Abstract
Objectives
To investigate whether CT-derived vascular parameters in primary breast cancer predict complete pathological response (pCR) to neoadjuvant chemotherapy (NAC).
Methods
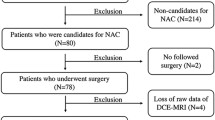
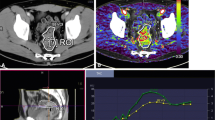
Twenty prospective patients with primary breast cancer due for NAC underwent volumetric helical perfusion CT to derive whole tumour regional blood flow (BF), blood volume (BV) and flow extraction product (FE) by deconvolution analysis. A pCR was achieved if no residual invasive cancer was detectable on pathological examination. Relationships between baseline BF, BV, FE, tumour size and volume, and pCR were examined using the Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed to assess the parameter best able to predict response. Intra- and inter-observer variability was assessed using Bland–Altman statistics.
Results
Seventeen out of 20 patients completed NAC with four achieving a pCR. Baseline BF and FE were higher in patients who achieved a pCR compared with those who did not (P = 0.032); tumour size and volume were not significantly different (P > 0.05). ROC analysis revealed that BF and FE were able to identify responders effectively (AUC = 0.87; P = 0.03). There was good intra- and inter-observer agreement.
Conclusions
Primary breast cancers which exhibited higher levels of perfusion before treatment were more likely to achieve a pCR to NAC.
Key Points
• CT-derived vascular parameters may be useful in breast cancer treatment.
• Perfusion CT can help predict response to neoadjuvant chemotherapy in breast cancer.
• Baseline blood flow and flow extraction product are higher in complete pathological responders.




Similar content being viewed by others
References
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Sledge GW Jr (2002) Vascular endothelial growth factor in breast cancer: biologic and therapeutic aspects. Semin Oncol 29:104–110
Jeswani T, Padhani AR (2005) Imaging tumour angiogenesis. Cancer Imaging 5:131–138
Kambadakone AR, Sahani DV (2009) Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am 47:161–178
Miles KA, Williams RE (2008) Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging 8:81–86
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42
Park CM, Goo JM, Lee HJ et al (2009) FN13762 murine breast cancer: region-by-region correlation of first-pass perfusion CT indexes with histologic vascular parameters. Radiology 251:721–730
Tateishi U, Kusumoto M, Nishihara H et al (2002) Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer 95:835–842
Li Y, Yang ZG, Chen TW et al (2008) Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer 61:44–53
Ma SH, Le HB, Jia BH et al (2008) Peripheral pulmonary nodules: relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression. BMC Cancer 8:186
Goh V, Halligan S, Daley F et al (2008) Colorectal tumor vascularity: quantitative assessment with multidetector CT—do tumor perfusion measurements reflect angiogenesis? Radiology 249:510–517
d'Assignies G, Couvelard A, Bahrami S et al (2009) Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 250:407–416
Wang JH, Min PQ, Wang PJ et al (2006) Dynamic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol 186:1423–1430
Jones RL, Lakhani SR, Ring AE et al (2006) Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer 94:358–362
Haberland U, Klotz E, Abolmaali N (2010) Performance assessment of dynamic spiral scan modes with variable pitch for quantitative perfusion computed tomography. Invest Radiol 45:378–386
Saddi KA, Chefd'hotel C, Cheriet F (2007) Large deformation registration of contrast-enhanced images with volume-preserving constraint. In: Pluim JPW, Reinhardt JM (eds) Medical imaging 2007: image processing. Proc SPIE, vol 6512. The International Society for Optical Engineering (SPIE), Bellingham
Goetti R, Leschka S, Desbiolles L et al (2010) Quantitative computed tomography liver perfusion imaging using dynamic spiral scanning with variable pitch: feasibility and initial results in patients with cancer metastases. Invest Radiol 45:419–426
Reiner CS, Goetti R, Eberli D et al (2012) CT perfusion of renal cell carcinoma: impact of volume coverage on quantitative analysis. Invest Radiol 47:33–40
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Mukherji SK, Castelijns JA (2010) CT perfusion of head and neck cancer: why we should care versus why should we care! AJNR Am J Neuroradiol 31:391–393
Gandhi D, Chepeha DB, Miller T et al (2006) Correlation between initial and early follow-up CT perfusion parameters with endoscopic tumor response in patients with advanced squamous cell carcinomas of the oropharynx treated with organ-preservation therapy. AJNR Am J Neuroradiol 27:101–106
Petralia G, Preda L, Giugliano G et al (2009) Perfusion computed tomography for monitoring induction chemotherapy in patients with squamous cell carcinoma of the upper aerodigestive tract: correlation between changes in tumor perfusion and tumor volume. J Comput Assist Tomogr 33:552–559
