Abstract
Objective
To determine the differences in motor pathways and selected non-motor pathways of the basal ganglia in Parkinson’s disease (PD) patients compared to healthy controls (HCs).
Methods
We analysed diffusion weighted imaging data of 24 PD patients and 26 HCs. We performed deterministic tractography analysis using the spherical deconvolution-based damped Richardson-Lucy algorithm and subcortical volume analysis.
Results
We found significantly increased fractional anisotropy (FA) in the motor pathways of PD patients: the bilateral corticospinal tract (right; corrected p = 0.0003, left; corrected p = 0.03), bilateral thalamus-motor cortex tract (right; corrected p = 0.02, left; corrected p = 0.004) and the right supplementary area-putamen tract (corrected p = 0.001). We also found significantly decreased FA in the right uncinate fasiculus (corrected p = 0.01) and no differences of FA in the bilateral supero-lateral medial forebrain bundles (p > 0.05) of PD patients compared to HCs. There were no subcortical volume differences (p > 0.05) between the PD patients and HCs.
Conclusion
These results can inform biological models of neurodegeneration and neuroplasticity in PD. We suggest that increased FA values in the motor tracts in PD may reflect compensatory reorganization of neural circuits indicative of adaptive or extended neuroplasticity.
Key points
• Fractional anisotropy was higher in motor pathways of PD patients compared to healthy controls.
• Fractional anisotropy was lower in the uncinate fasciculus of PD patients compared to healthy controls.
• Increased fractional anisotropy could suggest adaptive neuroplasticity or selective neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because the motor symptoms of Parkinson’s disease (PD) only arise after approximately 80 % depletion of striatal dopamine, it has long been thought that compensatory plasticity must occur both within and outside the basal ganglia as the pathology progresses [1]. Such compensatory processes, as well as any long-range deficits arising from degeneration of the basal ganglia pathways, should ultimately be reflected in the architecture of the fibre tracts connecting motor cortex, basal ganglia and thalamus.
To date most diffusion-based studies on PD have only considered changes within defined regions of interest (ROIs) in grey matter [2] or whole brain voxel-wise analysis of white matter using tract-based spatial statistics (TBSS) [3]. These approaches are prone to partial volume effects and entail difficulties of spatial alignment across participants. Given that there are crossing fibres in about 90 % of the voxels of the brain, allocation of voxel-based findings to specific tracts can be highly speculative [4]. To overcome these limitations, we conducted deterministic tractography using the damped Richardson-Lucy (dRL) algorithm which is more sensitive to detect group differences than voxel-based approaches and produces anatomically plausible white matter tracts [4]. This method improves fibre tracking in areas of complex fibre architecture and regions affected by partial volume [5].
We reconstructed the following motor pathways: (i) the corticospinal tract, (ii) fibre connections between the thalamus and the motor cortex, and (iii) fibre connections between the supplementary motor area and the putamen. The corticospinal tract is crucial for self-initiated movements and thus putatively involved in bradykinesia in PD [6]. Post-mortem and diffusion studies show direct white matter fibre connections from the supplementary motor area to the striatum [7]. It is assumed that these connections are relevant for motor performance in PD [8]. The thalamus, a relay centre for sensory and motor information, conveys motor inputs from the basal ganglia to the cortex and decreased dopaminergic innervation to the thalamus is assumed to contribute to the pathophysiology of PD [9]. These tracts are thus closely related to PD pathophysiology and symptoms, and potentially also to their remediation. We were also interested in potential changes in the non-motor tracts of (iv) the uncinate fasiculus and (v) the supero-lateral medial forebrain bundle. The uncinate fasiculus is assumed to be involved in language, emotional processing and episodic memory [10], while the supero-lateral medial forebrain bundle mediates reward-seeking [11], which are also potentially affected in PD. Hence our objective was to determine the differences in motor pathways and selected non-motor pathways of the basal ganglia in PD patients compared to healthy controls (HCs). Additionally we wanted to determine whether there were any group differences in basal ganglia volumes that could drive potential group differences in the white matter measures, therefore we also performed subcortical volume analysis [12].
