Abstract
Objective
With a region of interest (ROI)-based approach 2-year-old children after congenital diaphragmatic hernia (CDH) show reduced MR lung perfusion values on the ipsilateral side compared to the contralateral. This study evaluates whether results can be reproduced by segmentation of whole-lung and whether there are differences between the ROI-based and whole-lung measurements.
Methods
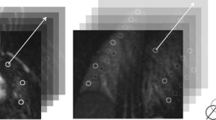
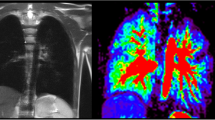
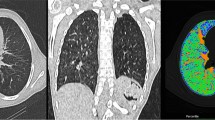
Using dynamic contrast-enhanced (DCE) MRI, pulmonary blood flow (PBF), pulmonary blood volume (PBV) and mean transit time (MTT) were quantified in 30 children after CDH repair. Quantification results of an ROI-based (six cylindrical ROIs generated of five adjacent slices per lung-side) and a whole-lung segmentation approach were compared.
Results
In both approaches PBF and PBV were significantly reduced on the ipsilateral side (p always <0.0001). In ipsilateral lungs, PBF of the ROI-based and the whole-lung segmentation-based approach was equal (p=0.50). In contralateral lungs, the ROI-based approach significantly overestimated PBF in comparison to the whole-lung segmentation approach by approximately 9.5 % (p=0.0013).
Conclusions
MR lung perfusion in 2-year-old children after CDH is significantly reduced ipsilaterally. In the contralateral lung, the ROI-based approach significantly overestimates perfusion, which can be explained by exclusion of the most ventral parts of the lung. Therefore whole-lung segmentation should be preferred.
Key Points
• Ipsilaterally, absolute lung perfusion after CDH is reduced in whole-lung analysis.
• Ipsilaterally, the ROI- and whole-lung-based approaches generate identical results.
• Contralaterally, the ROI-based approach significantly overestimates perfusion results.
• Whole lung should be analysed in MR lung perfusion imaging.
• MR lung perfusion measurement is a radiation-free parameter of lung function.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- CDH:
-
Congenital diaphragmatic hernia
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- ECMO:
-
Extracorporeal membrane oxygenation
- MTT:
-
Mean transit time
- PBF:
-
Pulmonary blood flow
- PBV:
-
Pulmonary blood volume
- ROI:
-
Region of interest
- TWIST:
-
Time-resolved angiography with stochastic trajectories (TWIST)
References
Stege G, Fenton A, Jaffray B (2003) Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 112:532–535
Wright JC, Budd JL, Field DJ, Draper ES (2011) Epidemiology and outcome of congenital diaphragmatic hernia: a 9-year experience. Paediatr Perinat Epidemiol 25:144–149
Skari H, Bjornland K, Haugen G, Egeland T, Emblem R (2000) Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg 35:1187–1197
Madderom MJ, Toussaint L, van der Cammen-van Zijp MHM et al (2012) Congenital diaphragmatic hernia with(out) ECMO: impaired development at 8 years. Archives of disease in childhood. Fetal and neonatal ed. doi:10.1136/archdischild-2012-303020
Spoel M, van der Cammen-van Zijp MHM, Hop WCJ, Tibboel D, de Jongste JC, Ijsselstijn H (2013) Lung function in young adults with congenital diaphragmatic hernia; a longitudinal evaluation. Pediatr Pulmonol 48:130–137
Stefanutti G, Filippone M, Tommasoni N et al (2004) Cardiopulmonary anatomy and function in long-term survivors of mild to moderate congenital diaphragmatic hernia. J Pediatr Surg 39:526–531
Panitch HB, Weiner DJ, Feng R et al (2014) Lung function over the first 3 years of life in children with congenital diaphragmatic hernia. Pediatr Pulmonol. doi:10.1002/ppul.23082
Escobar H, Carver TW Jr (2011) Pulmonary function testing in young children. Curr allergy asthma rep 11:473–481
Waag KL, Loff S, Zahn K et al (2008) Congenital diaphragmatic hernia: a modern day approach. Semin Pediatr Surg 17(4):244–254
Cruz-Martinez R, Moreno-Alvarez O, Hernandez-Andrade E et al (2011) Changes in lung tissue perfusion in the prediction of survival in fetuses with congenital diaphragmatic hernia treated with fetal endoscopic tracheal occlusion. Fetal Diagn Ther 29(1):101–107
Pal K, Gupta DK (2010) Serial perfusion study depicts pulmonary vascular growth in the survivors of non-extracorporeal membrane oxygenation-treated congenital diaphragmatic hernia. Neonatology 98(3):254–259
Ley S, Ley-Zaporozhan J (2012) Pulmonary perfusion imaging using MRI: clinical application. Insights into imaging 3(1):61–71
Weidner M, Zoellner FG, Hagelstein C et al (2014) High temporal versus high spatial resolution in MR quantitative pulmonary perfusion imaging of two-year old children after congenital diaphragmatic hernia repair. Eur Radiol 24(10):2427–2434
