Abstract
Purpose
To evaluate the prognostic value and molecular basis of a CT-derived pleural contact index (PCI) in early stage non-small cell lung cancer (NSCLC).
Experimental design
We retrospectively analysed seven NSCLC cohorts. A quantitative PCI was defined on CT as the length of tumour-pleura interface normalised by tumour diameter. We evaluated the prognostic value of PCI in a discovery cohort (n = 117) and tested in an external cohort (n = 88) of stage I NSCLC. Additionally, we identified the molecular correlates and built a gene expression-based surrogate of PCI using another cohort of 89 patients. To further evaluate the prognostic relevance, we used four datasets totalling 775 stage I patients with publically available gene expression data and linked survival information.
Results
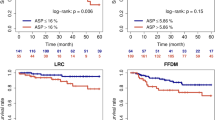
At a cutoff of 0.8, PCI stratified patients for overall survival in both imaging cohorts (log-rank p = 0.0076, 0.0304). Extracellular matrix (ECM) remodelling was enriched among genes associated with PCI (p = 0.0003). The genomic surrogate of PCI remained an independent predictor of overall survival in the gene expression cohorts (hazard ratio: 1.46, p = 0.0007) adjusting for age, gender, and tumour stage.
Conclusions
CT-derived pleural contact index is associated with ECM remodelling and may serve as a noninvasive prognostic marker in early stage NSCLC.
Key points
• A quantitative pleural contact index (PCI) predicts survival in early stage NSCLC.
• PCI is associated with extracellular matrix organisation and collagen catabolic process.
• A multi-gene surrogate of PCI is an independent predictor of survival.
• PCI can be used to noninvasively identify patients with poor prognosis.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2016. A Cancer Journal for Clinicians, CA
Aberle DR, DeMello S, Berg CD et al (2013) Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 369:920–931
Bradley JD, El Naqa I, Drzymala RE, Trovo M, Jones G, Denning MD (2010) Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 77:1146–1150
Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S (2012) Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 13:802–809
Neal JW, Gainor JF, Shaw AT (2015) Developing biomarker-specific end points in lung cancer clinical trials. Nat Rev Clin Oncol 12:135–146
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406
Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO reports:e201439246
Paszek MJ, Zahir N, Johnson KR et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254
Levental KR, Yu HM, Kass L et al (2009) Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 139:891–906
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219
Fraley SI, Feng YF, Krishnamurthy R et al (2010) A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12:598–U169
Neri S, Yoshida J, Ishii G et al (2014) Prognostic impact of microscopic vessel invasion and visceral pleural invasion in non–small cell lung cancer: a retrospective analysis of 2657 patients. Ann Surg 260:383–388
Travis WD, Brambilla E, Rami-Porta R et al (2008) Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 3:1384–1390
Shimizu K, Yoshida J, Nagai K et al (2005) Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 130:160–165
Lakha S, Gomez JE, Flores RM, Wisnivesky JP (2014) Prognostic significance of visceral pleural involvement in early-stage lung cancer. CHEST J 146:1619–1626
Huang H, Wang T, Hu B, Pan C (2015) Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 99:1130–1139
Hsu J-S, Han I-T, Tsai T-H et al (2015) Pleural Tags on CT Scans to Predict Visceral Pleural Invasion of Non–Small Cell Lung Cancer That Does Not Abut the Pleura. Radiology 279:590–596
Ebara K, Takashima S, Jiang B et al (2015) Pleural invasion by peripheral lung cancer: prediction with three-dimensional CT. Acad Radiol 22:310–319
Aerts HJWL, Velazquez, ER, Leijenaar RTH, Parmar C, Grossmann P, Cavalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans R, Dekker A, Quackenbush J, Gillies RJ, Lambin P (2015) Data from NSCLC-Radiomics. Cancer Imaging Archive
Aerts HJWL, Velazquez ER, Leijenaar RTH et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Clark K, Vendt B, Smith K et al (2013) The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging 26:1045–1057
Gentles AJ, Newman AM, Liu CL et al (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21:938–945
Goswami CP, Nakshatri H (2013) PROGgene: gene expression based survival analysis web application for multiple cancers. J Clin Bioinform 3:1