Materials and methods
Participants
Twenty-six PD patients in Hoehn and Yahr stages I-III were recruited from clinics in South Wales by H.M. and collaborating clinicians (see Acknowledgements). They were scanned before and after taking part in a trial that compared functional magnetic resonance imaging (fMRI)-based neurofeedback and exercise with a gaming console over 12 weeks (NCT01867827) and will be reported elsewhere. Here we use the data from the baseline scan, which were unaffected by the intervention. The study was approved by the local NHS research ethics committee, and all patients gave informed consent. Diffusion MRI data of 26 HCs from a previous study into the effects of ageing on white matter microstructure [13] which used the same diffusion imaging protocol as the current study were included to provide an age-matched healthy comparison group (Table 1). Two PD patients’ scans were excluded from the final analysis due to corrupted data files.
Magnetic resonance (MR) data acquisition
Diffusion weighted MRI data were acquired on the 3T GE Signa HDx system (General Electric Healthcare) in Cardiff University Brain Research and Imaging Centre (CUBRIC) using a peripherally gated twice-refocused pulse-gradient spin-echo echo-planar imaging sequence providing whole oblique axial (parallel to the commissural plane) brain coverage. Data were acquired from 60 slices of 2.4-mm thickness, with a field of view (FOV) of 23 cm, and an acquisition matrix of 96 × 96 (yielding isotropic voxels of 2.4 × 2.4 × 2.4 mm, reconstructed to a resolution of 1.9 × 1.9 × 2.4 mm). Echo time (TE) was 87 ms and parallel imaging (ASSET factor = 2) was used. Diffusion encoding gradients (b = 1,200 s/mm2) were applied along 30 isotropically-distributed directions [14] as the optimal b-value for deriving good estimates of fractional anisotropy (FA), mean diffusivity (MD) and fibre orientation is 750–1,300 s/mm2 [15, 16].
T1-weighted structural scans were acquired using an oblique-axial, 3D fast spoiled gradient recalled sequence (FSPGR) with the following parameters: 178 slices; TE = 3 ms, TR = 7.9 ms, voxel size 1.0 × 1.0 × 1.0 mm3, 256 × 256 FOV. These structural images were acquired for co-registration and anatomical localization of regions of interest for the tracts. These FSPGR images were also used as a template to draw ROIs for the virtual dissection of the tracts and for the subcortical volumes analyses.
Diffusion MR Imagining (MRI) data processing
The diffusion MRI data were corrected for subject motion, echo planar imaging (EPI) distortions and Eddy Current (EC) distortions in ExploreDTIv4.8.3 [17]. We performed an affine registration to the non-diffusion-weighted images, with appropriate re-orienting of the encoding vectors [18]. A single diffusion tensor model was fitted [19] to the data in order to compute the quantitative parameters: FA, MD, radial and axial diffusivity (RD and AD). The fibre orientation density function (fODF) was estimated with the dRL algorithm in each voxel [5]. Data were also corrected for cerebrospinal fluid (CSF)-based partial volume artefacts using the Free Water Elimination (FWE) method [20]. The rationale for this correction was that ageing and neurodegeneration are associated with grey and white matter tissue loss, and CSF-based partial volume effects have been shown to lead to artificial decreases in FA and increases in MD.
Deterministic tractography
Whole brain dRL-based deterministic tractography was performed in ExploreDTIv4.8.3 [17] using the spherical deconvolution-based dRL algorithm following peaks in the fODF reconstructed from dRL [4, 5]. The dRL algorithm preserves angular resolution and reduces the number of false positive fibre orientations. For each voxel in the data set, streamlines were initiated along any peak in the fODF that exceeded an amplitude of 0.05. This enables multiple fibre pathways to be generated from any voxel. Each streamline was continued in 0.5 mm steps following the peak in the ODF that subtended the smallest angle to the incoming trajectory. The termination criterion was set to an angle threshold greater than 45°, thus streamlines that had angles above this threshold were excluded from the final tracts. Three-dimensional fibre reconstructions were obtained by applying waypoint ROI gates (“AND”, “OR” and “NOT” gates following Boolean logic) to isolate specific tracts from the whole brain tractography data.
Corticospinal tract (CST)
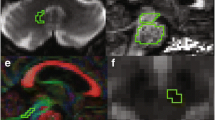
For the segmentation of the CST, the primary motor cortex was identified in the axial slice of a T1 image using a “SEED” region (in blue) (Fig. 1a (i)). Further, an “AND” region (in green) was drawn in the brain stem (identified as the blue colour of the pons in the anterior part of the brain stem) in the axial slice of an FA image (Fig. 1a (ii)). The CST of a representative subject on a sagittal slice of a T1 image is shown in (Fig. 1a (iii)).