Zoellner FG, Zahn K, Schaible T, Schoenberg SO, Schad LR, Neff KW (2012) Quantitative pulmonary perfusion imaging at 3.0 T of 2-year-old children after congenital diaphragmatic hernia repair: initial results. Eur Radiol 22:2743–2749
Zoellner FG, Weisser G, Reich M et al (2013) UMMPerfusion: an open source software tool towards quantitative MRI perfusion analysis in clinical routine. J Digit imaging : Off J Soc Comput Appl Radiol 26:344–352
Sourbron S, Dujardin M, Makkat S, Luypaert R (2007) Pixel-by-pixel deconvolution of bolus-tracking data: optimization and implementation. Phys Med Biol 52:429–447
Nikolaou K, Schoenberg SO, Brix G et al (2004) Quantification of pulmonary blood flow and volume in healthy volunteers by dynamic contrast-enhanced magnetic resonance imaging using a parallel imaging technique. Investig Radiol 39:537–545
Ijsselstijn H, van Heijst AF (2014) Long-term outcome of children treated with neonatal extracorporeal membrane oxygenation: increasing problems with increasing age. Semin Perinatol 38:114–121
Wright T, Filbrun A, Bryner B, Mychaliska G (2014) Predictors of early lung function in patients with congenital diaphragmatic hernia. J Pediatr Surg 49:882–885
Bjorkman KC, Kjellberg M, Bergstrom SE et al (2011) Postoperative regional distribution of pulmonary ventilation and perfusion in infants with congenital diaphragmatic hernia. J Pediatr Surg 46:2047–2053
Hayward MJ, Kharasch V, Sheils C et al (2007) Predicting inadequate long-term lung development in children with congenital diaphragmatic hernia: an analysis of longitudinal changes in ventilation and perfusion. J Pediatr Surg 42:112–116
Ohno Y, Hatabu H, Murase K et al (2004) Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: Preliminary experience in 40 subjects. J Magn Reson Imaging : JMRI 20:353–365
Ley-Zaporozhan J, Molinari F, Risse F et al (2011) Repeatability and reproducibility of quantitative whole-lung perfusion magnetic resonance imaging. J Thorac Imaging 26:230–239
Fink C, Puderbach M, Bock M et al (2004) Regional lung perfusion: assessment with partially parallel three-dimensional MR imaging. Radiology 231:175–184
Fink C, Ley S, Risse F et al (2005) Effect of inspiratory and expiratory breathhold on pulmonary perfusion: assessment by pulmonary perfusion magnetic resonance imaging. Investig Radiol 40:72–79
Heye T, Merkle EM, Reiner CS et al (2013) Reproducibility of dynamic contrastenhanced MR imaging. Part II. Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis. Radiology 266:812–821
Bauman G, Lutzen U, Ullrich M et al (2011) Pulmonary functional imaging: qualitative comparison of Fourier decomposition MR imaging with SPECT/CT in porcine lung. Radiology 260:551–559
Clark AR, Tawhai MH, Hoffman EA, Burrowes KS (2011) The interdependent contributions of gravitational and structural features to perfusion distribution in a multiscale model of the pulmonary circulation. J Appl Physiol (Bethesda, Md : 1985) 110:943–955
Hopkins SR, Arai TJ, Henderson AC, Levin DL, Buxton RB, Kim Prisk G (2010) Lung volume does not alter the distribution of pulmonary perfusion in dependent lung in supine humans. J Physiol 588:4759–4768
Bottger T, Grunewald K, Schobinger M et al (2007) Implementation and evaluation of a new workflow for registration and segmentation of pulmonary MRI data for regional lung perfusion assessment. Phys Med Biol 52:1261–1275
Ingrisch M, Dietrich O, Attenberger UI et al (2010) Quantitative pulmonary perfusion magnetic resonance imaging: influence of temporal resolution and signal-to-noise ratio. Investig Radiol 45:7–14
Zoellner FG, Daab M, Weidner M et al (2015) Semi-automatic lung segmentation of DCE-MRI data sets of 2-year old children after congenital diaphragmatic hernia repair: Initial results. Magn Reson Imaging 33(10):1345–1349
Acknowledgements
The scientific guarantor of this publication is Prof. Dr. K.W. Neff. The Insitute of Clinical Radiology and Nuclear Medicine Mannheim has research cooperations with Siemens Healthcare. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported in Weidner M, Zoellner FG, Hagelstein C, et al. (2014) High temporal versus high spatial resolution in MR quantitative pulmonary perfusion imaging of two-year old children after congenital diaphragmatic hernia repair. Eur Radiol 24(10):2427–34. Methodology: Performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weis, M., Sommer, V., Zöllner, F.G. et al. Region of interest-based versus whole-lung segmentation-based approach for MR lung perfusion quantification in 2-year-old children after congenital diaphragmatic hernia repair. Eur Radiol 26, 4231–4238 (2016). https://doi.org/10.1007/s00330-016-4330-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4330-6