet Q et al (2014) Genotype-phenotype correlations in hepatocellular adenoma: an update of MRI findings. Diagn Interv Radiol 20:193–199. https://doi.org/10.5152/dir.2013.13315
Thomeer MG, Gest B, van Beek H et al (2018) Quantitative analysis of hepatocellular adenoma and focal nodular hyperplasia in the hepatobiliary phase: external validation of LLCER method using gadobenate dimeglumine as contrast agent. J Magn Reson Imaging 47:860–861. https://doi.org/10.1002/jmri.25789
Roux M, Pigneur F, Luciani A (2018) Response to “Quantitative analysis of hepatocellular adenoma and focal nodular hyperplasia in the hepatobiliary phase: external validation of llcer method using gadobenate dimeglumine as contrast agent”. J Magn Reson Imaging 47:862–863. https://doi.org/10.1002/jmri.25788
Hata H, Inoue Y, Nakajima A, Komi S, Miyatake H (2017) Influence of the magnetic field strength on image contrast in Gd-EOB-DTPA-enhanced MR imaging: comparison between 1.5T and 3.0T. Magn Reson Med Sci 16:109–114. https://doi.org/10.2463/mrms.mp.2015-0158
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 40:715–724
Pascolo L, Cupelli F, Anelli PL et al (1999) Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun 257:746–752. https://doi.org/10.1006/bbrc.1999.0454
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maxime Ronot.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in a previous manuscript in the European Radiology focusing on a correlation between the quantitative analysis of benign hepatocellular tumor uptake on HBP imaging and the quantitative level of OATP expression [28].
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1258 kb)
Rights and permissions
About this article
Cite this article
Reizine, E., Ronot, M., Pigneur, F. et al. Iso- or hyperintensity of hepatocellular adenomas on hepatobiliary phase does not always correspond to hepatospecific contrast-agent uptake: importance for tumor subtyping. Eur Radiol 29, 3791–3801 (2019). https://doi.org/10.1007/s00330-019-06150-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06150-7