Reconstructed motor tracts. (a) Corticospinal tract: (i) Axial slice of T1 image showing “SEED” region. (ii) Axial slice of FA image showing “AND” region. (iii) Sagittal slice of T1 image showing the reconstructed CST. (b) Reconstructed supplementary area-putamen tract and (c) thalamus-motor cortex tract on sagittal slices of T1 image
Thalamus-motor cortex (THAL-MC) and supplementary area-putamen (SMA-PUT) tracts
The ROIs for segmentation of THAL-MC and SMA-PUT tracts were derived by creating masks chosen from the AAL (Anatomical Automatic Labelling) atlas in the MNI (Montreal Neurological Institute) space. These masks were warped to the individual native space of each subject using inverse parameters derived from warping the T1 image to the AAL template. These masks were then used as “SEED” and “AND” regions in ExploreDTI for segmenting out the respective tracts. Unwanted regions and spurious tracts were removed by using the “NOT” gate. The final SMA-PUT and THAL-MC tracts in a sagittal slice of a T1 image of a representative subject are shown in Fig. 1b and c, respectively.
Uncinate fasiculus (UNF)
For segmentation of the UNF, the most posterior slice in the coronal view of a T1 image where the frontal and the temporal lobes are separated was chosen. The “SEED” region (in blue) was drawn around the temporal lobe (Fig. 2d (i)) and the “AND” region (in green) was drawn around the basal forebrain (Fig. 2d (ii)). The UNF tract of a representative subject on a sagittal slice of a T1 image is shown in (Fig. 2d (iii)).
Reconstructed non-motor tracts. (d) Uncinate fasiculus tract: (i) Coronal slice of T1 image showing “SEED” region for uncinate fasiculus segmentation. (ii) Coronal slice of T1 image showing “AND” region. (iii) Sagittal slice of T1 image showing reconstructed uncinate fasiculus tract. (e) Supero-lateral medial forebrain bundle: (i) Axial slice of T1 image showing “AND” region. (ii) Coronal slice of T1 image showing “AND” region. (iii) Sagittal slice of T1 image showing reconstructed supero-lateral medial forebrain bundle
Supero-lateral medial forebrain bundle (slMFB)
For reconstruction of the slMFB one horizontal “AND” region was placed surrounding the ventral tegmental area. The anatomical borders for this AND region were laterally the substantia nigra, anteriorly the mammillary bodies and posteriorly the red nucleus (Fig. 2e (i)). Another “AND” region was drawn surrounding the caudate and putamen on a coronal section at the height of the nucleus accumbens (Fig. 2e (ii)) [21]. The slMFB tract of a representative subject on a sagittal slice of a T1 image is shown in (Fig. 2e (iii)).
Each reconstructed tract was visually inspected and any obvious outlier streamlines that were not consistent with their known anatomy were excluded by drawing “NOT” regions and the entire procedure was performed separately for both hemispheres.
Subcortical volumes
We measured volumes of subcortical brain structures using FSL's FIRST software (FMRIB Image Registration and Segmentation Tool) [12]. The structures of the thalamus, caudate, putamen, pallidum, hippocampus, amygdala, nucleus accumbens and ventricles were extracted for both hemispheres and their volumes measured for all T1-weighted MR images of the PD patients and HCs. The extracted volumes were normalized using the scaling factor obtained from brain tissue normalization for subject head size, using SIENAX (Structural Image Evaluation using Normalization of Atrophy) [22].
Statistical analysis
All the statistical analyses were carried out in the R statistical software v2.15.3 [23]. Before data analysis, all variables were checked for Gaussian distribution using the Shapiro-Wilk test (p < 0.05) and were transformed using appropriate transformations if necessary.
We performed multivariate analyses of covariance (MANCOVAs) in each hemisphere for each of the five tracts separately with FA, MD, AD and RD as dependent variables and group (PD and HC) as independent variable, along with age and gender as covariates. These analyses were corrected for multiple comparisons using Bonferroni correction with a corrected threshold of p < 0.05 (0.05/10 = 0.005). Significant results were further analysed using post-hoc univariate ANOVAs also corrected for multiple comparisons using Bonferroni correction with a corrected threshold of p < 0.05 (0.05/32 = 0.00156). The inter-rater reliability of diffusion tensor indices derived from the manually reconstructed tracts was investigated using the inter-class correlation coefficient (ICC).
