Abstract
Objective
To determine the neuroimaging pattern of cerebellar dysplasia (CD) and other posterior fossa morphological anomalies associated with mutations in tubulin genes and to perform clinical and genetic correlations.
Methods
Twenty-eight patients harbouring 23 heterozygous pathogenic variants (ten novel) in tubulin genes TUBA1A (n = 10), TUBB2B (n = 8) or TUBB3 (n = 5) were studied by a brain MRI scan performed either on a 1.5 T (n = 10) or 3 T (n = 18) MR scanner with focus on the posterior fossa.
Results
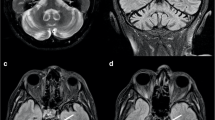
Cerebellar anomalies were detected in 24/28 patients (86%). CD was recognised in 19/28 (68%) including cortical cerebellar dysplasia (CCD) in 18/28, either involving only the cerebellar hemispheres (12/28) or associated with vermis dysplasia (6/28). CCD was located only in the right hemisphere in 13/18 (72%), including four TUBB2B-, four TUBB3- and five TUBA1A-mutated patients, while in the other five TUBA1A cases it was located only in the left hemisphere or in both hemispheres. The postero-superior region of the cerebellar hemispheres was most frequently affected.
Conclusions
The cerebellar involvement in tubulinopathies shows specific features that may be labelled as ‘tubulin-related CD’. This pattern is unique and differs from other genetic causes of cerebellar dysplasia.
Key Points
• Cortical cerebellar dysplasia without cysts is suggestive of tubulin-related disorder.
• Cerebellar dysplasia in tubulinopathies shows specific features labelled as ‘tubulin-related CD’.
• Focal and unilateral involvement of cerebellar hemispheres has important implications for counselling.



Similar content being viewed by others
Change history
12 September 2017
An erratum to this article has been published.
Abbreviations
- CD:
-
Cerebellar dysplasia
- CCD:
-
Cortical cerebellar dysplasia
- CVD:
-
Cerebellar vermian dysplasia
- GPR56:
-
G Protein-Coupled Receptor 56
- MCDs:
-
Malformations of cortical development
- MRI:
-
Magnetic resonance imaging
- PMG:
-
Polymicrogyria
- TUBA1A :
-
Tubulin, Alpha-1A
- TUBB2B :
-
Tubulin, Beta-2B
- TUBB3 :
-
Tubulin, Beta-3
- WNT1:
-
Wingless-Type Mmtv Integration Site Family, Member 1
References
Poretti A, Boltshauser E, Huisman TA (2016) Cerebellar and Brainstem malformations. Neuroimaging Clin N Am 26:341–357
Patel S, Barkovich AJ (2002) Analysis and classification of cerebellar malformations. AJNR Am J Neuroradiol 23:1074–1087
Demaerel P (2002) Abnormalities of cerebellar foliation and fissuration: classification, neurogenetics and clinicoradiological correlations. Neuroradiology 44:639–646
Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB (2012) A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135:1348–1369
Bosemani T, Orman G, Boltshauser E, Tekes A, Huisman TA, Poretti A (2015) Congenital abnormalities of the posterior fossa. Radiographics 35:200–220
Bahi-Buisson N, Poirier K, Fourniol F et al (2014) The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain 137:1676–1700
Kumar RA, Pilz DT, Babatz TD et al (2010) TUBA1A mutations cause wide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on alpha tubulins. Hum Mol Genet 19:2817–2827
Oegema R, Cushion TD, Phelps IG et al (2015) Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum Mol Genet 24:5313–5325
Richards S, Aziz N, Bale S, Bick D et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Romaniello R, Arrigoni F, Cavallini A et al (2014) Brain malformations and mutations in α- and β-tubulin genes: a review of the literature and description of two new cases. Dev Med Child Neurol 56:354–360
Romaniello R, Tonelli A, Arrigoni F et al (2012) A novel mutation in the β-tubulin gene TUBB2B associated with complex malformation of cortical development and deficits in axonal guidance. Dev Med Child Neurol 54:765–776
Keays DA, Tian G, Poirier K (2007) Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 128:45–57
Zanni G, Colafati GS, Barresi S (2013) Description of a novel TUBA1A mutation in Arg-390 associated with asymmetrical polymicrogyria and mid-hindbrain dysgenesis. Eur J Paediatr Neurol 17:361–365
Jaglin XH, Poirier K, Saillour Y et al (2009) Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet 41:746–752
Cushion TD, Dobyns WB, Mullins JG (2013) Overlapping cortical malformations and mutations in TUBB2B and TUBA1A. Brain 136:536–548
Cederquist GY, Luchniak A, Tischfield MA et al (2012) An inherited TUBB2B mutation alters a kinesin-binding site and causes polymicrogyria, CFEOM and axon dysinnervation. Hum Mol Genet 21:5484–5499