Bisdas S, Rumboldt Z, Surlan-Popovic K et al (2010) Perfusion CT in squamous cell carcinoma of the upper aerodigestive tract: long-term predictive value of baseline perfusion CT measurements. AJNR Am J Neuroradiol 31:576–581
Hermans R, Meijerink M, Van den Bogaert W et al (2003) Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys 57:1351–1356
Zima A, Carlos R, Gandhi D et al (2007) Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJNR Am J Neuroradiol 28:328–334
Wang J, Wu N, Cham MD, Song Y (2009) Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol 193:1090–1096
Tacelli N, Remy-Jardin M, Copin MC et al (2010) Assessment of non-small cell lung cancer perfusion: pathologic-CT correlation in 15 patients. Radiology 257:863–871
Ng QS, Goh V, Fichte H et al (2006) Lung cancer perfusion at multi-detector row CT: reproducibility of whole tumor quantitative measurements. Radiology 239:547–553
Lind JS, Meijerink MR, Dingemans AM et al (2010) Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol 20:2890–2898
Ng QS, Goh V, Carnell D et al (2007) Tumor antivascular effects of radiotherapy combined with combretastatin a4 phosphate in human non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 67:1375–1380
Goh V, Halligan S, Gharpuray A et al (2008) Quantitative assessment of colorectal cancer tumor vascular parameters by using perfusion CT: influence of tumor region of interest. Radiology 247:726–732
Goh V, Halligan S, Gartner L et al (2006) Quantitative colorectal cancer perfusion measurement by multidetector-row CT: does greater tumour coverage improve measurement reproducibility? Br J Radiol 79:578–583
Sahani DV, Kalva SP, Hamberg LM et al (2005) Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 234:785–792
Koukourakis MI, Mavanis I, Kouklakis G et al (2007) Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol 30:315–318
Bellomi M, Petralia G, Sonzogni A et al (2007) CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 244:486–493
Goh V, Halligan S, Wellsted DM, Bartram CI (2009) Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol 19:79–89
Park MS, Klotz E, Kim MJ et al (2009) Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology 250:110–117
Makari Y, Yasuda T, Doki Y et al (2007) Correlation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancers. J Surg Oncol 96:220–229
Ng CS, Wang X, Faria SC et al (2010) Perfusion CT in patients with metastatic renal cell carcinoma treated with interferon. AJR Am J Roentgenol 194:166–171
Fournier LS, Oudard S, Thiam R et al (2010) Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 256:511–518
Jinzaki M, Tanimoto A, Mukai M et al (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24:835–842
Hirasawa H, Tsushima Y, Hirasawa S et al (2007) Perfusion CT of breast carcinoma: arterial perfusion of nonscirrhous carcinoma was higher than that of scirrhous carcinoma. Acad Radiol 14:547–552
Willett CG, Boucher Y, di Tomaso E et al (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145–147
Xiong HQ, Herbst R, Faria SC et al (2004) A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs 22:459–466
Kerbel RS, Klement G, Pritchard KI, Kamen B (2002) Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol 13:12–15
Fisher ER, Wang J, Bryant J et al (2002) Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 95:681–695
Wang J, Buchholz TA, Middleton LP et al (2002) Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer 94:3107–3114
Davies MM, Burke D, Carnochan P et al (2002) Basic fibroblast growth factor infusion increases tumour vascularity, blood flow and chemotherapy uptake. Acta Oncol 41:84–90
Smith IC, Welch AE, Hutcheon AW et al (2000) Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 18:1676–1688
Park SH, Moon WK, Cho N et al (2010) Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 257:56–63
Mankoff DA, Dunnwald LK, Gralow JR et al (2002) Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med 43:500–509
Ah-See ML, Makris A, Taylor NJ et al (2008) Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 14:6580–6589
Martincich L, Montemurro F, De Rosa G et al (2004) Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat 83:67–76
Ng QS, Goh V, Klotz E et al (2006) Quantitative assessment of lung cancer perfusion using MDCT: does measurement reproducibility improve with greater tumor volume coverage? AJR Am J Roentgenol 187:1079–1084
Kalender WA, Beister M, Boone JM et al (2012) High-resolution spiral CT of the breast at very low dose: concept and feasibility considerations. Eur Radiol 22:1–8
Miles KA, Griffiths MR (2003) Perfusion CT: a worthwhile enhancement? Br J Radiol 76:220–231
Goh V, Halligan S, Hugill JA, Bartram CI (2006) Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol 187:164–169
Acknowledgements
Kings College London receives funding from the National Institute for Health Research (NIHR) as a Comprehensive Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S.P., Makris, A., Gogbashian, A. et al. Predicting response to neoadjuvant chemotherapy in primary breast cancer using volumetric helical perfusion computed tomography: a preliminary study. Eur Radiol 22, 1871–1880 (2012). https://doi.org/10.1007/s00330-012-2433-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2433-2