For the subcortical volumes analysis, we performed two sample t-tests comparing the corrected subcortical volumes of the PD patients and HCs.
We also extracted additional tractography measures of the number of reconstructed streamlines, number of voxels occupied by tract, tract volume and tract length for tracts with significant results from the MANCOVA also corrected for multiple comparisons using Bonferroni correction with a corrected threshold of p < 0.05 (0.05/32 = 0.00156).
The results of a complementary TBSS analysis are reported in the Supplementary material.
Results
In all PD patients and HCs, we obtained reliable reconstruction results of all investigated fibre tracts (Supplementary Table 1).
PD patients showed significantly higher FA in the motor (CST, SMA-PUT and THAL-MC) tracts but not in the non-motor (UNF and slMFB) tracts. These effects were statistically supported by significant multivariate effects for group, followed up by the relevant significant univariate effects.
Multivariate effects
The MANCOVAs demonstrated a significant effect of group (PD and HC) on the multivariate diffusion tensor indices (FA, MD, AD and RD) in the CST, SMA-PUT, THAL-MC and UNF, but not in the slMFB (Table 2).
Univariate group effects
Post-hoc analyses showed significantly increased FA and AD in right CST, and significantly increased FA in the left CST of the PD patients compared to HC (Table 3). In PD patients, there was significantly increased FA and AD in the right SMA-PUT tracts and significantly increased AD in the left SMA-PUT tracts. Similarly, there was significantly increased FA in both right and left THAL-MC tracts of the PD patients compared to HCs (Table 3). Conversely, in the UNF of PD patients, there was significantly decreased FA and significantly increased RD in the right UNF, and significantly increased MD in the left UNF (Table 3).
Subcortical volumes analysis
We found no significant volume changes between PD patients and HCs in the FSL FIRST analysis of subcortical and ventricular volumes (Supplementary Table 3).
Additional tractography measures
We found a significantly higher number of reconstructed streamlines, higher number of occupied voxels and larger tract volume in the tracts of PD patients compared to HCs (Supplementary Table 4).
Discussion
Patients with early-stage PD relative to age-matched controls demonstrated a specific increase in FA and AD in the white matter of motor tracts which was not present for two non-motor comparison tracts.
In the previous literature, neurodegenerative disorders have generally been associated with decreased FA in the major pathways, which has been attributed to primary white matter degeneration, demyelination, reduced gliosis or axonal damage as a result of grey matter loss [24–26]. Diffusion tensor-based ROIs and whole brain analyses in PD have shown reduced FA or increased MD in a priori regions such as the substantia nigra and putamen [2, 3, 27]. Thus only our finding of decreased FA and increased RD in the UNF, but not our findings of increased FA and AD in the CST and cortico-basal ganglia tracts, would be compatible with a general neurodegenerative process.
Neurodegenerative models
Our findings may reflect selective neurodegeneration. AD, which is the measure of diffusion in the principal fibre direction, is sensitive to the number of axons and their coherence [28] while RD, the measure of diffusion perpendicular to the principal diffusion direction, is thought to reflect decreased myelination [29]. Therefore, in our data the results of elevated FA in combination with elevated AD could suggest selective neurodegeneration causing lower neural branching, decreases of axonal diameter and thus higher coherence along the principal orientation. Increased FA in these tracts may also occur as a primary pathogenic consequence of altered pallido-thalamic activity in PD. A combined diffusion tensor and histology-based study of rat model of traumatic brain injury showed that an increase in FA in the cortical regions was correlated with gliosis [30]. It has been suggested that FA is more related to axonal package density and less to myelination [31]. Along these lines, our data could suggest a higher axonal package density of motor pathways as there were no significant differences in RD in our cohort.
Neuroplastic models
Alternatively, our findings might reflect early compensatory mechanisms associated with increases in axonal density in pathways of the cortico-basal-ganglia-thalamo-cortical loop. Such compensatory changes might preserve neural functions despite loss of the dopaminergic input [1]. A recent study in PD patients showed an increase in striato-cortical connectivity mediated by levodopa intake [32], hence it could also be suggested that increased FA in the SMA-PUT and THAL-MC tracts may be an adaptive response to abnormal dopaminergic modulation and levodopa intake.