Li C, Wong WH (2003) DNA-chip analyzer (dChip). In: Parmigiani G, Garrett E, Irizarry R, Zeger S, (eds) The Analysis of Gene Expression Data. Springer, 120–141
Imai K, Minamiya Y, Ishiyama K et al (2013) Use of CT to Evaluate Pleural Invasion in Non–Small Cell Lung Cancer: Measurement of the Ratio of the Interface between Tumor and Neighboring Structures to Maximum Tumor Diameter. Radiology 267:619–626
Koch GG (1983) Intraclass correlation coefficient. Encycl Stat Sci 4
Bland JM, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Park HS, Harder EM, Mancini BR, Decker RH (2015) Central versus Peripheral Tumor Location: Influence on Survival, Local Control, and Toxicity Following Stereotactic Body Radiotherapy for Primary Non–Small-Cell Lung Cancer. J Thorac Oncol 10:832–837
Tibshirani R, Hastie T, Narasimhan B, Chu G (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci 99:6567–6572
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Harrell FE, Lee KL, Califf RM, Pryor DB, Rosati RA (1984) Regression modelling strategies for improved prognostic prediction. Stat Med 3:143–152
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological):289–300
Pignon J-P, Tribodet H, Scagliotti GV et al (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552–3559
Strauss GM, Herndon JE, Maddaus MA et al (2008) Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non–small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26:5043–5051
Wang H, Schabath MB, Liu Y et al (2015) Semiquantitative computed tomography characteristics for lung adenocarcinoma and their association with lung cancer survival. Clin Lung Cancer 16:e141–e163
Wang H, Schabath MB, Liu Y et al (2015) Association between computed tomographic features and kirsten rat sarcoma viral oncogene mutations in patients with stage i lung adenocarcinoma and their prognostic value. Clin Lung Cancer 12:00266–00261
Hsu K-H, Chen K-C, Yang T-Y et al (2011) Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 6:1066–1072
Lee H-J, Kim YT, Kang CH et al (2013) Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology 268:254–264
Rizzo S, Petrella F, Buscarino V et al (2016) CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol 26:32–42
Hasegawa M, Sakai F, Ishikawa R, Kimura F, Ishida H, Kobayashi K (2016) CT Features of Epidermal Growth Factor Receptor–Mutated Adenocarcinoma of the Lung: Comparison with Nonmutated Adenocarcinoma. J Thorac Oncol 11:819–826
Liu Y, Kim J, Qu F et al (2016) CT Features Associated with Epidermal Growth Factor Receptor Mutation Status in Patients with Lung Adenocarcinoma. Radiology 280:271–280
Wang H, Schabath MB, Liu Y et al (2016) Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol 85:1934–1940
Zhou J, Zheng J, Yu Z et al (2015) Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 25:1257–1266
Paz H, Pathak N, Yang J (2014) Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene 33:4193–4202
Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14:430–439
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22:796–802
Win T, Miles KA, Janes SM et al (2013) Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non–small cell lung cancer. Clin Cancer Res 19:3591–3599
Grove O, Berglund AE, Schabath MB et al (2015) Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS One 10, e0118261
Coroller TP, Grossmann P, Hou Y et al (2015) CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol 114:345–350
Huang Y, Liu Z, He L et al (2016) Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non—Small Cell Lung Cancer. Radiology 152234
Liu Y, Balagurunathan Y, Atwater T et al (2016) Radiological Image traits Predictive of Cancer Status in Pulmonary Nodules. Clin Cancer Res: Clin. 3102.2016
de Bruin EC, McGranahan N, Mitter R et al (2014) Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346:251–256
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ruijiang Li.
Conflict of interest
The authors have no potential conflicts of interest.
Funding
This research was partially supported by the NIH grant number R00 CA166186, R01 CA193730.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross-sectional study
• multicentre study
Additional information
Juheon Lee and Yi Cui contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 689 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Cui, Y., Sun, X. et al. Prognostic value and molecular correlates of a CT image-based quantitative pleural contact index in early stage NSCLC. Eur Radiol 28, 736–746 (2018). https://doi.org/10.1007/s00330-017-4996-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4996-4