Tischfield MA, Baris HN, Wu C et al (2010) Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140:74–87
Alby C, Malan V, Boutaud L et al (2016) Clinical, genetic and neuropathological findings in a series of 138 fetuses with a corpus callosum malformation. Birth Defects Res A Clin Mol Teratol 106:36–46
Romaniello R, Arrigoni F, Bassi MT, Borgatti R (2015) Mutations in α- and β-tubulin encoding genes: implications in brain malformations. Brain Dev 37:273–280, Review
Bahi-Buisson N, Cavallin M. Tubulinopathies Overview (2016) In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2016.18
Saywell V, Cioni JM, Ango F (2014) Developmental gene expression profile of axon guidance cues in Purkinje cells during cerebellar circuit formation. Cerebellum 13:307–317
Bilimoria PM, Bonni A (2013) Molecular control of axon branching. Neuroscientist 19:16–24
Barallobre MJ, Pascual M, Del Río JA, Soriano E (2005) The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Rev 49:22–47
Buel GR, Rush J, Ballif BA (2010) Fyn promotes phosphorylation of collapsin response mediator protein 1 at tyrosine 504, a novel, isoform-specific regulatory site. J Cell Biochem 111:20–28
Lin PC, Chan PM, Hall C, Manser E (2011) Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J Biol Chem 286:41466–41478
Moreno-Flores MT, Martín-Aparicio E, Avila J, Díaz-Nido J, Wandosell F (2002) Ephrin-B1 promotes dendrite outgrowth on cerebellar granule neurons. Mol Cell Neurosci 20:429–446
Qu C, Dwyer T, Shao Q, Yang T, Huang H, Liu G (2013) Direct binding of TUBB3 with DCC couples netrin-1 signaling to intracellular microtubule dynamics in axon outgrowth and guidance. J Cell Sci 126:3070–3081
Breuss M, Morandell J, Nimpf S et al (2015) The Expression of Tubb2b Undergoes a Developmental Transition in Murine Cortical Neurons. J Comp Neurol 523:2161–2186
Fallet-Bianco C, Laquerrière A, Poirier K et al (2014) Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol Commun 2:69
Tischfield MA, Cederquist GY, Gupta ML Jr, Engle EC (2011) Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev 21:286–294
Aldinger KA, Mendelsohn NJ, Chung BH et al (2016) Variable brain phenotype primarily affects the brainstem and cerebellum in patients with osteogenesis imperfecta caused by recessive WNT1 mutations. J Med Genet 53:427–430
Acknowledgements
The authors are grateful to the patients involved in this study and their parents for their kind cooperation. We also acknowledge the PADAPORT project (to RB and EMV) funded by the Pierfranco and Luisa Mariani Foundation. We are also grateful to Dr. Pascal Joset, Institute of Medical Genetics, Zürich, for mutation analysis of one patient.
During the revision process of the manuscript our colleague Andrea Poretti suddenly passed away. Not only was he one of the most important authors of this study, he was first and foremost a dear friend. During the past years his enthusiastic and tireless efforts into the study of cerebellum and brain malformations has influenced all of us deeply. This paper is in memory of Andrea Poretti.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Renato Borgatti.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
This study was supported by the Italian Ministry of Health (Grant 5X1000-2012 to RB Grant 5X1000-2014 to RR; Ricerca Finalizzata grant NET-2013-02356160 to RB and EMV), and by the European Research Council (Starting Grant StG260888 to EMV).
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Romaniello, Arrigoni, Cavallini, et al. [10] and Romaniello, Tonelli, Arrigoni, et al. [11].
Methodology
• retrospective
• observational
• multicentre study
Additional information
The original version of this article was revised: The first name of the author Raffaella Cusmai was rendered incorrectly and has now been corrected.
During the revision process of the manuscript our colleague Andrea Poretti sadly suddenly passed away.
An erratum to this article is available at https://doi.org/10.1007/s00330-017-4986-6.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 26 kb)
Supplementary Table 2
(DOCX 24 kb)
Supplementary Table 3
(DOCX 23 kb)
Supplementary Figure
(DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Romaniello, R., Arrigoni, F., Panzeri, E. et al. Tubulin-related cerebellar dysplasia: definition of a distinct pattern of cerebellar malformation. Eur Radiol 27, 5080–5092 (2017). https://doi.org/10.1007/s00330-017-4945-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4945-2