Our results agree with the suggested compensation stages of PD where structures outside the basal ganglia, especially the SMA, would undergo change to counterbalance the putative abnormal activity in the thalamus and basal ganglia [1]. Considering the long time-course from the onset of PD-related pathology to the onset of clinical symptoms [33], the subcortical and cortical motor pathways may well have undergone compensatory and adaptive structural and functional reorganization long before PD patients have been diagnosed clinically. Such a model would be supported by our observation (Supplementary table 2) that changes in the diffusion metrics were not confined to the hemisphere contralateral to the side of dominant symptoms.
Recent evidence from PD rodent models showed axonal sprouting as compensatory mechanism [34], but this has not been studied in detail outside the nigrostriatal system. Based on our present findings, we suggest that there may be compensatory sprouting in motor tracts in response to decreased input from the thalamus and striatum as a consequence of PD pathology. We would further suggest that sprouting should also be assessed in corticospinal and thalamo-cortical motor tracts, both in animal models and in human brains post-mortem.
Methodological considerations
Diffusion tensor metrics are highly sensitive to white matter microstructural changes; however, they are non-specific indices with complex measures, hence one has to be cautious when inferring any specific biological mechanisms underlying changes in these measures [35]. Before interpreting our findings in terms of putatively increased structural connections in PD we need to exclude the possibility that they are influenced by methodological artefacts. We used the dRL algorithm which reduces the number of spurious fibre orientations that can produce artefactual reconstructions of tracts and is said to be robust in mapping cortical connectivity [5]. In patient and clinical studies, presence of degeneration makes the estimated tract reconstructions prone to isotropic partial volume effects [36] but the spherical deconvolution methods make it possible to obtain accurate white matter fibres that are corrected for partial volume effects and crossing fibres. Additionally, we found a higher number of reconstructed streamlines in PD patients than in HCs in the motor tracts and the UNF even though only the motor tracts showed increased FA whereas the UNF showed decreased FA. We can thus exclude that higher FA values were driven by the increase in number of reconstructed streamlines or number of occupied voxels or tract volume.
Because FA is a normalized measure it can be affected by changes in any of the compartments of white matter. For a better understanding of the specific contributions of axonal microstructural changes and myelination to changes in the white matter architecture in PD, future studies can apply complex diffusion microstructural models such as Composite Hindered and Restricted Model of Diffusion (CHARMED) or AxCaliber [26]. However, their clinical application in patients with movement disorders is limited by the long duration of scanning protocols. Recently developed myelin water fraction mapping and quantitative magnetization transfer techniques could also be used in future studies to better quantify myelination [37].
Conclusion
This first comprehensive deterministic tractography study of motor and non-motor tracts in PD revealed increased FA in the cortico-basal ganglia and CSTs, which may indicate compensatory mechanisms or structural changes related to altered pallido-thalamic activity in PD. We suggest that tractography be incorporated in longitudinal imaging studies of PD in order to evaluate the role of white matter changes in neurodegenerative and neuroplastic processes.
Abbreviations
- AD:
-
Axial diffusivity
- CST:
-
Corticospinal tract
- dRL:
-
Damped Richardson-Lucy
- FA:
-
Fractional anisotropy
- PD:
-
Parkinson’s disease
- slMFB:
-
Supero-lateral medial forebrain bundle
- SMA-PUT:
-
Supplementary motor area-putamen tract
- THAL-MC:
-
Thalamus-motor cortex tract
- UNF:
-
Uncinate fasiculus
References
Bezard E, Gross CE, Brotchie JM (2003) Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci 26:215–221
Meijer FJ, Bloem BR, Mahlknecht P, Seppi K, Goraj B (2013) Update on diffusion MRI in Parkinson's disease and atypical parkinsonism. J Neurol Sci 332:21–29
Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB (2012) White matter pathology in Parkinson's disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage 62:1675–1684
Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766
Dell'acqua F, Scifo P, Rizzo G et al (2010) A modified damped Richardson-Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage 49:1446–1458
Phillips O, Squitieri F, Sanchez-Castaneda C et al (2014) The corticospinal tract in Huntington's disease. Cereb Cortex. doi:10.1093/cercor/bhu065
Vergani F, Lacerda L, Martino J et al (2014) White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry 85:1377–1385
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425
Lindenbach D, Bishop C (2013) Critical involvement of the motor cortex in the pathophysiology and treatment of Parkinson's disease. Neurosci Biobehav Rev 37:2737–2750
Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136:1692–1707
Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B (2012) Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 24:223–236
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922
Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ (2011) Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci 31:13236–13245
Jones DK, Horsfield MA, Simmons A (1999) Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42:515–525
Jones DK, Williams SC, Gasston D, Horsfield MA, Simmons A, Howard R (2002) Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum Brain Mapp 15:216–230
(2011) Diffusion MRI: theory, methods and applications. Oxford University Press, Oxford
Leemans A, Jeurissen B, Sijbers J, Jones DK (2009) ExploreDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. Proceedings of the International Society for Magnetic Resonance in Medicine 17th Annual Meeting, Honolulu, Hawaii, 3536
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62:717–730
Bracht T, Doidge AN, Keedwell PA, Jones DK (2015) Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol Med 45:865–874
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Team RDC (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Concha L, Gross DW, Wheatley BM, Beaulieu C (2006) Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 32:1090–1099
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055
Assaf Y (2008) Can we use diffusion MRI as a bio-marker of neurodegenerative processes? Bioessays 30:1235–1245
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP (2013) Diffusion tensor imaging of nigral degeneration in Parkinson's disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin 3:481–488
Takahashi M, Ono J, Harada K, Maeda M, Hackney DB (2000) Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology 216:881–885
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436
Budde MD, Janes L, Gold E, Turtzo LC, Frank JA (2011) The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265
Herz DM, Haagensen BN, Christensen MS et al (2015) Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 21:2042–2051
Arkadir D, Bergman H, Fahn S (2014) Redundant dopaminergic activity may enable compensatory axonal sprouting in Parkinson disease. Neurology 82:1093–1098
Jones DK, Knosche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage 73:239–254
Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A (2008) Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol 7:715–727
Levesque IR, Giacomini PS, Narayanan S et al (2010) Quantitative magnetization transfer and myelin water imaging of the evolution of acute multiple sclerosis lesions. Magn Reson Med 63:633–640
Acknowledgments
We thank Dr Alistair Church, Dr Biju Mohammed, Dr Chris Thomas and Mrs Debbie Davies for their help with patient recruitment, and all patients, carers and control participants for their participation. We also thank Dr Nils Muhlert for his help with the subcortical volumes analysis.
The scientific guarantor of this publication is Prof. David E J Linden. The authors of this manuscript declare relationships with the following companies: Co-author Huw Morris has relationships with companies; Abbie, Teva, UCB, Boehringer-Ingelheim, GSK, Ipsen Fund, Medtronic, MNDA, PSP Association. This study has received funding by: Author Jilu Princy Mole is grateful for the support from Cardiff University's Overseas Research Scholarship (2014-2017) and MRes International Scholarship (2013-2014). This study received funding from the Wellcome Trust through the Institutional Strategic Support Fund to Cardiff University. The patient data were acquired as part of the study "Real-time fMRI Neurofeedback for Treatment of Parkinson's Disease" (NCT01867827). The aging study was supported by funding from the Medical Research Council, UK, via a Clinician Scientist Fellowship to Dr. Michael J. O'Sullivan. (G0701912). This work was supported by the Swiss National Science Foundation (SNF), Switzerland (to Tobias Bracht, Grant number PBBEP3_144797). Authors Jilu Princy Mole, Claudia Metzler-Baddeley and David E J Linden have statistical expertise in the analysis of neuroimaging data. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients and controls) in this study. Some study subjects or cohorts (the controls) have been previously reported in Metzler-Baddeley, C, Jones, DK, Belaroussi, B, Aggleton, JP and O'Sullivan, M (2011). Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. The Journal of Neuroscience, 31(37), 13236-13245 and Metzler-Baddeley, C, Jones, DK, Steventon, J, Westacott, L, Aggleton, JP and O'Sullivan, M (2012). Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. Journal of Neuroscience, 32(49), 17612-17619.
Methodology: case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 857 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mole, J.P., Subramanian, L., Bracht, T. et al. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur Radiol 26, 3327–3335 (2016). https://doi.org/10.1007/s00330-015-4178-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4178-1